Abstract
Considerable adverse side effects and cytotoxicity of highly potent drugs for healthy tissue require the development of novel drug delivery systems to improve pharmacokinetics and result in selective distribution of the loaded agent. The introduction of targeted liposomal formulations has provided potential solutions for improved drug delivery to cancer cells, penetrating delivery through blood–brain barrier and gene therapy. A large number of investigations have been developed over the past few decades to overcome pharmacokinetics and unfavorable side effects limitations. These improvements have enabled targeted liposome to meet criteria for successful and improved potent drug targeting. Promising in vitro and in vivo results for liposomal-directed delivery systems appear to be effective for vast variety of highly potent therapeutics. This review will focus on the past decade’s potential use and study of highly potent drugs using targeted liposomes.
Introduction
Liposomes abundance utilizations for therapeutic benefit have been continuously expanded due to their flexible structures and practical function since their introduction in 1960s (Balazs and Godbey Citation2011). Liposomes advantages over other drug delivery systems are their capability for transporting and protecting diverse therapeutic biomolecules including both hydrophilic and hydrophobic drugs, their desirable biocompatibility and biodegradability, different range of morphologies and sizes based on easy manipulation of their composition, low toxicity, nonimmunogenicity and low costs (Tiwari et al. Citation2012, Voinea and Simionescu Citation2002). Hence, there are numerous lipid formulations for liposomal drug and gene delivery application. Moreover, constant improvement and modification to control their biological properties and promote their pharmacokinetics characteristics have been investigated via addition of different lipids and targeting moieties (Kong et al. Citation2012, Sercombe et al. Citation2015).
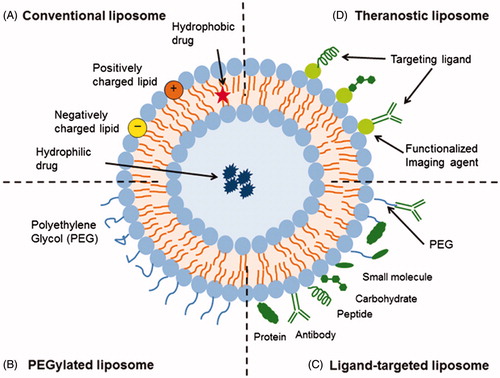
Among variety of liposome modifications, active targeting emerges as a useful and common strategy in order to enhance liposomal delivery system’s desired properties such as targeting ability, increased selectivity, increased cellular internalization, prolonged duration of exposure, minimized adverse effects, and improved therapeutic index (Puri et al. Citation2009, Zamboni Citation2005). As a result, expanding number of receptors has been significantly progressed to target therapeutic cargo’s delivery in a preferred manner (Deshpande et al. Citation2013). The different types of liposomal drug delivery systems including conventional, PEGylated, ligand targeted and theranostic liposomes have shown in . All types of liposomes enclose an aqueous core and based on their versatile structure, they are capable of carrying both hydrophobic drugs (in their phospholipid bilayer) and hydrophilic drugs (in their lumen) (Daraee et al. Citation2016, Sercombe et al. Citation2015). However, low encapsulation efficiency of hydrophilic drugs resulted in undertaking many strategies in order to increase encapsulation such as the preparation method of the liposome, particularly and expansively the cycles of freeze and thaw and the reverse phase evaporation, the proper selection of the composition, the formulation of liposomes with larger particle size, the adjustment of the zeta potential to maximize electrostatic interaction between the charged drug (Eloy et al. Citation2014).
Figure 1. Schematic representation of the different types of liposomal drug delivery systems including (A) conventional (B) PEGylated (C) ligand-targeted and (D) theranostic liposomes. In addition, the site of hydrophobic and hydrophilic drug placing has shown. (Taken from Susan Hua work, with permission from author and journal) (Sercombe et al., Citation2015).
In addition to these statements, rapid advances in medicine and biotechnology result in growing number of highly potent compounds to overcome incurable and fatal diseases such as cancers, diabetes, etc. Despite the fast discovery and development race in this field, many highly potent drug candidates have encountered serious barriers because of their limited bioavailability, low solubility, poor efficacy, rapid clearance, low stability, high nonspecific toxicity to healthy organs and increased occurrence of side effects (Basavaraj and Betageri Citation2014, Naseri et al. Citation2015). Therefore, the essence for designing and creating of novel drug delivery systems to improve the pharmacokinetics and pharmacodynamics of each therapeutic and to overcome stated delivery obstacles was widely felt. Among available drug delivery platforms, the inherent advantages of liposomes that are mentioned earlier make them the most commonly studied candidate to deliver drugs, proteins, polypeptides, genes, and nucleic acids (Chime and Onyishi Citation2013). The focus of this review is to provide detailed descriptions of different lately investigated surfaced modified liposomes as a promising targeted delivery system for potent drugs because of recent increased interests in the use of targeted liposomes.
Anticancer drugs
Growing use of potent drugs to overcome fatal disease has become a serious concern. According to their unwanted side effects and damages on healthy and normal tissues, their common and unavoidable usages have resulted in fundamental restriction. Hence, with the advent of extensive adverse effects of highly potent chemical drugs and the significant need to improve their therapeutics, closed phospholipid bi-layered structures called liposome have received a substantial attention as a pharmaceutical carrier during the past 30 years (Deshpande et al. Citation2013). Using liposome-mediated delivery reduces toxicity and increases efficacy by modifying therapeutic index. Furthermore, merging the concept of passive and active targeting for liposomes has shown promising results such as reduced systemic toxicity, increased cellular uptake, selective drug targeting and controlled release in vitro, in vivo and clinical data. Passive targeting (enhanced permeability and retention (EPR) effect mediated) requires the longevity of the pharmaceutical carrier in the blood by incorporating into a macromolecule or nanoparticle and its accumulation in pathological sites and active targeting requires the conjugation of specific ligand to the surface of pharmaceutical carriers to recognize and bind pathological cells (Rahman et al. Citation2016, Singh and Lillard Citation2009). There are several encouraging reports from early clinical applications and clinical trials based on using liposomes for different cancers treatment, Alzheimer's and Parkinson's disease, inflammatory diseases, parasitic diseases, fungal infections, and bacterial infections (Lopez-Berestein and Fidler Citation1989, Spuch and Navarro Citation2011).
Cisplatin is a highly potent alkylating antineoplastic agent and commonly used for the treatment of many cancers including melanoma, lung, lymphoma, ovarian, testicular, cervical, bladder, testicular, head and neck cancers. Due to its high toxicity (nephrotoxicity, ototoxicity, and neurotoxicity), designing proper delivery systems during treatments remains still as significant challenges (Apps et al. Citation2015). Wang et al. have developed conjugated sodium alginate (SA) to cisplatin, incorporated into PEGylated liposomes and were modified with epidermal growth factor (EGF) to specifically target EGFR-expressing tumors (Wang et al. Citation2014). The in vitro experiments on SKOV3 cells and in vivo xenograft experiments have revealed enhanced delivery of cisplatin and its antitumor efficacy to EGFR-positive ovarian tumor cells that is caused tumor-specific accumulation and induced cell-cycle arrest and apoptosis in a time-dependent manner, as well as reducing nephrotoxicity and lower body weight loss in mice. In addition, Hirai et al. have established a novel in vivo antitumor therapy with Sialyl LewisX conjugated cisplatin-loaded liposome to selectively target tumors and to substantially reduce the toxic side effects of high dose of cisplatin (Hirai et al. Citation2010). In another study, Lv et al. have investigated cisplatin liposome modified with transferrin (TF) in order to target glioma aggressive type of primary intracranial neoplasm and subsequently result into passing of drug through the blood–brain barrier (Lv et al. Citation2013). Their novel drug delivery system has exhibited potential ability to cross the BBB in a clathrin-mediated endocytosis way and subsequently was targeted to glioma cells and also was resulted in a higher cytotoxicity and much more potent sequential-target to glioma cells in vitro.
Oxaliplatin (trans-l-diaminocyclohexane oxalatoplatinum, l-OHP) is a cisplatin derivative that was designed to improve side effects such as toxicity to the kidney and peripheral nerve system (Apps et al. Citation2015). Suzuki et al. have described novel target-sensitive TF–PEG-liposomes, called pendant-type PEG immunoliposomes against solid tumors (Suzuki et al. Citation2008). Their investigation has showed high concentration of l-OHP being maintained in mouse tumor tissue and that tumor growth was more suppressed in various types of tumors that overexpress TF receptors.
Paclitaxel, a potent chemotherapeutic agent and a microtubule-stabilizing drug, is used for the treatment of breast, ovarian, colon, head and neck and nonsmall cell lung cancers (Peltier et al. Citation2006). A novel float receptor (FR)-targeted liposomal paclitaxel formulation by Wu et al. was composed of DPPC/DMPG/mPEG-DSPE/folate-PEG-DSPE has exhibited excellent colloidal stability and good drug loading properties in addition to the prolonged circulation time and efficiently taken up by FR + KB, human oral carcinoma cells (Wu et al. Citation2006). In another study, the in vitro and in vivo drug delivery characteristics of a paclitaxel-loaded anti-HER2 PEGylated immunoliposomal formulation have been prepared by Yang et al. which showed efficient and specific delivery of paclitaxel, slower disease progression, and appropriate response rate in patients with human epidermal growth factor receptor-2 (HER2)-overexpressing tumors (Yang et al. Citation2007).
In the earlier stages of targeting of liposomes with HER2 receptor, one of the thoroughly studied models for immunoliposomal formulations was the application of the whole part of mAb as a target moiety, but advances in antibody engineering have allowed the use of antibody fragments such as Fab′ and single-chain fragment variable (scFv) as targeting agents (Kontermann Citation2006, Park et al. Citation2002). scFvs are recombinant molecules in which the variable regions of heavy and light immunoglobulin chains encoding antigen-binding domains are engineered into a single polypeptide (Nelson Citation2010). In recent years, scFv has gained popularity over traditional targeting agents because of several potential advantages including slower clearance, reduce immunogenic risk (engineer fully human fragments), lower production cost, using phage display and select desired affinity and specificity, better identification and purification with engineering tags into scFv constructs (Cheng and Allen Citation2010).
Tamoxifen (TAM) is an antagonist of the estrogen receptor in breast tissue. It has been used in hormone (antiestrogen) therapy for early breast cancer and other types of cancer to block the actions of estrogen and also is able to reverse multidrug resistance (MDR) protein efflux of drugs (Sugimoto et al. Citation2003). Epirubicin is a new potent anthracycline derivative of doxorubicin (DOX) used for chemotherapy and a broad-spectrum antineoplastic agent. Epirubicin has been clinically used in treating parenchymal organ cancers, breast cancer, ovarian cancer, non-Hodgkin’s lymphomas, pancreatic cancer, soft-tissue sarcomas, acute leukemia, gastric cancer, small-cell lung cancer plus inhibiting brain tumor cells in vitro (Safra Citation2003). Tian et al. have developed a new kind of functionalized epirubicin liposomes, modified with two targeting ligands, TAM and TF which significantly transport drug-loaded liposomes across the blood–brain barrier (BBB) and then target the brain glioma cells in vitro and in animals (Tian et al. Citation2010). Another newly liposomal nanostructure of functional targeting epirubicin, dually modified with aminophenyl glucose and cyclic pentapeptide, was made by Zhang et al. to treat brain glioblastoma (Zhang et al. Citation2015). The designated nanocarrier have exhibited the ability to be transported across the blood–brain barriers via the carrier-mediated transcytosis, efficient in killing glioblastoma cells and in destroying glioblastoma neovasculature in vitro and in vivo, targeting effects via the integrin β3 receptor and prolonged circulation in the blood system which presented functional targeting epirubicin liposomes as an effective therapy for disabling neovascularization in the brain glioblastoma region and treating brain glioblastoma.
Lonidamine a derivative of indazole-3-carboxylic acid is a potent apoptotic inducer of cancer cells. It had been used to treat nonsmall-cell lung carcinoma (NSCLC), benign prostate hypertrophy (BPH) metastatic, brain tumors, and breast cancer. It has revealed a serious side effect on liver. Li et al. have developed mitochondria-specific targeting lonidamine liposomes modified with a dequalinium polyethylene glycol distearoylphosphatidylethanolamine (DQA-PEG2000-DSPE) conjugate as a cotherapy with targeting epirubicin liposomes to circumvent drug-resistant lung cancer and NSCLC (Li et al. Citation2013). The results have suggested enhanced accumulation in tumor tissue via the long-circulatory effect and reduced immediate aggregation in live tissue.
Doxorubicin, an anthracycline antitumor antibiotic used in chemotherapy for the treatment of wide range of cancers, including breast, ovary, gastric, liver, kidney, endometrial, blood cancers like leukemia and lymphoma and many types of carcinoma (solid tumors) and soft-tissue sarcomas. It has showed serious adverse effects including threatening heart damage, nausea or vomiting, low blood counts, hair loss which were consider as its limiting factor for the usage of this common anticancer drug (Safra Citation2003). Gabizon et al. and Li et al. have prepared doxorubicin-loaded stealth liposomes conjugated transferrin that have revealed prolonged residence time in the circulation and low reticulo-endothelial system (RES) uptake in tumor-bearing mice. All these were resulted in enhanced extravasation of the liposomes into the solid tumor tissue and enhanced intracellular uptake of the entrapped DOX and improved therapeutic efficacy of liver cancer (Gabizon et al. Citation2004, Li et al Citation2009). Based on Tuscano et al. report, xenograft mouse model of delivering CD22-targeted PEGylated liposomal doxorubicinin a B-cell non-Hodgkin's lymphoma have exhibited improved cytotoxicity, higher drug accumulation in tumor and greater tumor volume reduction, so that survival was occurred more effectively than nontargeted liposomal DOX (Tuscano et al. Citation2010). Moreover, in recent years, aptamers with the highest binding affinity and specificity as novel strategies and promising molecules in drug delivery are the most frequently used ligands for targeting (Nimjee et al. Citation2005). In this regard, Song et al. have developed a novel DOX-loaded DOTAP:DOPE liposomes delivery system by conjugating a target tumor cell-specific aptamer that was selected among 10 potential generated aptamers. In vitro and in vivo studies on the selected aptamer (SRZ1) have revealed promoted uptake efficiency, enhanced DOX accumulation, highest and specific binding affinity to 4T1 cells and significant suppression of tumor growth in tumor-bearing mice (Song et al. Citation2015).
Additionally phase-1 clinical study of anti-EGFR immunoliposomes loaded with DOX (anti-EGFR ILs-DOX) in patients with solid tumors was assessed by Mamot et al. where produced nanobody-targeted liposomes in the dose of 50 mg doxorubicin per m2 have showed promising antitumor activity and the drug was well tolerated (Mamot et al. Citation2012). Since nanobodies discovery in 1993, a number of variable domain of heavy-chain antibodies with (1) high specificity and affinity (2) more stability and selectivity (3) low toxicity and immunogenicity (4) ease of production, are being utilized as therapeutic targets in many fields of medicine (Oliveira et al. Citation2013, Steeland et al. Citation2016).
In addition, Van der Meel et al. have utilized simultaneous liposomal targeting, using EGFR and insulin-like growth factor 1 receptor (IGF-1R) loaded with kinase inhibitor (AG538). This multivalent nanobody liposomal system loaded with kinase inhibitor for cancer treatment was able to block both EGFR and IGF-1R activation and induce a strong inhibition of tumor cell proliferation (van der Meel et al. Citation2012).
According to serious side effects of DOX and its extensive use, the necessity of designing effective delivery system and improving selective localization of this commonly used chemotherapy drug to reduce its cytotoxicity, plenty of researches have been reported for innovative targeted liposomal doxorubicin formulation (Chang et al. Citation2013, Mamot et al. Citation2003, Medina et al. Citation2011, Paliwal et al. Citation2011, Pastorino et al. Citation2003, Wang et al. Citation2012).
Daunorubicinis chemotherapeutic of the anthracycline antitumor antibiotic family and widely used in treating a variety of malignancies and specific types of leukemia (acute myeloid leukemia and acute lymphocytic leukemia). However, its clinical utility is hampered by the myelosuppression, cardiotoxicity and resistance. Ying et al. have prepared novel dual-targeting daunorubicin PEGylated liposomes by conjugating with p-aminophenyl-α-D-mannopyranoside (MAN) and TF which transported drug across the BBB to treat brain glioma (Ying et al. Citation2010). This liposomal systems have exhibited significant increase in transport of the drug-loaded liposomes across the BBB model and antineoplastic effect to C6 glioma cells in vitro and enhanced median survival time on C6 glioma-bearing rats in vivo. In another study, Zhang et al. have developed mitochondrial-targeting liposomes that incorporate daunorubicin plus quinacrine for relapsed breast cancer treatment which is arised from the cancer stem cells (Zhang et al. Citation2012). The designated system has showed well-distributed particle size with high encapsulation efficiency and delayed drug release besides long circulatory effect and selective accumulation in mitochondria that eventually cause apoptosis of MCF-7 cancer stem cells. Daunorubicin-loaded folate polyethylene glycol–cholesterol hemisuccinate (folate–PEG–CHEMS) anchored liposomes is another study that have developed by Xiong et al. (Citation2011). The novel targeted liposomal daunorubicin has showed improved in vitro cytotoxicity effect on L1210JF cells and in vivo significant prolonged life span on an L1210JF murine leukemia tumor model and enhanced in vitro and in vivo endocytosis of liposomes into L1210JF cells via FR cell-specific delivery.
Deferoxamine (DFO), a potent iron chelator used to remove excess iron from the body. With its increased usage due to its short half-life and poor absorption, considerable toxicity for cardiovascular and pulmonary has been seen. Thus, an efficient delivery system that intensifies its cell-specific bioavailability is essential. Schuster et al. have developed DFO-loaded PEGylated immunoliposomes that specifically targets fibroblast activation protein (FAP) by single-chain fragment variable (scFv) (Schuster et al. Citation2015). It has exhibited specific binding and uptake into FAP-expressing cells and significant reduction in the collagen deposition. There are also further examples of various highly potent drugs that were applied in the form of immunoliposomes to (1) increase specificity of cells, (2) decrease cytotoxicity (3) provide high rates of uptake (4) enhance drug delivery, such as indinavir, vincristine, ceramides, mitoxantrone, mitoxantrone, irinotecan that were explained briefly in .
Table 1. Highly potent chemical drugs delivered using targeted liposomes.
Peptide/protein drugs
Until now, US Food and Drug Administration (FDA) have approved more that 200 proteins and peptides as therapeutic modalities to treat variety of human diseases based on their high potency and specificity besides less toxicity to normal tissues (Cryan Citation2005). Hence, designing an appropriate delivery system for them become a challenge due to their (1) immunogenicity, (2) inadequate stability and rapid clearance, (3) short plasma half-life and shelf-life, (4) low penetration across biological membranes (Lu et al. Citation2006). Therefore, among different novel drug delivery systems, liposomes are one of the most promising systems that have the potential to overcome current limitations and consider as next generation protein therapeutics (Pisal et al. Citation2010). Protein and peptide drugs can be either encapsulated or chemically conjugated to the surface group of liposomes (Martins et al. Citation2007). Long circulation and target sensitive liposomes are two approaches that were followed extensively to obtain liposomal protein and peptide delivery; however, considerable researches have been created for active and antibody-targeted structures (Huang and Zhou Citation1992, Lu et al. Citation2006).
Hua et al. developed tissue plasminogen activator (tPA) loaded microbubbles and targeted by arginine–glycine–aspartic acid–serine peptide for the ultrasound-assisted drug thrombolysis. Results have represented that in vivo tPA release and thrombolytic efficacy were promising which was lead to significant hemorrhagic risk decreases (Hua et al. Citation2014). Mastrobattista et al. have also entrapped pH-dependent fusogenic peptide (diINF-7) together with diphtheria toxin A chain (DTA) inside immunoliposomes, targeted to the epidermal growth factor receptor (EGFR) on the surface of ovarian carcinoma cells. Their results have showed great enhance in cytosolic delivery of immunoliposome-entrapped proteins by pH-dependent destabilization of endosomal membrane after cellular uptake of liposomes (Mastrobattista et al. Citation2002).
As already mentioned elsewhere passive targeting besides pegylation of liposomal protein and peptide are prevalent and well-worked structures to overcome their instability and short half-life (Deshpande et al. Citation2013, Milla et al. Citation2012). The preclinical efficacy of the cytokine-loaded liposomes to deliver interleukin-12 (IL-12) into several human tumors and to reactive tumor-associated T cells in situ by Simpson-Abelson et al. have exhibited intratumoral liposome IL-12 delivery and local and sustained release of IL-12 from liposomes, which causes reactivates resident lymphocytes to secrete IFN-γ in different epithelial tumor types (Simpson-Abelson et al. Citation2009). Based on Kanaoka et al. report, liposomal IL-2 resulted in better distribution, enhanced the duration time in the systemic circulation and improved therapeutic effect against tumor after subcutaneous administration to mice (Kanaoka et al. Citation2003).
In another study, Kisel et al. have prepared liposomal carrier for oral delivery of insulin. In vitro oral administration of liposomes resulted in hyperinsulinemia that was attended by a decrease in blood glucose concentration as a consequence of interlocking of receptors with highly resistant lipid–insulin complexes (Kisel et al. Citation2001).
Also another recent crucial development in the field of liposomal protein delivery systems is simultaneous administration of multiple therapeutic agents such as chemotherapeutics and proteins for achieving enhanced therapeutic effects and elevated tumor inhibition efficiency in treatment of different types of cancers (Eldar-Boock et al. Citation2013, He et al. Citation2016). Cabanes et al. have assessed in vivo therapeutic effect of Doxil and liposomal IL-2 which was resulted in long-term survivors against M109 pulmonary metastases in mice. Their research has showed that synergistic liposome-based chemoimmunotherapy is highly efficacious approach to eradicate regionally spread tumors and metastasis (Cabanes et al. Citation1999). Accordingly, delivery of the dual or multiple cargos, such as protein and peptides beside chemotherapeutic drugs, will enhance sensitivity of tumors to therapeutic agents and reduced dose of toxic drugs and adverse effects (Mo et al. Citation2014). In , there are given some extra examples of targeted and non-targeted liposomal protein delivery systems.
Table 2. Highly potent protein and peptide drugs delivered using targeted liposomes.
miRNA, siRNA, and DNA therapeutics
The potential use of gene therapy via viral and nonviral vectors holds great promise and has been equipped modern medicine with new achievements that were unthinkable two decades ago (Mali Citation2013). High safety, low immunogenicity, large capacity and also possibility of repeat administration of nonviral vectors in comparison to virus-derived vectors, has made them as a popular choice in targeted transgenic delivery system (Ramamoorth and Narvekar Citation2015).
The first common nonviral gene transfer vectors were naked-DNA or plasmid DNA (pDNA) that was placed in liposomes in order to enhance cellular DNA uptake (Al-Dosari and Gao Citation2009). Recently, siRNAs, microRNAs, single-stranded antisense oligonucleotides (ODNs) and ribozymes as four major types of anti-mRNA strategies have been broadly used in the treatment of cancers, infections, hematological diseases, antiviral diseases, neurodegenerative diseases, dominantly inherited genetic disorders, cardiovascular disorders, and pain research and many other illnesses (Majzoub et al. Citation2016, Movahedi et al. Citation2015). Since application of small interfering RNA (siRNA) as a potent sequence-selective inhibitors of transcription, encountered with serious limitation due to (1) strong electrostatic repulsion between anionic cell surface charge and anionic phosphate backbone charge of naked siRNA (2), activating immunogenic responses (3), poor tissue and cell membrane penetration (4), low transfection efficiency (5), rapid clearance and poor pharmacokinetic property (6), arising unfavorable biological responses such as off-target effects and interferon response (Kanasty et al. Citation2013). Therefore, the essence of designing efficient delivery system in order to deliver and internalize the siRNA to the cells of target tissue still remains a challenge (Shim and Kwon Citation2010). Hence, various successful examples of liposomal delivery systems have been developed contain using cationic or ionizable lipids, shielding lipids, cholesterol, and targeting ligands in order to achieve cell-specific delivery system (Huang and Liu Citation2011, Whitehead et al. Citation2009). Among them, the ability of targeted anti-mRNA liposomal delivery systems in covering protection of entrapped anti-mRNA formulation from renal clearance and nuclease degradation besides providing efficient cellular uptake and endosomal escape is of utmost importance (Gandhi et al. Citation2014, Pereira et al. Citation2013).
Asai et al. have developed tetraethylenepentamine-based polycation liposomes, active targeted with Ala-Pro-Arg-Pro-Gly (APRPG-TEPA-PCL) for the systemic delivery of siRNA used for targeting angiogenic endothelium. Intravenous injection of APRPG–TEPA–PCL into Colon26 NL-17 carcinoma-bearing mice have showed liposome accumulation in the tumor, which have confirmed APRPG–PEG conjugation to liposome and it was a useful strategy for both drug- and gene-targeted delivery to angiogenic vessels in tumors (Asai et al. Citation2011). In another study, Sato et al. have demonstrated potential therapeutic efficacy of encapsulated siRNA, vitamin A–coupled liposomes for reversing human liver cirrhosis. Their study has exhibited prolonged survival time and liver fibrosis resolve in rat (Sato et al. Citation2008). In addition, Feng et al. have concluded that folate receptor-targeted liposomes, delivering MYCN siRNA into LA-N-5 cells, have efficient suppressive effect on MYCN mRNA expression both in vitro and in vivo and capable of treating various tumors (Feng et al. Citation2010). In another study, Yu-Wai-Man et al. have optimized receptor-targeted liposome peptide nanoparticles incorporating MRTF-B siRNA to develop a safe and efficient nonviral siRNA delivery system to prevent fibrosis after glaucoma filtration surgery and other contractile scarring conditions in the eye. Their results suggested that after a single transfection treatment the MRTF-B gene in human Tenon’s fibroblasts was efficiently silenced and collagen matrix contraction was completely blocked (Yu-Wai-Man et al. Citation2016). Furthermore, Li et al. have developed aptamer-targeted PEGylated liposome (AS1411ePEG-liposome) to deliver anti-BRAF siRNA (siBraf) for melanomas treatment. AS1411, an aptamer as a targeting probe was resulted in the inhibition of the melanoma growth in vitro and in vivo because of higher selective accumulation of the siRNA in tumor cells comparing with normal cells and significant siRNA silencing activity in A375 tumor xenograft mice (Li et al. Citation2014).
Over the past decade, among small RNAs, microRNAs (miRNAs) approximately 21-nucleotide-long, have become a major focus of researches in target gene therapy by gene silencing via the recruitment of corresponded mRNA into the RNA-induced silencing complex and cleavage of the target mRNA resulting in selective inhibition of expressed unwanted protein (Mourelatos Citation2008, Zhang et al. Citation2012). The most common nonviral strategy for disease treatment thorough miRNAs transfection is liposomal delivery system that frequently has been used both in vitro and in vivo however understanding the molecular mechanisms of these therapeutic vehicles needs substantial investigation in order to be employed in clinical trials (Zhang et al. Citation2012). Cyclic RGD peptide (cRGD)-functionalized a PEGylated LPH (liposome–polycation–hyaluronic acid) nanoparticle for the targeted delivery of anti-miRNA antisense oligonucleotides for antiangiogenesis therapy has been reported by Liu et al. In vitro and in vivo assessments of cRGD-modified LPH nanoparticles have exhibited effective antiangiogenesis activities of suppressing endothelial cell migration and blood tube formulation (Liu et al. Citation2010). Also Liu et al. have investigated in vitro and in vivo tumor-targeting ability of IL-4Rα aptamer–liposome–CpG oligodeoxynucleotide (ODN) delivery system. The IL-4Rα–aptamer–liposome–CpG has demonstrated better tumor distribution and enhanced antitumor activity compared to the control group in vitro and in vivo. The developed immunotherapy system has inhibited distinct myeloid-derived suppressor cell populations in tumors and bone marrow and improved immunotherapy efficacy (Liu et al. Citation2016). In another study, Pore et al. have described new strategy for gene therapy by using glucocorticoid receptor (GR)-targeted liposome, delivering a specially designed artificial miRNA-plasmid against Hsp90 (amiR-Hsp90). Their straightforward strategy for simultaneous targeting of multiple pro-cancerous factors, have shown significant anticancer effect without eliciting nonspecific side effects on two different tumor models in mice (Pore et al. Citation2013).
Similar to siRNA, naked-DNA or pDNA allows the treatment of many diseases at their root cause. However, they also suffer from the low efficiency of penetration into the nucleus (Moghimi et al. Citation2016). Therefore, much progress has been made in recent decades after investigation of lipid-mediated gene delivery systems (Felgner et al. Citation1987). As a result, highly efficient formulations for in vitro applications of liposomal-based gene delivery system have been evaluated; however; designing appropriate synthetic vectors for in vivo applications remains a challenge (Ewert et al. Citation2016). Reddy et al. have demonstrated folate-targeted cationic liposomal vectors containing protamine-condensed plasmid DNA, can significantly enhance folate-mediated endocytosis and target transgenes to FR expressing cancer cells both in vitro and in vivo. They have also concluded from gene therapy of peritoneal cancers with folic acid (FA)-targeted cationic lipid that this novel liposomal vector can be a more efficient alternative to conventional chemotherapy (Reddy et al. Citation2002). In another study, Gira˜o da Cruz et al. have designed transferrin-associated lipoplexes to transfer DNA into C6 glioma cells and primary hippocampal and cortical neurons. Their results were promising for enhanced internalization and transfection of Tf-associated lipoplexes by neuronal cells and approved usefulness of these lipid-based carriers to transfer gene to cells (da Cruz et al. Citation2004). In addition, Simo˜es et al. have applied transferring with cationic liposome/DNA complex to gene therapy. Their study have revealed high levels of transfection besides having the advantage of being active in the presence of serum and being nontoxic due to application of the ternary complexes of transferrin/cationic liposomes/DNA (Simoes et al. Citation1998). And in the last example, Charoensit et al. have demonstrated incorporation of All-trans–retinoic acid with IL-12 plasmid DNA into cationic liposome (ATRA-cationic liposome/IL-12 pDNA complexes) which was significantly improved therapeutic efficacy in metastatic lung tumor after intravenous injection in a mouse model (Charoensit et al. Citation2010).
In addition to targeted liposomal gene delivery systems, targeted codelivery of potent drugs (especially chemotherapeutic drugs for cancer treatment) and therapeutic gene in order to suppress cancer growth besides reducing adverse effects of chemotherapeutics and improve the chemo-sensitivity, have been shown to be successful (Glasgow and Chougule Citation2015). Chen Wang et al. have investigated nanoscale liposomal codelivery of siRNA and DOX targeted with NGR (aspargine–glycine–arginine) peptide as a new strategy for cancer therapy and to overcome the drug resistance of cancer cells. They have accomplished novel therapeutic agent against drug-resistant tumors, which was successfully enhance drug potency (Chen et al. Citation2010). Based on Wang et al. report, codelivery of DNA and DOX incorporated into float-coated PEGylated liposomes, have showed effective DNA-binding ability, high gene transfection efficiency and sustained drug release profile (Wang et al. Citation2010). In another study, Skandrani et al. have prepared lipid nanoliposome functionalized with polyethyleneimine (PEI) moieties for codelivery of pDNA and paclitaxel. They have demonstrated the codelivery of paclitaxel with PEI/pDNA, efficiently internalized both cargo into cells and loading nanovectors with fluorescent molecules create multiactive vectors for theranostics in gene therapy (Skandrani et al. Citation2014). In , there are given several other important examples of liposomal potent and specific gene delivery systems which also include miRNA and oligonucleotides.
Table 3. Highly potent nucleic acid therapeutics delivered using targeted liposomes.
Targeted liposomes under clinical trials
Immunoliposomes are one of the pioneer examples used in clinical trials and they have had the greatest impact especially in cancer treatment to date. Although there are some drawbacks for immunoliposome-based drug delivery systems in clinical development, prospects of further clinical acceptance of them are gradually evaluated by newer approaches (Basavaraj and Betageri Citation2014, Eloy et al. Citation2014). Examples of ligand-targeted nanoliposome formulations undergoing clinical evaluation were presented briefly in .
Table 4. Examples of ligand-targeted nanoliposome formulations undergoing clinical evaluation (Goodall et al. Citation2015, van der Meel et al. Citation2013).
Conclusions
Highly standard approaches in pharmacology have been achieved via current progressions in the field of liposomal drug delivery systems. Targeted liposomal formulations for highly potent drugs present an ideal strategy to overcome potent therapeutics barriers such as off-target distribution and lack of efficacy. Feasibility of novel therapeutic concepts with desired attributes have been provided via substantial improvements in liposomal-based delivery system’s preclinical studies. Continual advances in application of targeted liposomal delivery systems for novel potent therapeutics point to the need for consideration of penetration and localization of sufficient drug content to modify therapeutic impacts. Therefore, with new targets and drugs being evaluated, necessity for improving drug stability and exposure besides drug accumulation enhancement in the target tissue is expanding. In this context, advance in the design of novel-directed liposomal delivery platform facilitates fast and effective therapeutic response and led to further improvement in liposome’s future clinical applications concept for potent drug, protein, and gene carriers. In the near future with new targets and drugs being evaluated, the applications of directed liposomes will undergo a growth; however, practical and affordable application of them are still remained a challenge in late-stage clinical trials.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Al-Ahmady ZS, Chaloin O, Kostarelos K. 2014. Monoclonal antibody-targeted, temperature-sensitive liposomes: in vivo tumor chemotherapeutics in combination with mild hyperthermia. J Control Release. 196:332–343.
- Al-Dosari MS, Gao X. 2009. Nonviral gene delivery: principle, limitations, and recent progress. AAPS J. 11:671–681.
- Apps MG, Choi EH, Wheate NJ. 2015. The state-of-play and future of platinum drugs. Endocr Relat Cancer. 22:R219–R233.
- Asai T, Matsushita S, Kenjo E, Tsuzuku T, Yonenaga N, Koide H, et al. 2011. Dicetyl phosphate-tetraethylenepentamine-based liposomes for systemic siRNA delivery. Bioconjug Chem. 22:429–435.
- Baek SE, Lee KH, Park YS, Oh DK, Oh S, Kim KS, Kim DE. 2014. RNA aptamer-conjugated liposome as an efficient anticancer drug delivery vehicle targeting cancer cells in vivo. J Control Release. 196:234–242.
- Balazs DA, Godbey W. 2011. Liposomes for use in gene delivery. J Drug Deliv. 2011:326497.
- Bardania H, Shojaosadati SA, Kobarfard F, Dorkoosh F, Zadeh ME, Naraki M, Faizi M. 2016a. Encapsulation of eptifibatide in RGD-modified nanoliposomes improves platelet aggregation inhibitory activity. J Thromb Thrombolysis. [Epub ahead of print]. doi: 10.1007/s11239-016-1440-6
- Bardania H, Shojaosadati SA, Kobarfard F, Dorkoosh F. 2016b. Optimization of RGD-modified nano-liposomes encapsulating eptifibatide. Iran J Biotechnol. 14:33–40.
- Basavaraj S, Betageri GV. 2014. Can formulation and drug delivery reduce attrition during drug discovery and development—review of feasibility, benefits and challenges. Acta Pharmaceutica Sinica B. 4:3–17.
- Belogurov AA, Stepanov AV, Smirnov IV, Melamed D, Bacon A, Mamedov AE, et al. 2013. Liposome-encapsulated peptides protect against experimental allergic encephalitis. FASEB J. 27:222–231.
- Bi R, Shao W, Wang Q, Zhang N. 2008. Spray-freeze-dried dry powder inhalation of insulin-loaded liposomes for enhanced pulmonary delivery. J Drug Target. 16:639–648.
- Cabanes A, Even-Chen S, Zimberoff J, Barenholz Y, Kedar E, Gabizon A. 1999. Enhancement of antitumor activity of polyethylene glycol-coated liposomal doxorubicin with soluble and liposomal interleukin 2. Clin Cancer Res. 5:687–693.
- Cao Z, Tong R, Mishra A, Xu W, Wong GC, Cheng J, Lu Y. 2009. Reversible cell-specific drug delivery with aptamer-functionalized liposomes. Angew Chem Int Ed Engl. 48:6494–6498.
- Chang DK, Li PC, Lu RM, Jane WN, Wu HC. 2013. Peptide-mediated liposomal Doxorubicin enhances drug delivery efficiency and therapeutic efficacy in animal models. PLoS One. 8:e83239.
- Charoensit P, Kawakami S, Higuchi Y, Yamashita F, Hashida M. 2010. Enhanced growth inhibition of metastatic lung tumors by intravenous injection of ATRA-cationic liposome/IL-12 pDNA complexes in mice. Cancer Gene Ther. 17:512–522.
- Chen Y, Bathula SR, Li J, Huang L. 2010. Multifunctional nanoparticles delivering small interfering RNA and doxorubicin overcome drug resistance in cancer. J Biol Chem. 285:22639–22650.
- Chen Y, Wu JJ, Huang L. 2010. Nanoparticles targeted with NGR motif deliver c-myc siRNA and doxorubicin for anticancer therapy. Mol Ther. 18:828–834.
- Chen Y, Zhu X, Zhang X, Liu B, Huang L. 2010. Nanoparticles modified with tumor-targeting scFv deliver siRNA and miRNA for cancer therapy. Mol Ther. 18:1650–1656.
- Cheng W, Allen T. 2010. The use of single chain Fv as targeting agents for immunoliposomes: an update on immunoliposomal drugs for cancer treatment. Expert Opin Drug Deliv. 7:461–478.
- Chime SA, Onyishi IV. 2013. Lipid-based drug delivery systems (LDDS): recent advances and applications of lipids in drug delivery. Afr J Pharm Pharmacol. 7:3034–3059.
- Cryan SA. 2005. Carrier-based strategies for targeting protein and peptide drugs to the lungs. AAPS J. 7:E20–E41.
- da Cruz MTG, Simões S, de Lima MCP. 2004. Improving lipoplex-mediated gene transfer into C6 glioma cells and primary neurons. Exp Neurol. 187:65–75.
- Daraee H, Eatemadi A, Abbasi E, Fekri Aval S, Kouhi M, Akbarzadeh A. 2016. Application of liposomes in medicine and drug delivery. Artif Cells Nanomed Biotechnol. 44:381–391.
- Deshpande PP, Biswas S, Torchilin VP. 2013. Current trends in the use of liposomes for tumor targeting. Nanomedicine (Lond). 8:1509–1528.
- Eldar-Boock A, Polyak D, Scomparin A, Satchi-Fainaro R. 2013. Nano-sized polymers and liposomes designed to deliver combination therapy for cancer. Curr Opin Biotechnol. 24:682–689.
- Eliaz RE, Szoka FC. 2001. Liposome-encapsulated doxorubicin targeted to CD44 a strategy to kill CD44-overexpressing tumor cells. Cancer Res. 61:2592–2601.
- Eloy JO, Claro de Souza M, Petrilli R, Barcellos JP, Lee RJ, Marchetti JM. 2014. Liposomes as carriers of hydrophilic small molecule drugs: strategies to enhance encapsulation and delivery. Colloids Surf B Biointerfaces. 123:345–363.
- Ewert KK, Kotamraju VR, Majzoub RN, Steffes VM, Wonder EA, Teesalu T, Ruoslahti E, Safinya CR. 2016. Synthesis of linear and cyclic peptide–PEG–lipids for stabilization and targeting of cationic liposome–DNA complexes. Bioorg Med Chem Lett. 26:1618–1623.
- Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M, et al. 1987. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci USA. 84:7413–7417.
- Feng C, Wang T, Tang R, Wang J, Long H, Gao X, Tang S. 2010. Silencing of the MYCN gene by siRNA delivered by folate receptor-targeted liposomes in LA-N-5 cells. Pediatr Surg Int. 26:1185–1191.
- Gabizon A, Shmeeda H, Horowitz AT, Zalipsky S. 2004. Tumor cell targeting of liposome-entrapped drugs with phospholipid-anchored folic acid–PEG conjugates. Adv Drug Deliv Rev. 56:1177–1192.
- Gagné JF, Désormeaux A, Perron S, Tremblay MJ, Bergeron MG. 2002. Targeted delivery of indinavir to HIV-1 primary reservoirs with immunoliposomes. Biochim Biophys Acta. 1558:198–210.
- Gandhi NS, Tekade RK, Chougule MB. 2014. Nanocarrier mediated delivery of siRNA/miRNA in combination with chemotherapeutic agents for cancer therapy: current progress and advances. J Control Release. 194:238–256.
- Gandhi R, Khatri N, Baradia D, Vhora I, Misra A. 2015. Surface-modified Epirubicin-HCl liposomes and its in vitro assessment in breast cancer cell-line: MCF-7. Drug Deliv. 23:1152–1162.
- Glasgow MD, Chougule MB. 2015. Recent developments in active tumor targeted multifunctional nanoparticles for combination chemotherapy in cancer treatment and imaging. J Biomed Nanotechnol. 11:1859–1898.
- Goodall S, Jones ML, Mahler S. 2015. Monoclonal antibody‐targeted polymeric nanoparticles for cancer therapy–future prospects. J Chem Technol Biotechnol. 90:1169–1176.
- Goren D, Horowitz AT, Tzemach D, Tarshish M, Zalipsky S, Gabizon A. 2000. Nuclear delivery of doxorubicin via folate-targeted liposomes with bypass of multidrug-resistance efflux pump. Clin Cancer Res. 6:1949–1957.
- Haller CA, Cui W, Wen J, Robson SC, Chaikof EL. 2006. Reconstitution of CD39 in liposomes amplifies nucleoside triphosphate diphosphohydrolase activity and restores thromboregulatory properties. J Vasc Surg. 43:816–823.
- Hattori Y, Maitani Y. 2004. Enhanced in vitro DNA transfection efficiency by novel folate-linked nanoparticles in human prostate cancer and oral cancer. J Control Release. 97:173–183.
- He C, Tang Z, Tian H, Chen X. 2016. Co-delivery of chemotherapeutics and proteins for synergistic therapy. Adv Drug Deliv Rev. 98:64–76.
- He Y, Zhang L, Song C. 2010. Luteinizing hormone-releasing hormone receptor-mediated delivery of mitoxantrone using LHRH analogs modified with PEGylated liposomes. Int J Nanomedicine. 5:697–705.
- Hirai M, Minematsu H, Hiramatsu Y, Kitagawa H, Otani T, Iwashita S, et al. 2010. Novel and simple loading procedure of cisplatin into liposomes and targeting tumor endothelial cells. Int J Pharm. 391:274–283.
- Hua X, Zhou L, Liu P, He Y, Tan K, Chen Q, Gao Y, Gao Y. 2014. In vivo thrombolysis with targeted microbubbles loading tissue plasminogen activator in a rabbit femoral artery thrombus model. J Thromb Thrombolysis. 38:57–64.
- Huang L, Liu Y. 2011. In vivo delivery of RNAi with lipid-based nanoparticles. Ann Rev Biomed Eng. 13:507–530.
- Huang L, Zhou F. 1992. Liposome and immunoliposome mediated delivery of proteins and peptides. In: Gregoriadis G, Florence AT, Poste G, Eds. Targeting of Drugs 3. New York: Springer, pp. 45–50.
- Hyndman L, Lemoine JL, Huang L, Porteous DJ, Boyd AC, Nan X. 2004. HIV-1 Tat protein transduction domain peptide facilitates gene transfer in combination with cationic liposomes. J Control Release. 99:435–444.
- Iinuma H, Maruyama K, Okinaga K, Sasaki K, Sekine T, Ishida O, Ogiwara N, et al. 2002. Intracellular targeting therapy of cisplatin-encapsulated transferrin-polyethylene glycol liposome on peritoneal dissemination of gastric cancer. Int J Cancer. 99:130–137.
- Ishii T, Asai T, Oyama D, Fukuta T, Yasuda N, Shimizu K, et al. 2012. Amelioration of cerebral ischemia–reperfusion injury based on liposomal drug delivery system with asialo-erythropoietin. J Control Release. 160:81–87.
- Ishiwatari H, Sato Y, Murase K, Yoneda A, Fujita R, Nishita H, et al. 2013. Treatment of pancreatic fibrosis with siRNA against a collagen-specific chaperone in vitamin A-coupled liposomes. Gut. 62:1328–1339.
- Iwase Y, Maitani Y. 2010. Octreotide-targeted liposomes loaded with CPT-11 enhanced cytotoxicity for the treatment of medullary thyroid carcinoma. Mol Pharm. 8:330–337.
- Jain AK, Chalasani KB, Khar RK, Ahmed FJ, Diwan PV. 2007. Muco-adhesive multivesicular liposomes as an effective carrier for transmucosal insulin delivery. J Drug Target. 15:417–427.
- Javadi M, Pitt WG, Tracy CM, Barrow JR, Willardson BM, Hartley JM, Tsosie NH. 2013. Ultrasonic gene and drug delivery using eLiposomes. J Control Release. 167:92–100.
- Ju RJ, Li XT, Shi JF, Li XY, Sun MG, Zeng F, et al. 2014. Liposomes, modified with PTD HIV-1 peptide, containing epirubicin and celecoxib, to target vasculogenic mimicry channels in invasive breast cancer. Biomaterials. 35:7610–7621.
- Kanaoka E, Takahashi K, Yoshikawa T, Jizomoto H, Nishihara Y, Hirano K. 2003. Continuous release of interleukin-2 from liposomal IL-2 (mixture of interleukin-2 and liposomes) after subcutaneous administration to mice. Drug Dev Ind Pharm. 29:1149–1153.
- Kanaoka E, Takahashi K, Yoshikawa T, Jizomoto H, Nishihara Y, Hirano K. 2001. A novel and simple type of liposome carrier for recombinant interleukin-2. J Pharm Pharmacol. 53:295–302.
- Kanaoka E, Takahashi K, Yoshikawa T, Jizomoto H, Nishihara Y, Uchida N, Maekawa R, Hirano K. 2002. A significant enhancement of therapeutic effect against hepatic metastases of M5076 in mice by a liposomal interleukin-2 (mixture). J Control Release. 82:183–187.
- Kanasty R, Dorkin JR, Vegas A, Anderson D. 2013. Delivery materials for siRNA therapeutics. Nat Mater. 12:967–977.
- Kisel M, Kulik LN, Tsybovsky IS, Vlasov AP, Vorob'yov MS, Kholodova EA, Zabarovskaya ZV. 2001. Liposomes with phosphatidylethanol as a carrier for oral delivery of insulin: studies in the rat. Int J Pharm. 216:105–114.
- Kohara N, Kitaoka F, Komuta K, Yamamoto M, Motojima K, Kanematsu T. 1995. Effective treatment of liver metastases from colon cancer with a combination of γ-interferon and cisplatin chemotherapy: report of a case. Surg Today. 25:357–360.
- Kong F, Zhou F, Ge L, Liu X, Wang Y. 2012. Mannosylated liposomes for targeted gene delivery. Int J Nanomedicine. 7:1079–1089.
- Kontermann RE. 2006. Immunoliposomes for cancer therapy. Curr Opin Mol Ther. 8:39.
- Koshkaryev A, Piroyan A, Torchilin VP. 2012. Increased apoptosis in cancer cells in vitro and in vivo by ceramides in transferrin-modified liposomes. Cancer Biol Ther. 13:50–60.
- Krieger ML, Eckstein N, Schneider V, Koch M, Royer HD, Jaehde U, Bendas G. 2010. Overcoming cisplatin resistance of ovarian cancer cells by targeted liposomes in vitro. Int J Pharm. 389:10–17.
- Lajavardi L, Camelo S, Agnely F, Luo W, Goldenberg B, Naud MC, et al. 2009. New formulation of vasoactive intestinal peptide using liposomes in hyaluronic acid gel for uveitis. J Control Release. 139:22–30.
- Li L, Hou J, Liu X, Guo Y, Wu Y, Zhang L, Yang Z. 2014. Nucleolin-targeting liposomes guided by aptamer AS1411 for the delivery of siRNA for the treatment of malignant melanomas. Biomaterials. 35:3840–3850.
- Li N, Zhang CX, Wang XX, Zhang L, Ma X, Zhou J, et al. 2013. Development of targeting lonidamine liposomes that circumvent drug-resistant cancer by acting on mitochondrial signaling pathways. Biomaterials. 34:3366–3380.
- Li X, Ding L, Xu Y, Wang Y, Ping Q. 2009. Targeted delivery of doxorubicin using stealth liposomes modified with transferrin. Int J Pharm. 373:116–123.
- Liu XQ, Song WJ, Sun TM, Zhang PZ, Wang J. 2010. Targeted delivery of antisense inhibitor of miRNA for antiangiogenesis therapy using cRGD-functionalized nanoparticles. Mol Pharm. 8:250–259.
- Liu YJ, Dou XQ, Wang F, Zhang J, Wang XL, Xu GL, et al. 2016. IL-4Rα aptamer-liposome-CpG oligodeoxynucleotides suppress tumour growth by targeting the tumour microenvironment. J Drug Target. [Epub ahead of print]. doi: http://dx.doi.org/10.1080/1061186X.2016.1258569
- Lopez-Berestein G, Fidler IJ. 1989. Liposomes in the Therapy of Infectious Diseases and Cancer. New York: Alan R. Liss, pp. 245–262.
- Lu Y, Yang J, Sega E. 2006. Issues related to targeted delivery of proteins and peptides. AAPS J. 8:E466–E478.
- Lv Q, Li LM, Han M, Tang XJ, Yao JN, Ying XY, Li FZ, Gao JQ. 2013. Characteristics of sequential targeting of brain glioma for transferrin-modified cisplatin liposome. Int J Pharm. 444:1–9.
- Majzoub RN, Chan CL, Ewert KK, Silva BF, Liang KS, Jacovetty EL, et al. 2014. Uptake and transfection efficiency of PEGylated cationic liposome-DNA complexes with and without RGD-tagging. Biomaterials. 35:4996–5005.
- Majzoub RN, Ewert KK, Safinya CR. 2016. Cationic liposome–nucleic acid nanoparticle assemblies with applications in gene delivery and gene silencing. Phil Trans R Soc A. 374:20150129.
- Mali S. 2013. Delivery systems for gene therapy. Indian J Hum Genet. 19:3.
- Mamot C, Drummond DC, Greiser U, Hong K, Kirpotin DB, Marks JD, Park JW. 2003. Epidermal growth factor receptor (EGFR)-targeted immunoliposomes mediate specific and efficient drug delivery to EGFR-and EGFRvIII-overexpressing tumor cells. Cancer Res. 63:3154–3161.
- Mamot C, Drummond DC, Noble CO, Kallab V, Guo Z, Hong K, Kirpotin DB, Park JW. 2005. Epidermal growth factor receptor–targeted immunoliposomes significantly enhance the efficacy of multiple anticancer drugs in vivo. Cancer Res. 65:11631–11638.
- Mamot C, Ritschard R, Wicki A, Stehle G, Dieterle T, Bubendorf L, et al. 2012. Tolerability, safety, pharmacokinetics, and efficacy of doxorubicin-loaded anti-EGFR immunoliposomes in advanced solid tumours: a phase 1 dose-escalation study. The Lancet Oncol. 13:1234–1241.
- Martins S, Sarmento B, Ferreira DC, Souto EB. 2007. Lipid-based colloidal carriers for peptide and protein delivery-liposomes versus lipid nanoparticles. Int J Nanomed. 2:595.
- Maruyama K, Takizawa T, Takahashi N, Tagawa T, Nagaike K, Iwatsuru M. 1997. Targeting efficiency of PEG-immunoliposome-conjugated antibodies at PEG terminals. Adv Drug Deliv Rev. 24:235–242.
- Maruyama K. 2011. Intracellular targeting delivery of liposomal drugs to solid tumors based on EPR effects. Adv Drug Deliv Rev. 63:161–169.
- Mastrobattista E, Koning GA, van Bloois L, Filipe AC, Jiskoot W, Storm G. 2002. Functional characterization of an endosome-disruptive peptide and its application in cytosolic delivery of immunoliposome-entrapped proteins. J Biol Chem. 277:27135–27143.
- Mathias CJ, Wang S, Lee RJ, Waters DJ, Low PS, Green MA. 1996. Tumor-selective radiopharmaceutical targeting via receptor-mediated endocytosis of gallium-67-deferoxamine-folate. J Nucl Med. 37:1003–1008.
- Medina OP, Haikola M, Tahtinen M, Simpura I, Kaukinen S, Valtanen H, et al. 2011. Liposomal tumor targeting in drug delivery utilizing MMP-2-and MMP-9-binding ligands. J Drug Deliv. 2011:160515.
- Mendonça LS, Firmino F, Moreira JN, Pedroso de Lima MC, Simões S. 2009. Transferrin receptor-targeted liposomes encapsulating anti-BCR-ABL siRNA or asODN for chronic myeloid leukemia treatment. Bioconjug Chem. 21:157–168.
- Milla P, Dosio F, Cattel L. 2012. PEGylation of proteins and liposomes: a powerful and flexible strategy to improve the drug delivery. Curr Drug Metab. 13:105–119.
- Mo R, Jiang T, Gu Z. 2014. Recent progress in multidrug delivery to cancer cells by liposomes. Nanomedicine (Lond). 9:1117–1120.
- Moghimi HR, Saffari M, Dass CR. 2016. Barriers to liposomal gene delivery: from application site to the target. Iranian Journal of Pharmaceutical Research. 15:3–17.
- Mourelatos Z. 2008. Small RNAs: the seeds of silence. Nature. 455:44–45.
- Movahedi F, Hu RG, Becker DL, Xu C. 2015. Stimuli-responsive liposomes for the delivery of nucleic acid therapeutics. Nanomedicine. 11:1575–1584.
- Naseri N, Valizadeh H, Zakeri-Milani P. 2015. Solid lipid nanoparticles and nanostructured lipid carriers: structure, preparation and application. Adv Pharm Bull. 5:305.
- Nelson AL. 2010. Antibody fragments: hope and hype. MAbs. 2:77–83.
- Nimjee SM, Rusconi CP, Sullenger BA. 2005. Aptamers: an emerging class of therapeutics. Annu Rev Med. 56:555–583.
- Oliveira S, Heukers R, Sornkom J, Kok RJ, van Bergen En Henegouwen PM. 2013. Targeting tumors with nanobodies for cancer imaging and therapy. J Control Release. 172:607–617.
- Paliwal SR, Paliwal R, Pal HC, Saxena AK, Sharma PR, Gupta PN, Agrawal GP, Vyas SP. 2011. Estrogen-anchored pH-sensitive liposomes as nanomodule designed for site-specific delivery of doxorubicin in breast cancer therapy. Mol Pharm. 9:176–186.
- Park J, Wrzesinski SH, Stern E, Look M, Criscione J, Ragheb R, et al. 2012. Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat Mater. 11:895–905.
- Park JW, Hong K, Kirpotin DB, Colbern G, Shalaby R, Baselga J, et al. 2002. Anti-HER2 immunoliposomes enhanced efficacy attributable to targeted delivery. Clin Cancer Res. 8:1172–1181.
- Pastorino F, Brignole C, Marimpietri D, Sapra P, Moase EH, Allen TM, Ponzoni M. 2003. Doxorubicin-loaded Fab′ fragments of anti-disialoganglioside immunoliposomes selectively inhibit the growth and dissemination of human neuroblastoma in nude mice. Cancer Res. 63:86–92.
- Peltier S, Oger JM, Lagarce F, Couet W, Benoît JP. 2006. Enhanced oral paclitaxel bioavailability after administration of paclitaxel-loaded lipid nanocapsules. Pharm Res. 23:1243–1250.
- Pereira DM, Rodrigues PM, Borralho PM, Rodrigues CM. 2013. Delivering the promise of miRNA cancer therapeutics. Drug Discov Today. 18:282–289.
- Pisal DS, MP, Kosloski SV, Balu I. 2010. Delivery of therapeutic proteins. J Pharm Sci. 99:2557–2575.
- Pore SK, Choudhary A, Rathore B, Ganguly A, Sujitha P, Kumar CG, et al. 2013. Hsp90-targeted miRNA-liposomal formulation for systemic antitumor effect. Biomaterials. 34:6804–6817.
- Puri A, Loomis K, Smith B, Lee JH, Yavlovich A, Heldman E, Blumenthal R. 2009. Lipid-based nanoparticles as pharmaceutical drug carriers: from concepts to clinic. Crit Rev Ther Drug Carrier Syst. 26:523–580.
- Rahman M, Kumar V, Beg S, Sharma G, Katare OP, Anwar F. 2016. Emergence of liposome as targeted magic bullet for inflammatory disorders: current state of the art. Artif Cells Nanomed Biotechnol. 44:1597–1608.
- Ramadas M, Paul W, Dileep KJ, Anitha Y, Sharma CP. 2000. Lipoinsulin encapsulated alginate-chitosan capsules: intestinal delivery in diabetic rats. J Microencapsul. 17:405–411.
- Ramamoorth M, Narvekar A. 2015. Non viral vectors in gene therapy – an overview. J Clin Diagn Res. 9:GE01–GE06.
- Ravar F, Saadat E, Gholami M, Dehghankelishadi P, Mahdavi M, Azami S, Dorkoosh FA. 2016. Hyaluronic acid-coated liposomes for targeted delivery of paclitaxel, in-vitro characterization and in-vivo evaluation. J Control Release. 229:10–22.
- Reddy J, Abburi C, Hofland H, Howard SJ, Vlahov I, Wils P, Leamon CP. 2002. Folate-targeted, cationic liposome-mediated gene transfer into disseminated peritoneal tumors. Gene Ther. 9:1542–1550.
- Reulen SW, Brusselaars WW, Langereis S, Mulder WJ, Breurken M, Merkx M. 2007. Protein-liposome conjugates using cysteine-lipids and native chemical ligation. Bioconjug Chem. 18:590–596.
- Rhim T, Lee M. 2016. Targeted delivery of growth factors in ischemic stroke animal models. Exp Opin Drug Deliv. 13:709–723.
- Rivest V, Phivilay A, Julien C, Bélanger S, Tremblay C, Emond V, Calon F. 2007. Novel liposomal formulation for targeted gene delivery. Pharm Res. 24:981–990.
- Safra T. 2003. Cardiac safety of liposomal anthracyclines. The Oncologist. 8:17–24.
- Sapra P, Moase EH, Ma J, Allen TM. 2004. Improved therapeutic responses in a xenograft model of human B lymphoma (Namalwa) for liposomal vincristine versus liposomal doxorubicin targeted via anti-CD19 IgG2a or Fab′ fragments. Clin Cancer Res. 10:1100–1111.
- Sato Y, Murase K, Kato J, Kobune M, Sato T, Kawano Y, et al. 2008. Resolution of liver cirrhosis using vitamin A–coupled liposomes to deliver siRNA against a collagen-specific chaperone. Nat Biotechnol. 26:431–442.
- Schuster L, Seifert O, Vollmer S, Kontermann RE, Schlosshauer B, Hartmann H. 2015. Immunoliposomes for targeted delivery of an antifibrotic drug. Mol Pharm. 12:3146–3157.
- Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. 2015. Advances and challenges of liposome assisted drug delivery. Front Pharmacol. 6:286.
- Shim MS, Kwon YJ. 2010. Efficient and targeted delivery of siRNA in vivo. FEBS J. 277:4814–4827.
- Simoes S, Slepushkin V, Gaspar R, de Lima MC, Düzgüneş N. 1998. Gene delivery by negatively charged ternary complexes of DNA, cationic liposomes and transferrin or fusigenic peptides. Gene Ther. 5:955–964.
- Simoes S, Slepushkin V, Pires P, Gaspar R, de Lima MP, Düzgüneş N. 1999. Mechanisms of gene transfer mediated by lipoplexes associated with targeting ligands or pH-sensitive peptides. Gene Ther. 6:1798–1807.
- Simpson-Abelson MR, Purohit VS, Pang WM, Iyer V, Odunsi K, Demmy TL, et al. 2009. IL-12 delivered intratumorally by multilamellar liposomes reactivates memory T cells in human tumor microenvironments. Clin Immunol. 132:71–82.
- Singh R, Lillard JW. 2009. Nanoparticle-based targeted drug delivery. Exp Mol Pathol. 86:215–223.
- Skandrani N, Barras A, Legrand D, Gharbi T, Boulahdour H, Boukherroub R. 2014. Lipid nanocapsules functionalized with polyethyleneimine for plasmid DNA and drug co-delivery and cell imaging. Nanoscale. 6:7379–7390.
- Song X, Ren Y, Zhang J, Wang G, Han X, Zheng W, Zhen L. 2015. Targeted delivery of doxorubicin to breast cancer cells by aptamer functionalized DOTAP/DOPE liposomes. Oncol Rep. 34:1953–1960.
- Spuch C, Navarro C. 2011. Liposomes for targeted delivery of active agents against neurodegenerative diseases (Alzheimer's disease and Parkinson's disease). J Drug Deliv. 2011:469679.
- Steeland S, Vandenbroucke RE, Libert C. 2016. Nanobodies as therapeutics: big opportunities for small antibodies. Drug Discov Today. 21:1076–1113.
- Sugimoto Y, Tsukahara S, Imai Y, Ueda K, Tsuruo T. 2003. Reversal of breast cancer resistance protein-mediated drug resistance by estrogen antagonists and agonists. Mol Cancer Ther. 2:105–112.
- Suzuki R, Takizawa T, Kuwata Y, Mutoh M, Ishiguro N, Utoguchi N, et al. 2008. Effective anti-tumor activity of oxaliplatin encapsulated in transferrin–PEG-liposome. Int J Pharm. 346:143–150.
- Takeuchi H, Matsui Y, Yamamoto H, Kawashima Y. 2003. Mucoadhesive properties of carbopol or chitosan-coated liposomes and their effectiveness in the oral administration of calcitonin to rats. J Control Release. 86:235–242.
- Ten Hagen TL, Van Der Veen AH, Nooijen PT, Van Tiel ST, Seynhaeve AL, Eggermont AM. 2002. Pegylated liposomal tumor necrosis factor‐α results in reduced toxicity and synergistic antitumor activity after systemic administration in combination with liposomal doxorubicin (Doxil®) in soft tissue sarcoma‐bearing rats. Int J Cancer. 97:115–120.
- Tian W, Ying X, Du J, Guo J, Men Y, Zhang Y, Li RJ, et al. 2010. Enhanced efficacy of functionalized epirubicin liposomes in treating brain glioma-bearing rats. Eur J Pharm Sci. 41:232–243.
- Tiwari G, Tiwari R, Sriwastawa B, Bhati L, Pandey S, Pandey P, et al. 2012. Drug delivery systems: an updated review. Int J Pharm Investig. 2:2.
- Torchilin VP, Levchenko TS, Rammohan R, Volodina N, Papahadjopoulos-Sternberg B, D'Souza GG. 2003. Cell transfection in vitro and in vivo with nontoxic TAT peptide-liposome-DNA complexes. Proc Natl Acad Sci USA. 100:1972–1977.
- Tuscano JM, Martin SM, Ma Y, Zamboni W, O’Donnell RT. 2010. Efficacy, biodistribution, and pharmacokinetics of CD22-targeted pegylated liposomal doxorubicin in a B-cell non–Hodgkin's lymphoma xenograft mouse model. Clin Cancer Res 16:2760–2768.,
- Vaidya B, Agrawal G, Vyas SP. 2011. Platelets directed liposomes for the delivery of streptokinase: development and characterization. Eur J Pharm Sci. 44:589–594.
- van der Meel R, Oliveira S, Altintas I, Haselberg R, van der Veeken J, Roovers RC, et al. 2012. Tumor-targeted nanobullets: anti-EGFR nanobody-liposomes loaded with anti-IGF-1R kinase inhibitor for cancer treatment. J Control Release. 159:281–289.
- van der Meel R, Vehmeijer LJ, Kok RJ, Storm G, van Gaal EV. 2013. Ligand-targeted particulate nanomedicines undergoing clinical evaluation: current status. Adv Drug Deliv Rev. 65:1284–1298.
- Voinea M, Simionescu M. 2002. Designing of ‘intelligent’ liposomes for efficient delivery of drugs. J Cell Mol Med. 6:465–474.
- Wang F, Chen L, Zhang R, Chen Z, Zhu L. 2014. RGD peptide conjugated liposomal drug delivery system for enhance therapeutic efficacy in treating bone metastasis from prostate cancer. J Control Release. 196:222–233.
- Wang H, Zhao P, Su W, Wang S, Liao Z, Niu R, Chang J. 2010. PLGA/polymeric liposome for targeted drug and gene co-delivery. Biomaterials. 31:8741–8748.
- Wang Y, Zhou J, Qiu L, Wang X, Chen L, Liu T, Di W. 2014. Cisplatin–alginate conjugate liposomes for targeted delivery to EGFR-positive ovarian cancer cells. Biomaterials. 35:4297–4309.
- Wang Z, Yu Y, Dai W, Lu J, Cui J, Wu H, et al. 2012. The use of a tumor metastasis targeting peptide to deliver doxorubicin-containing liposomes to highly metastatic cancer. Biomaterials. 33:8451–8460.
- Whitehead KA, Langer R, Anderson DG. 2009. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 8:129–138.
- Wu J, Liu Q, Lee RJ. 2006. A folate receptor-targeted liposomal formulation for paclitaxel. Int J Pharm. 316:148–153.
- Wu J, Lu Y, Lee A, Pan X, Yang X, Zhao X, Lee RJ. 2007. Reversal of multidrug resistance by transferrin-conjugated liposomes co-encapsulating doxorubicin and verapamil. J Pharm Pharm Sci. 10:350–357.
- Xiong S, Yu B, Wu J, Li H, Lee RJ. 2011. Preparation, therapeutic efficacy and intratumoral localization of targeted daunorubicin liposomes conjugating folate-PEG-CHEMS. Biomed Pharmacother. 65:2–8.
- Xu L, Huang CC, Huang W, Tang WH, Rait A, Yin YZ, Cruz I, Xiang LM. 2002. Systemic tumor-targeted gene delivery by anti-transferrin receptor scFv-immunoliposomes 1 This work was supported in part by National Cancer Institute Grant R01 CA45158 (to EC), National Cancer Institute Small Business Technology Transfer Phase I Grant R41 CA80449 (to EC), and a grant from SynerGene Therapeutics, Inc. 1. Mol Cancer Ther. 1:337–346.
- Xu X, Costa A, Burgess DJ. 2012. Protein encapsulation in unilamellar liposomes: high encapsulation efficiency and a novel technique to assess lipid-protein interaction. Pharm Res. 29:1919–1931.
- Yang T, Choi MK, Cui FD, Lee SJ, Chung SJ, Shim CK, Kim DD. 2007. Antitumor effect of paclitaxel-loaded PEGylated immunoliposomes against human breast cancer cells. Pharm Res. 24:2402–2411.
- Yang T, Li B, Qi S, Liu Y, Gai Y, Ye P, et al. 2014. Co-delivery of doxorubicin and Bmi1 siRNA by folate receptor targeted liposomes exhibits enhanced anti-tumor effects in vitro and in vivo. Theranostics. 4:1096–1111.
- Ying X, Wen H, Lu WL, Du J, Guo J, Tian W, et al. 2010. Dual-targeting daunorubicin liposomes improve the therapeutic efficacy of brain glioma in animals. J Control Release. 141:183–192.
- Yonenaga N, Kenjo E, Asai T, Tsuruta A, Shimizu K, Dewa T, Nango M, Oku N. 2012. RGD-based active targeting of novel polycation liposomes bearing siRNA for cancer treatment. J Control Release. 160:177–181.
- Yu B, Mao Y, Bai L, Herman SEM, Wang X, Ramanunni A, et al. 2013. Targeted nanoparticle delivery overcomes off-target immunostimulatory effects of oligonucleotides and improves therapeutic efficacy in chronic lymphocytic leukemia. Blood. 121:136–147.
- Yu-Wai-Man C, Tagalakis AD, Manunta MD, Hart SL, Khaw PT. 2016. Receptor-targeted liposome-peptide-siRNA nanoparticles represent an efficient delivery system for MRTF silencing in conjunctival fibrosis. Scientific Rep. 6:21881.
- Zalba S, Contreras AM, Haeri A, Ten Hagen TL, Navarro I, Koning G, Garrido MJ. 2015. Cetuximab-oxaliplatin-liposomes for epidermal growth factor receptor targeted chemotherapy of colorectal cancer. J Control Release. 210:26–38.
- Zamboni WC. 2005. Liposomal, nanoparticle, and conjugated formulations of anticancer agents. Clin Cancer Res. 11:8230–8234.
- Zhang CX, Zhao WY, Liu L, Ju RJ, Mu LM, Zhao Y, et al. 2015. A nanostructure of functional targeting epirubicin liposomes dually modified with aminophenyl glucose and cyclic pentapeptide used for brain glioblastoma treatment. Oncotarget. 6:32681.
- Zhang L, Gao H, Chen L, Wu B, Zheng Y, Liao R, Jiang Y, He F. 2008. Tumor targeting of vincristine by mBAFF-modified PEG liposomes in B lymphoma cells. Cancer Lett. 269:26–36.
- Zhang L, Yao HJ, Yu Y, Zhang Y, Li RJ, Ju RJ, et al. 2012. Mitochondrial targeting liposomes incorporating daunorubicin and quinacrine for treatment of relapsed breast cancer arising from cancer stem cells. Biomaterials. 33:565–582.
- Zhang X, Koh CG, Yu B, Liu S, Piao L, Marcucci G, Lee RJ, Lee LJ. 2009. Transferrin receptor targeted lipopolyplexes for delivery of antisense oligonucleotide g3139 in a murine k562 xenograft model. Pharmaceu Res. 26:1516–1524.
- Zhang Y, Satterlee A, Huang L. 2012. In vivo gene delivery by nonviral nectors: overcoming hurdles. Mol Ther. 20:1298–1304.
- Zhang Z, Yao J. 2012. Preparation of irinotecan-loaded folate-targeted liposome for tumor targeting delivery and its antitumor activity. AAPS PharmSciTech. 13:802–810.
- Zhao H, Wang JC, Sun QS, Luo CL, Zhang Q. 2009. RGD-based strategies for improving antitumor activity of paclitaxel-loaded liposomes in nude mice xenografted with human ovarian cancer. J Drug Target. 17:10–18.
