Abstract
A novel polyvinyl alcohol-amino multi-walled carbon nanotube (PVA-AMWCNT) nanocomposite microsphere was prepared successfully for the first time and used for endotoxin removal. The resulting AMWCNT modified PVA microsphere was characterized by SEM, Raman spectrum and fluorescence image, which indicated AMWCNT was dispersed into the macropores of PVA microsphere uniformly. The PVA-AMWCNT microspheres showed better adsorption capability and faster adsorption equilibrium for endotoxin in aqueous solution when compared to the PVA microsphere with polymyxin B (PMB) as ligand. More noteworthy, the PVA based microspheres had little nonspecific adsorption in simulated serum. Therefore, PVA-AMWCNT nanocomposite microsphere with an excellent haemocompatibility has a great potential application in clinical blood purification.
Introduction
Endotoxin, which is derived from the outer cell wall of Gram-negative bacteria, consists of a polysaccharide and a terminal lipid A moiety. Lipid A is the most conserved part of endotoxin which is responsible for most of the biological activities of endotoxin [Citation1]. As we all know, endotoxin plays an important role on the structural stability and biological activity of bacteria [Citation2]. Even at 1 ng/kg in the body, endotoxin may cause potential pyrogenic and shock reactions in mammals. In addition, endotoxin is a major concern during the purification process of target protein, because Escherichia coli is a common expression host. However, the removal of endotoxin from biomedical products or fluids is always a challenge. Affinity adsorption has been proved to be the most effective technique among various methods for endotoxin removal [Citation3,Citation4].
Up to date, many researches focus on the development of the affinity adsorbent for endotoxin removal from protein fluids and endotoxemia patients [Citation5–10]. As a commercial product, polymyxin B (PMB) immobilized polystyrene fibres (PMX-F) can effectively remove endotoxin by direct haemoperfusion, which reduced the mortality in septic patients [Citation11,Citation12]. However, the patient has to take the risk, if PMB, which has nephrotoxicity, leaks off from PMX-F will be harmful to the patients’ health.
In recent years, CNTs have aroused extensive attention to scientists due to their excellent sorption, electronic, thermal, mechanical and field emission properties. CNT-based nanocomposite adsorbents were reported for low-density lipoprotein and bilirubin removal in blood purification [Citation13–16]. However, it is rarely seen that CNT-based materials was used for endotoxin removal [Citation17].
Due to the amphiphilic structure property of endotoxin, it is believed that electrostatic interaction and hydrophobic intermolecular interaction are the main affinity forces that exist in the adsorption of endotoxin. Amino multi-walled carbon nanotube (AMWCNT) has a positive charge and can react well with the negative charge on endotoxin molecule. Theoretically, AMWCNT can be an excellent affinity ligand for endotoxin removal [Citation18]. Polyvinyl alcohol (PVA) microsphere is considered as an ideal carrier for biomedical applications due to its macroporous structure, easy modification of the hydroxyl group, good biocompatibility and mechanical properties [Citation19,Citation20]. Therefore, PVA microspheres modified with AMWCNT has several advantages for endotoxin adsorption in blood purification.
In this manuscript, PVA-AMWCNT nanocomposite microsphere was prepared under ultrasonic condition, in which PVA and AMWCNT was coupled via epoxy with amino groups. Our results demonstrated that AMWCNT was successfully immobilized on PVA microsphere, and PVA-AMWCNT microspheres exhibited more excellent adsorption capacity than that of PVA microspheres with PMB as ligand. Thus, PVA-AMWCNT nanocomposite microspheres have a good prospect as an endotoxin adsorbent in haemoperfusion.
Materials and methods
Materials
Multi-walled carbon nanotube (MWCNT), manufactured by the chemical vapour deposition (CVD) method and modified with amino group, was purchased from Beijing DK nano technology Co., Ltd (Beijing, PR China). The mean diameter of the AMWCNT is 8–15 nm and its length is about 50 um. Polyvinyl pyrrolidone (PVP-K30) was from Tianjin Dingguo Biotech Co., Ltd (Tianjin, PR China). Fluorescein isothiocyanate isomer I (FITC) and epichlorohydrin was obtained from Aladdin Co., Ltd (Seoul, Korea). All other analytical grade chemicals were purchased locally. Ultrapure water was used to prepare AMWCNT dispersion.
Preparation of macroporous PVA microspheres
Macroporous PVA microspheres was synthesized by a free-radical suspension polymerization method according to the procedure reported previously [Citation20]. Briefly, vinyl acetate, TAIC, ethyl acetate, n-heptane and AIBN were thoroughly mixed and poured into an aqueous solution containing 1% of PVA. The mixture was stirred with proper speed to obtain uniform droplets. Then the temperature was raised for suspension polymerization. The resultant microspheres of poly(vinyl acetate-triallylisocyanurate) was extracted with methyl alcohol to remove the unreacted reagents. After the follow-up alcoholysis, filtering, washing and drying treatment, the PVA macroporous microspheres was prepared successfully.
Immobilization of AMWCNT on macroporous PVA microspheres
The macroporous PVA microspheres was activated by epichlorohydrin in the alkaline (dimethyl sulfoxide) DMSO solution at 40 °C for 5 h. The activated PVA microspheres was rinsed and stored in ultrapure water before use. The preparation of PVA-AMWCNT composite microspheres that was described in the literature was presently conducted with minor adjustment [Citation21]. First, the homogeneous dispersion of AMWCNT with the concentration of 4 mg/ml was prepared by ultrasonically dispersing AMWCNT into PVP solution. Second, the epoxy PVA microspheres was immersed in the above dispersion and sonicated at room temperature for 4 h to immobilize AMWCNT into the pores of PVA microspheres. Then, the prepared PVA-AMWCNT was washed with ultrapure water to remove unreacted AMWCNT and stored in ultrapure water for further use. AMWCNT labelled with FITC was immobilized on PVA microspheres by the same procedure of PVA-AMWCNT microspheres (avoid light). For comparison, the PVA modified with PMB (PVA-PMB) was prepared with the same method as mentioned above for PVA-AMWCNT.
Determination of ligand contents
The content of AMWCNT was determined by the dry weight change of the microspheres before and after immobilization. The concentration of PMB was assayed by ninhydrin colorimetry [Citation22] and the coupling amount of PMB was calculated according to the different concentrations before and after immobilization.
Characterization of PVA-AMWCNT microspheres
The skeleton density, apparent density, porosity, water content and swelling ratio were calculated by the method reported previously [Citation23–25]. Scanning electron microscopy (SEM) (Gemin SEM 500, ZEISS) was employed to observe the surface morphology of the modified PVA microspheres and unmodified PVA microspheres which were dried in vacuum drying chamber and sprayed gold before observing. The immobilization of AMWCNT on PVA was also proved by fluorescence images (Axio Imager Z1, Carl Zeiss AG, Oberkochen, Germany). Raman spectroscopy (RenishawinVia, Gloucestershire, UK) was performed to assess the presence of AMWCNT within the microspheres.
Leakage of AMWCNT
The leakage of AMWCNT test was performed as follows: 1 ml wet PVA-AMWCNT was added to 10 ml distilled water. After that, the mixture was shaken at 160 rpm for 72 h, and the supernatant was detected by UV–vis spectrophotometer at 266 nm [Citation26]. For comparison, the unmodified PVA was also tested with the same procedure as above for PVA-AMWCNT.
Adsorption experiment
Endotoxin adsorption experiments were carried out to evaluate the adsorption performances of the adsorbent. All glassware was treated with detergent in ultrasonic cleaning device and then was baked at 250 °C for at least 3 h before use. The experiments were performed using endotoxin-free water and equipment.
Static adsorption of endotoxin and BSA was studied on PVA-AMWCNT, PVA-PMB and unmodified PVA. Fifteen milligram wet microspheres was added in 2 ml endotoxin solution and shaken at 37 °C for 2 h. After that, endotoxin concentration was analysed by LAL assay and the BSA concentration was determined by the absorbance at 280 nm by UV–vis spectrophotometer. Adsorption kinetics was studied as follows: 15 mg-wet microspheres was added to 2 ml endotoxin solution and shaken at 37 °C. The concentrations of endotoxin were measured at 30, 45, 60, 90 and 120 min, respectively.
Blood compatibility
Blood routine and haemolysis analysis were performed to evaluate the blood compatibility of the PVA-AMWCNT microspheres. Fresh blood of healthy rabbits was anticoagulated by adding 2% potassium oxalate and diluted with 0.9% NaCl solution in order to produce the red blood cells (RBCs) suspension. For haemolysis assays [Citation27], the RBCs suspensions (0.1 ml for each sample) were mixed with: (a) 5 ml 0.9% NaCl solution as a negative control; (b) 5 ml deionized water as a positive control; (c) 5 m 0.9% NaCl solution and 2 g adsorbents. After incubating all the tubes at 37 °C for 60 min, blood cells were removed by centrifugation (2500 rpm) and the supernatants were evaluated at 545 nm for the release of haemoglobin. Experiments were performed in triplicate.
In order to study the influence of microspheres on blood components, fresh anticoagulant blood from healthy rabbits (2 ml for each sample) were mixed with different microspheres (200 mg for each sample), respectively [Citation28]. All the adsorbents were fully swelled overnight in physiological saline. After incubating at 37 °C for 60 min, blood was separated. Platelet, erythrocyte and leukocyte counts were measured using an Automatic Blood Cell Analyzer BC2600 (Shenzhen, China).
Results
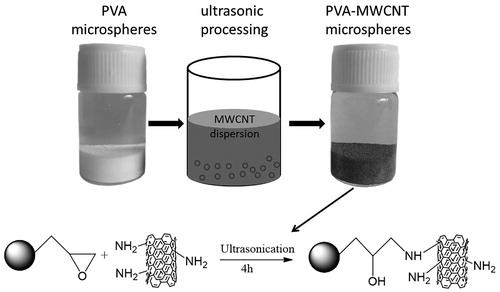
The PVA-AMWCNT microspheres were prepared by mixing AMWCNTs dispersion with PVA microspheres under ultrasonic condition for a preset time, in which the covalent bond linkage was formed between the epoxidized PVA and the primary amino group of the CNT. As shown in , the colour of PVA microspheres changed from white to dark grey, indicating that the AMWCNTs were successfully incorporated into the PVA microspheres. From SEM images and Raman spectrum, we can conclude that the AMWCNT was successfully immobilized on PVA microspheres.
The synthesis scheme of PVA-AMWCNT composite microspheres is shown in . The stable coupling between PVA microspheres and AMWCNT was obtained by epoxy group interacting with amino groups.
Physical parameters of PVA-AMWCNT composite microspheres
From , results indicate the apparent density, skeleton density, porosity, water content and swelling degree of PVA-AMWCNT composite microspheres are slightly higher than that of unmodified PVA microspheres.
Table 1. Physical parameters of PVA and PVA-AMWCNT microspheres.
The AMWCNT content in the composite microspheres is 19.9 ± 3.5 mg/ml and the coupling amount of PMB is 1.58 ± 0.28 mg/ml.
SEM analysis
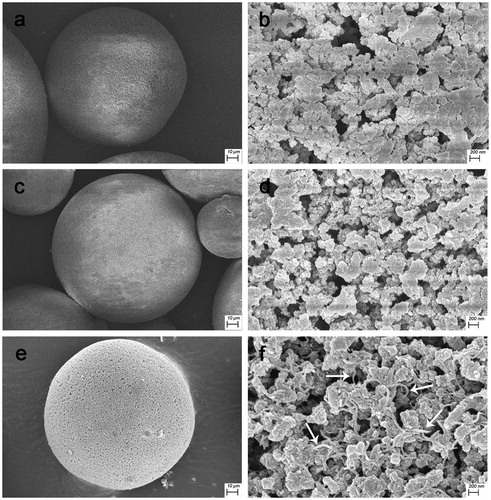
SEM shows that the PVA, PVA-PMB and PVA-AMWCNT microspheres are uniformly of spherical shape (). As shown in , a number of AMWCNT (white arrow) was well dispersed on the microspheres (). In addition, comparing , the latter shows a more developed macroporous structure which facilitates the diffusion of endotoxin into the interior of the microspheres.
Fluorescence spectrum
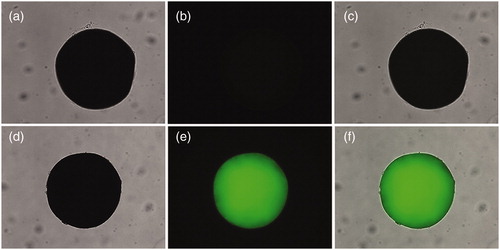
Fluorescence microscope was used to detect the presence and distribution of FITC-AMWCNT on PVA microspheres. shows the images of FITC-AMWCNT immobilized PVA microspheres compared with unmodified PVA microspheres. As shown in , the original PVA microspheres cannot be detected under a dark field. However, it can be seen that nanocomposite microspheres still maintains its normal morphology after being treated with FITC-AMWCNT in , and FITC-AMWCNT are distributed on the macroporous of microsphere, which suggests AMWCNT has ability to enter the pores of PVA microspheres.
Raman spectrum
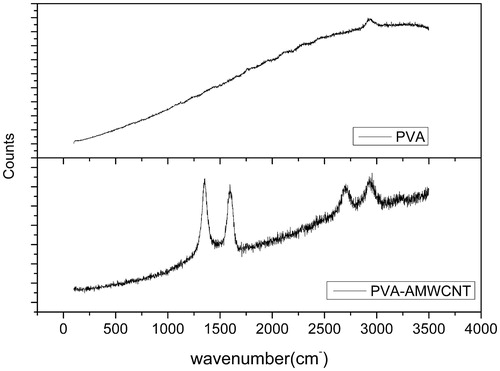
shows the Raman spectrum of CNTs which is characterized by the D and G bands. The D band around 1350 cm characterizes the disorder of the CNTs and the G band around 1590 cm is assigned to the graphite structure, both of which are specific to CNT [Citation29]. However, the spectrum of PVA microsphere shows no D and G band. The Raman spectrum shows the presence of AMWCNT in the composite microspheres.
The leakage of AMWCNT
After the PVA-AMWCNT microspheres was treated for 72 h, no AMWCNT leakage was detected in the supernatant (data is not shown). It indicated that the AMWCNT was stably immobilized on the PVA microspheres.
Adsorption experiment
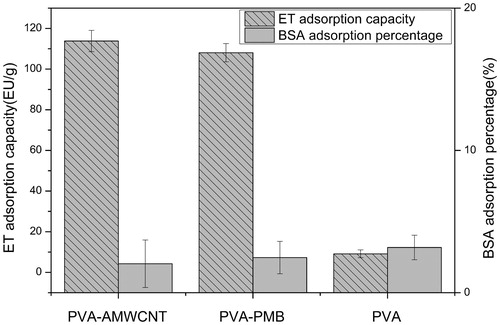
The adsorption capacity of AMWCNT on endotoxin was investigated with unmodified PVA microspheres as negative control and PVA-PMB as positive control, respectively. The results are shown in . It indicated that both PVA-AMWCNT and PVA-PMB can efficiently remove endotoxin, and PVA-AMWCNT(114EU/g) performed better results than PVA-PMB(108EU/g). However, the unmodified PVA hardly adsorbed endotoxin. Moreover, the adsorption capacity of PVA, PVA-AMWCNT and PVA-PMB for BSA has no significant differences, which are all about 3%.
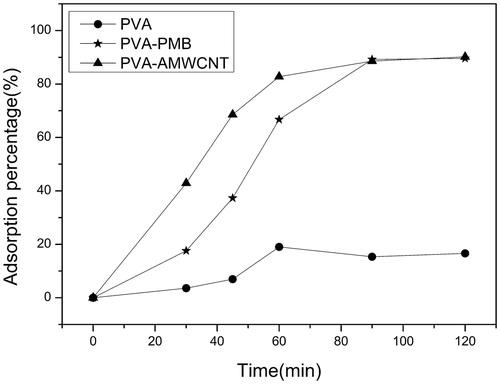
The time-dependent adsorption kinetic of endotoxin in aqueous solution is shown in . The curves demonstrated that the uptake of endotoxin by PVA-AMWCNT and PVA-PMB microspheres was more rapid than unmodified PVA microspheres, and PVA-AMWCNT reached equilibrium 30 min ahead of PVA-PMB. The difference in adsorption speed indicated that the introduction of AMWCNT accelerated the adsorption for endotoxin. After 90 min the endotoxin adsorption percentage of PVA-AMWCNT and PVA-PMB reached 90.2 and 89.6%, respectively, both of which were much higher than that of unmodified PVA microspheres (16.6%).
Blood compatibility
By haemolysis test we can determine the volume of haemoglobin released from RBCs. Haemolysis rate was calculated using the following equation, in which, ODsample, ODpositive and ODnegative stand for the absorbances of the sample, positive control and negative control, respectively.
(1)
shows the haemolysis degree of PVA microspheres and PVA/AMWCNT microspheres which both exhibit a low degree of haemolysis (<1%). We can conclude that the composite microspheres impregnated with CNTs did not increase the haemolysis degree. In addition, from the data in the , we can see the quantity of WBC, RBC, PLT and Lymph in the rabbit blood has a little decrease after incubated with PVA microspheres and PVA/AMWCNT composite microspheres. It can be concluded that the incorporation of AMWCNT into the composite microspheres did not affect the blood components.
Table 2. Haemolysis degree and blood routine of PVA and PVA/AMWCNT composite microspheres (mean ± SD, n = 3).
shows haemolysis degree and blood routine of PVA and PVA/AMWCNT composite microspheres (mean ± SD, n = 3).
Discussion
In the past decades, nanocomposite materials have been developed rapidly in different fields. CNT is one of the promising nanomaterials to enhance the properties of different materials [Citation30]. Multi-walled CNTs with abundant primary amino groups have amphiphilic structures similar to PMB, and are suitable as affinity ligands for endotoxin adsorption. Due to the amphiphilic property of endotoxin, both hydrophobic and ionic/polar interactions are considered to be beneficial for its affinity binding [Citation18]. PVA microsphere is considered an ideal carrier for biomedical application due to its macroporous structure, ease of hydroxyl group modification, good biocompatibility and high rigidity [Citation20]. Therefore, the PVA-AMWCNT nanocomposite microsphere for endotoxin adsorption have advantages as follows: First, macroporous structure of PVA microsphere is conducive to the diffusion and adsorption of endotoxin molecules. Second, the amphiphilic AMWCNT is favourable for endotoxin adsorption. Third, AMWCNT were covalently bonded to PVA microspheres, thus avoiding the leakage of AMWCNT. The PVA-AMWCNT microsphere which combines both the advantages of AMWCNT and PVA, should be an excellent endotoxin affinity adsorbent.
The PVA/AMWCNT nanocomposite adsorbent described in this manuscript was prepared by sonication and capillarity reaction [Citation21], in which AMWCNTs was trapped and immobilized into the macropores of the PVA microspheres. In order to avoid the aggregation of AMWCNT, biocompatible PVP was used to disperse AMWCNT homogeneously before the nanocomposite microsphere was prepared. Compared with the conventional blending method, ultrasonic processing facilitates uniform distribution of AMWCNT in the macropores of PVA microspheres.
The physical structure parameters of PVA-AMWCNT microspheres showed that apparent density, skeleton density, porosity, water content and swelling degree of PVA-AMWCNT composite microspheres are slightly higher than that of unmodified PVA microspheres. The increase of porosity may be one of the important factors to improve the adsorption of endotoxin, which is consistent with the results of the subsequent adsorption test. SEM, Raman spectrum and fluorescence images have demonstrated the successful trapping and immobilization of AMWCNT on PVA microsphere. From SEM images, AMWCNT can be observed on the macropores of PVA microspheres clearly. Raman spectrum and fluorescence images further demonstrated the presence of AMWCNT. Leakage test result indicated that AMWCNT did not leak off the composite microspheres prepared by ultrasonic treatment and thus avoid the potential toxicity of nanomaterial on the blood system.
The endotoxin adsorption capacity was evaluated in aqueous solution. Compared with unmodified PVA microspheres, the endotoxin adsorption capacity of PVA-AMWCNT composite microspheres increased significantly, even slightly better than that of PVA-PMB microspheres. The adsorption results indicated that AMWCNT enhanced the adsorption capacity of composite microsphere for endotoxin. The possible reasons are as follows: (1) The AMWCNT increased the porosity of the PVA microspheres, which increases the effective adsorption site of the endotoxin molecule. (2) The amphiphilic structure of AMWCNT is favourable for endotoxin adsorption [Citation18]. The low adsorption percentage of BSA (<3%) shows that the PVA based microspheres had little nonspecific adsorption in simulated serum. Adsorption dynamic for endotoxin showed the adsorption equilibrium time of PVA-AMWCNT microspheres was shorter than that of PVA-PMB microspheres, which indicated that the amphiphilic interaction between AMWCNT and endotoxin is a favourable factor for rapid removal of endotoxin [Citation18].
From the test result of the blood compatibility, we can conclude that the incorporation of AMWCNT into PVA microspheres did not affect blood cell compositions and haemolysis rate remarkably. According to previous report [Citation31], AMWCNT can be classified as non-haemolytic materials. In this study, the haemolysis rate of composite microspheres had almost no significant differences compared with unmodified PVA microspheres, both of which stayed within an acceptable range. The incorporation of AMWCNT into PVA microsphere did not affect blood cells remarkably. The blood compatibility of PVA-AMWCNT composite microspheres is acceptable.
Conclusions
In this study, PVA/AMWCNT composite microspheres were prepared successfully for the first time and used for endotoxin removal. SEM images showed that AMWCNTs was well dispersed in the macropores of PVA microspheres. Raman spectrum and fluorescence images further demonstrated the presence of AMWCNTs in composite microspheres. The PVA-AMWCNT microspheres showed slightly better adsorption capability and faster adsorption equilibrium for endotoxin in aqueous solution, than the PVA microsphere with PMB as ligand. More noteworthy, the PVA based microspheres had little nonspecific adsorption for albumin and an excellent blood compatibility property. It suggests that the PVA-AMWCNT nanocomposite microspheres have great potential in clinical application.
Acknowledgements
The support of National Natural Science Foundation of China (No. 81271710) and Natural Science Foundation of Tianjin City (No. 12ZCDZSY20000, 14ZCZDSY00011, 14JCTPJC00487, 13JCQNJC14200) are all well acknowledged.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- Raetz CRH. Biochemistry of Endotoxins. Annu Rev Biochem. 1990;59:129–170.
- Beutler B, Rietschel ET. Innate immune sensing and its roots: the story of endotoxin. Nat Rev Immunol. 2003;3:169–176.
- Umeda M, Niwa M, Yamagami S, et al. Novel endotoxin adsorbing materials, polymyxin-sepharose and polyporous polyethylene membrane for removal of endotoxin from dialysis systems. Biomater Artificial Cells Artificial Organs. 1990;18:491–497.
- Hou G, Liu T, Wang H, et al. Preparation of adsorbents for the removal of endotoxin. Artif Cells Blood Substit Immobil Biotechnol. 2005;33:227–237.
- Anspach FB. Endotoxin removal by affinity sorbents. J Biochem Biophys Methods. 2001;49:665–681.
- Anspach FB, Hilbeck O. Removal of endotoxins by affinity sorbents. J Chromatogr A. 1995;711:81–92.
- Matsumae H, Minobe S, Kindan K, et al. Specific removal of endotoxin from protein solutions by immobilized histidine. Biotechnol Appl Biochem. 1990;12:129–140.
- Sakata M, Kawai T, Ohkuma K, et al. Reduction of endotoxin contamination of various crude vaccine materials by gram-negative bacteria using aminated poly(gamma-methyl L-glutamate) spherical particles . Biol Pharm Bull. 1993;16:1065–1068.
- Chandy T, Rao GHR. Evaluation of heparin immobilized chitosan-peg microbeads for charcoal encapsulation and endotoxin removal. Artif Cell Blood Sub Biotechnol. 2000;28:65–77.
- Tani T, Chang TMS, Kodama M, et al. Endotoxin removed from hemoglobin solution using polymyxin-B immobilized fibre (PMX-F) followed by a new Tdrbidohetric Endotoxin Assay. Biomater Artif Cell Immobilization Biotechnol. 1992;20:457–462.
- Tani T, Hanasawa K, Endo Y, et al. Therapeutic apheresis for septic patients with organ dysfunction: hemoperfusion using a polymyxin B immobilized column. Artif Organs. 1998;22:1038–1044.
- Tani T, Shoji H, Guadagni G, Perego A. Extracorporeal removal of endotoxin: the polymyxin B-immobilized fiber cartridge. In: Ronco C, Piccinni P, Rosner MH, editors. Endoxemia and endotoxin shock: disease, diagnosis and therapy. Contributions to nephrology. Basel: Karger; 2010. p. 35–44.
- Lu Y, Gong Q, Lu F, et al. Preparation of sulfonated porous carbon nanotubes/activated carbon composite beads and their adsorption of low density lipoprotein. J Mater Sci Mater Med. 2011;22:1855.
- Ouyang A, Gong Q, Liang J. Carbon nanotube–chitosan composite beads with radially aligned channels and nanotube-exposed walls for bilirubin adsorption. Adv Eng Mater. 2015;17:460–466.
- Ye C, Gong Q-M, Lu F-P, et al. Preparation of carbon nanotubes/phenolic-resin-derived activated carbon spheres for the removal of middle molecular weight toxins. Sep Purif Technol. 2008;61:9–14.
- Zong W, Chen J, Han W, et al. Preparation of chitosan/amino multiwalled carbon nanotubes nanocomposite beads for bilirubin adsorption in hemoperfusion. J Biomed Mater Res B Appl Biomater. 2016: [Epub ahead of print]. DOI: 10.1002/jbm.b.33806
- Nie C, Ma L, Xia Y, et al. Novel heparin-mimicking polymer brush grafted carbon nanotube/PES composite membranes for safe and efficient blood purification. J. Membr Sci. 2015;475:455–468.
- Zhang Y, Yang H, Zhou K, et al. Synthesis of an affinity adsorbent based on silica gel and its application in endotoxin removal. React Funct Polym. 2007;67:728–736.
- Yu YT, Zhu H, Wang S. Amphiphilic polyvinyl alcohol adsorbent for the removal of low-density lipoprotein. Artif Cells Nanomed Biotechnol. 2015;43:117–123.
- Weichao W, Hui X, Lisha S, et al. Macroporous poly(vinyl alcohol) microspheres bearing phosphate groups as a new adsorbent for low-density lipoprotein apheresis. Biomed Mater. 2009;4:065007.
- Ha W, Song X-y, Chen J, et al. A physical entrapment method for the preparation of carbon nanotube reinforced macroporous adsorption resin with enhanced selective extraction performance. Nanoscale. 2015;7:18619–18627.
- Petsch D, Beeskow TC, Anspach FB, et al. Membrane adsorbers for selective removal of bacterial endotoxin. J Chromatogr B Biomed Sci Appl. 1997;693:79–91.
- He B, Huang W. Ion Exchange and Adsorption Resin. Shanghai: Shanghai Science and Technology Education Press. 1995. p. 182.
- Wan Y, Huang W, Wang Z, et al. Preparation and characterization of high loading porous crosslinked poly (vinyl alcohol) resins. Polymer 2004;45:71–77.
- Cheng Y, Wang S, Yu Y, et al. In vitro, in vivo studies of a new amphiphilic adsorbent for the removal of low-density lipoprotein. Biomaterials. 2003;24:2189–2194.
- Wang B, Liu S, Zhu Y, et al. Influence of polyvinyl pyrrolidone on the dispersion of multi-walled carbon nanotubes in aqueous solution. Russ J Phys Chem. 2014;88:2385–2390.
- Guo L, Zhang J, He Q, et al. Preparation of millimetre-sized mesoporous carbon spheres as an effective bilirubin adsorbent and their blood compatibility. Chem Commun (Camb). 2010;46:7127–7129.
- Sideman S, Mor L, Brandes JM, et al. Preparation of a biocompatible albumin-coated ion exchange resin for bilirubin removal from the blood of jaundiced newborns. J Biomed Mater Res. 1983;17:91–107.
- Rao AM, Bandow S, Richter E, et al. Raman spectroscopy of pristine and doped single wall carbon nanotubes. Thin Solid Films. 1998;331:141–147.
- Spitalsky Z, Tasis D, Papagelis K, et al. Carbon nanotube–polymer composites: chemistry, processing, mechanical and electrical properties. Prog. Polym. Sci. 2010;35:357–401.
- Zhao ML, Li DJ, Yuan L, et al. Differences in cytocompatibility and hemocompatibility between carbon nanotubes and nitrogen-doped carbon nanotubes. Carbon. 2011;49:3125–3133.