?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
To avoid potential systematical toxicity, solid lipid nanoparticles (SLNs) were prepared as a vehicle for transdermal delivery of ivermectin (IVM) using hot homogenisation followed by ultrasonic method. The as-prepared SLNs were approximately spherical shape with good stability. IVM was encapsulated in amorphous form within SLNs and displayed prolonged release from SLNs without burst release due to high encapsulation efficiency (EE). The cumulative permeation of IVM across excised rat skin from SLNs was significantly increased compared to the ivermection suspension. These results indicated that the proposed SLNs can be considered as an efficient carrier for dermal delivery of IVM to effectively treat scabies.
Introduction
Scabies is a common parasitic infection caused by Sarcoptes scabiei mite variety hominis and is transmitted primarily through skin-to-skin contact [Citation1]. According to the World Health Organisation (WHO), scabies was amongst the main tropical diseases in 2013, globally affecting more than 130 million people [Citation2]. In addition to itching and sleeping disturbances, scabies is also a major underlying cause of high rates of bacterial skin infections and their consequences [Citation3].
Ivermectin (IVM) is an important broad-spectrum antiparasitic drug against a variety of endo- and ecto- parasites (endectocide) belonging to the macrocyclic lactone family [Citation4]. However, IVM has been reported to cause serious side effects when the drug is used in high doses, such as when it is accidentally ingested [Citation5,Citation6]. The topical treatment of skin diseases appears to be favourable to avoid the systemic side effects. Topical IVM is an effective and cost-comparable agent in the treatment of scabies infection, and it may be particularly useful in the treatment of severely crusted scabies lesions in immune-compromised patients or when other topical therapies have failed [Citation7].
Solid lipid nanoparticles (SLNs) have emerged as an alternative carrier system to traditional carriers [Citation8], attracting great attention as an innovative colloidal drug carrier for topical application [Citation9]. It has been reported that the SLNs can retain beneficial properties of other colloidal carriers and have no disadvantages in terms of physical and chemical storage stability, toxicity, loading capacity (LC), production scale, target-oriented releasing properties and feasibility [Citation10]. In addition, the SLNs have occlusive properties that can increase skin hydration [Citation11]. Meanwhile, small sized lipid particles ensures close association with stratum corneum [Citation12], thus increasing the quantity of the drug penetrating into the skin. The SLNs seem to be well suited for application on damaged or inflamed skin [Citation13], hence they have been used for improving the skin/dermal uptake of several drugs, such as penciclovir, isotretinoin, fluocinolone acetonide and fluconazole [Citation14–17], supporting the fact that the SLNs may be employed as a topical delivery system for IVM.
In the present work, attempts were made to develop and optimise the ivermectin loaded SLNs (IVM-SLNs) using hot homogenisation and ultrasonic methods. The characterisation of optimised formulation was assessed using several important parameters, such as hydrodynamic diameter, zeta potential, drug entrapment efficiency (EE), transmission electron microscopy (TEM), differential scanning calorimetry (DSC) and in vitro drug release studies. The skin permeation of IVM-SLNs was also examined to explore the potential application of SLNs as an IVM topical formulation.
Materials and methods
Materials
IVM was obtained from Anhui Huaao Biotechnology Co., Ltd. (Hefei, China). IVM standard (91%) was purchased from China Institute of Veterinary Drug Control (Beijing, China). Palmitic acid and polyvinyl alcohol (PVA) were provided by Aladdin® (Shanghai, China). Ten polyglycerol fatty acid ester (P10) was supplied by Mitsubishi Chemical Corporation (Tokyo, Japan). Ultra-filtration centrifuge tube was obtained from Millipore Corporation (Bedford, USA). Solvents, such as methyl alcohol and acetonitrile in HPLC grade were available from TEDIA® (Fairfield, USA). Triton X-100 was bought from Sigma (St Louis, USA). The de-ionised (DI) water was purified using a Milli-Q system with 18.2 MΩ·cm−1 (Millipore, Billerica, USA). All other chemicals were of analytical reagent grade without further purification. SD rats (female, 12 weeks old, 200 ± 20 g) were purchased from the Comparative Medicine Centre of Yangzhou University (Yangzhou, China). All animal studies were carried out according to the Guideline for Animal Experimentation with the approval of the Animal Care Committee of Nanjing Agricultural University, Nanjing, China.
Preparation of IVM SLNs
SLNs were prepared by hot homogenisation, followed by ultrasonic method. Briefly, palmitic acid (0.5 g) was melted in a 30-ml glass vial by a magnetic stirrer at 75 °C. IVM (0.09 g) was then dissolved in the melted lipid. The lipid phase was subsequently poured into 15 mL of boiling aqueous solution with 1% PVA (w/v) under magnetic stirring at 300 rpm for 10 min to form a coarse oil-in-water emulsion. After that, the emulsion was sonicated using a 13 mm microprobe at 35% amplitude (Ningbo Xingzhi Biotechnology Co., Ltd., Ningbo, China) for 5 min, and then 15 mL of cold water (4 °C) was immediately poured into to obtain the IVM-SLNs. Similarly, the control SLNs were prepared without the addition of IVM.
Measurements of hydrodynamic diameter, polydispersity index and zeta potential
The hydrodynamic diameter, polydispersity index (PDI) and zeta potential of IVM-SLNs were characterised by dynamic light scattering (DLS) using a Malvern ZetaSizer Nano ZS (Malvern Instruments Ltd., Worcestershire, UK). The samples were appropriately diluted with de-ionised water before measurement.
TEM analysis
The morphology of IVM-SLNs was examined by TEM (H-7650, Japan). A few drops of IVM-SLNs were placed on carbon-coated copper grids and negatively stained with a 2% (v/v) phosphotungstic acid solution for 30 s. The solvent was then allowed to dry overnight at room temperature prior to TEM visualisation.
Fourier transforms-infrared (FT-IR) analysis
The optimised formulations were freeze-dried and converted into solid form. A Perkin Elmer FT-IR spectrophotometer (model 1600, Perkin-Elmer, San Diego, USA) was used to identify any changes in the molecular levels of IVM-SLNs, SLNs and IVM from 500 to 4000 cm−1. The samples were prepared with KBr and compressed into a suitable-size disk for measurement.
DSC analysis
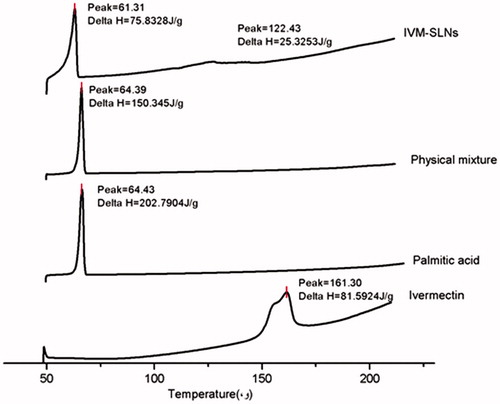
IVM-SLNs dispersions were lyophilised prior to DSC (Pyris 1 DSC, PerkinElmer, Francisco, USA) analysis. Solid lipid (palmitic acid), drug (IVM), lipid/drug (7:3) physical mixture and IVM-SLNs were subjected to DSC analysis. Prior to heating, approximately 3 mg samples were equilibrated in the DSC pan (hermetic crimped aluminium pans) at 45 °C for 30 min and then heated up to 200 °C at a scanning rate of 10 °C/min under N2 atmosphere.
Drug encapsulation efficiency and LC
The encapsulation efficiency (EE) of IVM-SLNs was calculated by measuring the amounts of free drug using a filtration technique. Two microliter IVM-SLNs dispersion was added into a centrifugal filter device (molecular weight cutoff, MWCO, 100 kDa, USA) and centrifuged at 3000 rpm for 5 min at 25 °C (Sigma 3K15, Harz, Germany). IVM was absorbed on the ultrafiltration membrane to reach saturation. Subsequently, IVM-SLNs dispersion was added again and the filtrate was collected. The unencapsulated IVM in the filtrate was measured using HPLC method. The total drug content in the IVM-SLNs was determined by demulsification method. Briefly, 2 mL of demulsifier (5% Triton X-100 ethanol solution) was added into 1 mL of IVM-SLNs dispersion to release the trapped IVM together with ultrasonic treatment. The resulting solution was quantified by HPLC method. The LC of IVM was the ratio of incorporated drug to lipid (w/w). LC and EE were calculated using the following formulas:
Where Winitial was the amounts of drug added in the formulation, Wtotal was the total amounts of IVM-SLNs and Wfree was the amounts of drug in supernatant.
In vitro release of IVM from IVM-SLNs
The dissolution behaviour of IVM from the SLNs was evaluated using dialysis bag technique. The dialysis bag was thoroughly washed with boiling water twice for 15 min to remove impurities, followed by overnight soaking in the release medium. The IVM-SLNs formulation (equivalent to 21 mg IVM) was placed in the cellulose membrane bags (molecular weight cut off 3.5 kDa) and dialyzed against 600 mL of ethanol/water mixture (50:50) in a 1000 mL beaker at 37 °C under magnetic stirring at 500 rpm. To determine the IVM amounts diffused through the dialysis bag, the samples (1 mL) were taken from the receiver solution and the same amount of fresh mixture was added to keep a constant volume at the fixed time points. IVM in the samples was measured by HPLC at 245 nm. The sink conditions were maintained for release study. The saturated concentration of IVM in the de-ionised water (pH 6.2) was 296.26 μg/mL in this test and the drug concentration in the release medium was 35 μg/mL when the IVM was released completely. IVM suspension were treated similarly and used as control for the measurement.
In vitro skin permeation study
In vitro skin permeation study was performed using a Franz diffusion cell. The effective diffusion area was 0.636 cm2 and the receiver chamber had a capacity of 5 mL. The excised rat epidermis from abdomen of female donor was mounted between the donor and acceptor compartments of the diffusion cell. The formulation weights with 4.54 mg IVM were applied to maintain a constant concentration in the donor compartment which was occluded during the whole experiment. The acceptor compartment was filled with physiological saline including 2% sodium dodecyl sulfate (SDS) and 20% alcohol and continuously stirred by a stirring bar at 1000 rpm. The whole assembly was fixed in a constant temperature at 37 °C. The samples (1 mL) were withdrawn at different time intervals and analysed by HPLC for drug content. The sink conditions were maintained and receptor phase was replenished with equal volume of fresh acceptor phase at each sample withdrawal. The amounts of drug permeating per unit area of the skin were plotted against time.
The cumulative amount of IVM (Qn, μg/cm2) that permeated through the rat skin was plotted as a function of time (t, h) and calculated based on the following EquationEq. (1)(1)
(1) [Citation18], according to Fick’s second law of diffusion. The EquationEquation (2)
(2)
(2) was expressed when the solution was in the steady state [Citation19]. The steady permeation flux (J) was calculated from the slope of the steady state portion of the amount of permeating drug divided by A versus time. The enhancement ratio (Er) was calculated using the following EquationEq. (3)
(3)
(3) :
(1)
(1)
(2)
(2)
(3)
(3)
Where Cn stands for the drug concentration of the receiver medium at each sampling time, Ci for drug concentration of the i sample, A for effective diffusion area and V0 and Vi for the volumes of the receiver solution and sample, respectively. C0 in EquationEq. (2)(2)
(2) represents the drug concentration which remained constant in the donor vehicle, while D is the diffusion coefficient and L is the thickness of the membrane and K is the partition coefficient of drug between membrane and vehicle. Kp stands for permeability coefficient. Js and J0 in EquationEq. (3)
(3)
(3) represent the steady permeation flux of IVM-SLNs and IVM suspension, respectively.
Data analysis
Statistical analysis of the obtained data was performed using SPSS software (version 16.0, Chicago, USA), and all values were represented as the means ± SD of the three replicates. The results were subjected to one-way ANOVA using the Duncan test to analyse the difference, in which p < .05 was considered to be statistically significant.
Results and discussion
Characterisation of IVM -SLNs
In the current study, IVM-SLNs were prepared through hot homogenisation coupled with ultrasonic process. The choice of lipid was crucial in this method, for formulation design based on its ability to dissolve the chemical substances [Citation20] Palmitic acid was selected as solid lipid due to its high dissolution for IVM (0.2 g/g). IVM was subsequently dispersed homogeneously in the melted plamitic acid, followed by mixing with 1% PVA aqueous solution to form a pre-emulsion. Ultrasonic process was then employed to reduce the particle size. The detailed characterisation by TEM revealed that the as-synthesised IVM-SLNs had approximately spherical shape and were quite uniform in shape (). The corresponding size distribution is represented by histogram from the IVM-SLNs, with 270.34 ± 0.35 nm mean diameter ranging from 150 to 400 nm ().
Figure 1. The morphology and size distribution histogram of ivermectin-loaded solid lipid nanoparticles (IVM-SLNs). (A) The morphology of IVM-SLNs was depicted by transmission electron microscopy (TEM). Scale bar was 2 μm. (B) Size distribution histogram was obtained by size analysis of several TEM images of particles. The mean diameter was 270.34 nm.
The hydrodynamic diameter and zeta potential for the IVM-SLNs were measured by DLS using a Malvern Zetasizer Nano. The average size of the IVM-SLNs in colloidal solution was 312.8 ± 2.40 nm () with a PDI of 0.082 ± 0.005 (). The optimised hydrodynamic diameter of the formulation was below 400 nm, which was available for the topical administration. It has been reported that a desirable value of particle size for NLCs is established as ≤500 nm, in order to penetrate the skin epithelium [Citation21]. The PDI is an important indication regarding sample homogeneity, as value below 0.25 reflects relatively homogeneous nanoparticles with minimum predisposition to aggregation [Citation22]. The zeta potential is also an important parameter that allows predictions on the physical stability of colloidal dispersions [Citation23]. In theory, higher values of zeta potential, either positive or negative, tend to stabilise the suspension and aggregation are less likely to occur for charged particles with pronounced zeta potential (>|30|). This is due to electrostatic repulsion between particles with same electrical charge [Citation24]. In this study, zeta potential of the IVM-SLNs was −30.5 ± 1.51 mV (), which predicted a long-term stability for the particles. The physical stability of the formulated IVM-SLNs were further evaluated by examining changes of hydrodynamic diameters, PDI and zeta potential during storage for 31 d at 4, 20 and 40 °C (). The results showed that narrow interval of values was maintained for the hydrodynamic diameter and zeta potential upon 31 d storage at 4 °C, indicating that the IVM-SLNs kept their stability at low temperature. In addition, the slight decrease in zeta potential for the IVM-SLNs may have been attributed to the presence of some free drug in the dispersion, which is anionic in nature. The cellular uptake of nanoparticles is through electrostatic attraction between the oppositely charged particles. Large negatively charged domain is present on the cell surface where cationic nanoparticles can be attracted leading to increased cellular uptake of either nanoparticles or released drug [Citation25]. It has been described that only negatively charged particles are able to permeate the skin [Citation21]. Altogether, the storage stability study indicated good physical stability for the lipid nanoparticles, most probably due to the surfactant used in their preparation [Citation26].
Figure 2. Hydrodynamic diameter and zeta potential of IVM-SLNs. (A) Hydrodynamic diameter and (B) zeta potential of IVM-SLNs in aqueous solution were measured by dynamic light scattering (DLS), using a Malvern Zetasizer Nano, and were 312.8 nm and −30.5 mV, respectively.
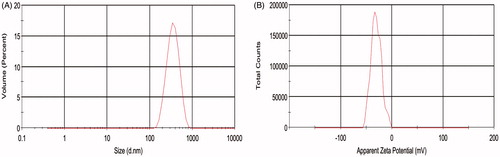
Figure 3. Storage stability of IVM-SLNs. (A) Size change during storage of 31 d at 4, 20 and 40 °C, (B) polydispersity index and (C) zeta potential change during the storage of 31 d at 4 °C.
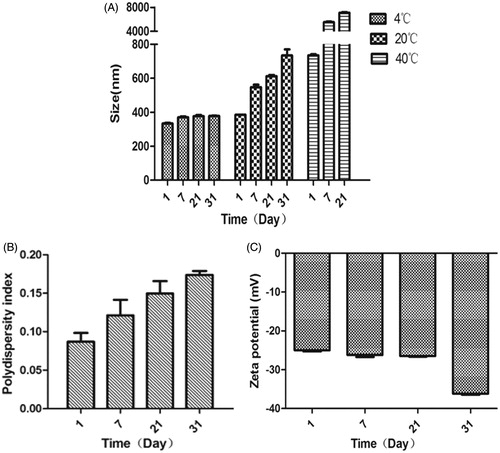
Table 1. Characterisation of IVM-SLN formulation.
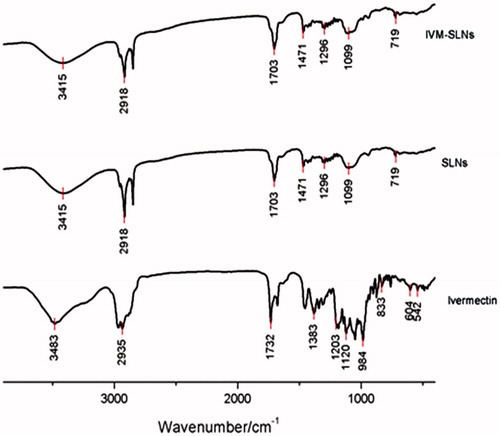
FT-IR studies were carried out to validate the substantial entrapment of IVM into the SLNs. shows the infrared spectra for IVM alone, SLNs, and IVM-SLNs. In the case of IVM alone, wave number at 3483 cm−1 depicted O–H stretching and a sharp absorption band appearing around 2935 cm−1 corresponded to C–H stretching, while 1732–1703 cm−1 could be attributed to the stretching band of C6-ring (stretching). The drug-free SLNs and IVM-SLNs exhibited the same spectra, which was completely different from IVM. These results thus confirmed total encapsulation of IVM into the SLNs.
Figure 4. FT-IR spectra for IVM-SLNs, solid lipid nanoparticles (SLNs) and ivermectin (IVM) on KBr disks.
DSC is a tool for investigating the melting and re-crystallisation behaviour of crystalline material like the SLNs [Citation27]. The IVM alone, palmitic acid, physical mixture (with the same composition) and IVM-SLNs powder were examined by DSC (). Pure IVM and solid lipid (palmitic acid) showed single sharp endothermic melting peaks at 161.3 and 64.43 °C, respectively. The DSC traces of IVM-SLN and physical mixture revealed the endothermic peak around palmitic acid melting point but the melting of drug disappeared. The most likely reason for the disappearance of the IVM peak in the physical mixtures could be due to the dissolution of IVM in the molten palmitic acid, suggesting the lipid may inhibit the crystallisation of IVM during the nanoparticle formation. In addition, the DSC trace of IVM-SLN described a depression in melting point and broadening peak could be attributed to the two stage of formulation melting. The initial event was indicative of re-crystallisation of an unstable polymorph and the latter event showed the melting of the most stable form [Citation28].
EE and LC
IVM is a hydrophobic drug that shows maximum solubility in palmitic acid and this lipid was thus selected to achieve highest encapsulation and minimum drug leakage in the current study. In addition, it was found that the addition of PVA (co-emulsifier) to oil phase led to increased EE due to reduction in particle crystallinity imparting improved stability and sustaining the release of encapsulated drug [Citation29]. The drug EE of the IVM-SLNs formulation was 98.48 ± 0.052% and the average drug loading of three batches of IVM-SLNs were 11.99 ± 0.13% ().
In vitro IVM release assay
There are two kinds of forms in lipid nanoparticles systems, incorporated in the lipid matrix either in dissolved or in dispersed form [Citation30]. The dissolvability of the drug in the lipid matrix therefore became a crucial controlling factor for the release of drug from SLNs. The release of entrapped IVM from the IVM- SLNs was evaluated by using a dialysis diffusion technique. The dissolution profiles of the IVM-SLNs and IVM suspension are shown in .
Figure 6. In vitro drug release profiles of IVM suspension and IVM-SLNs. Data were expressed as the mean ± SD (n = 3). *Ssignificant difference (p < .05).
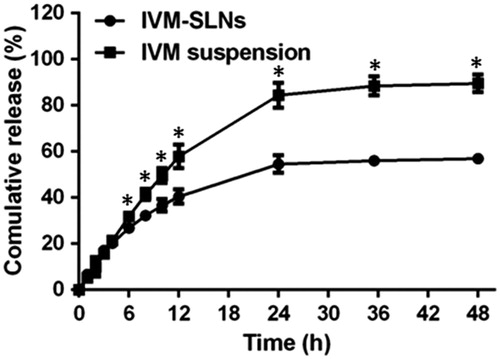
Different release profiles from the SLNs have been reported [Citation31]. During preparation of NLCs, cooling from high to room temperature favoured the enrichment of drug in the outer layers of the particles, resulting in superficial entrapment causing initial burst release [Citation32]. But in this study, IVM suspension and IVM-SLNs showed approximately 95% and 55% release, respectively (). This suggested that the IVM release from the SLNs displayed a slow and sustained release pattern without burst release, which was attributed to the higher EE of the IVM-SLNs.
In vitro drug skin penetration studies
In the current study, Franz diffusion cell was used to evaluate the penetration of IVM-SLNs through the skin. The cumulative amounts of IVM at different time intervals in the receptor solution are shown in . The amounts of IVM in the receptor chamber exhibited a steady increase as a function of time. Obviously, compared to the IVM suspension, the IVM-SLNs formulation resulted in more IVM penetrating through the rat skin at each time point of detection. The steady-state flux (Js) and permeation coefficient (Kp) for 12 h are shown in . Their values about Js and Kp were higher than those of IVM suspension, indicating that it was easier for the SLNs to penetrate through the rat skin, as suggested by the Er of IVM-SLNs (). In addition to the nanoparticle’ small size, the dosage forms of drug delivery system can significantly influence the drug and its related compounds performance through rat skin [Citation33]. Another reason is probably because of the hydrogen bond between the IVM and SLNs detected by FT-IR. It has been evidenced that the SLNs have higher occlusivity and increased hydration for the stratum corneum and therefore they influence the percutaneous absorption of active component contained in the formulation [Citation11,Citation12]. The intact rat skin was used in this study and the nanoparticles penetration may have been considerably enhanced when the skin barrier was compromised artificially or due to pathological processes [Citation34]. Taken all together, the SLNs are a promising novel vehicle for IVM topical application.
Figure 7. Cumulative amounts of ivermectin from IVM suspension and IVM-SLNs that permeated across rat skin. Data were expressed as the mean ± SD (n = 3). *Significant difference (p < .05).
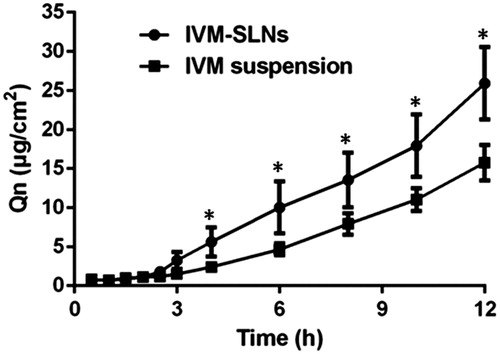
Table 2. Skin permeation parameters from different formulations of ivermection.
Conclusions
The IVM-SLNs formulations were successfully prepared through a hot homogenisation method combined with ultrasonic method for topical delivery of IVM. The IVM-SLNs showed relatively high EE with narrow particle size range. The release study displayed slow and sustained release patterns for the SLNs containing IVM, which could be an advantage for their topical application. The in vitro drug skin penetration study indicated that the IVM-SLNs easily penetrated the rat skin than ivermection suspension. Solid state analysis of SLNs and physical mixtures showed that the IVM had amorphous and crystalline form in the SLNs and physical mixtures, respectively. The present study opens up a window for topical application of SLNs loaded with IVM.
Disclosure statement
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- Romani L, Steer AC, Whitfeld MJ, et al. Prevalence of scabies and impetigo worldwide: a systematic review. Lancet Infect Dis. 2015a;15:960–967.
- Currie BJ. Scabies and global control of neglected tropical diseases. N Engl J Med. 2015;373:2371–2372.
- Romani L, Whitfeld MJ, Koroivueta J, et al. Mass drug administration for scabies control in a population with endemic disease. N Engl J Med. 2015b;373:2305–2313.
- Moreno L, Dominguez P, Farias C, et al. Ivermectin pharmacokinetics, metabolism, and tissue/egg residue profiles in laying hens. J Agric Food Chem. 2015;63:10327–10332.
- Goldust M, Rezaee E, Raghifar R, et al. Treatment of scabies: the topical ivermectin vs. permethrin 2.5% cream. Ann Parasitol. 2013;59:79–84.
- Panahi Y, Poursaleh Z, Goldust M. The efficacy of topical and oral ivermectin in the treatment of human scabies. Ann Parasitol. 2015;61:11–16.
- Steer AC, Kearns T, Andrews RM, McCarthy JS, Carapetis JR, Currie BJ. Ivermectin worthy of further investigation. Bull World Health Organ. 2009;87:A–B.
- Nuruzzaman M, Rahman MM, Liu Y, et al. Nanoencapsulation, nano-guard for pesticides: a new window for safe application. J Agric Food Chem. 2016;64:1447–1483.
- Pardeike J, Hommoss A, Müller RH. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int J Pharm. 2009;366:170–184.
- Mehnert W, Mader K. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev. 2001;47:165–196.
- Lauterbach A, Muller-Goymann CC. Applications and limitations of lipid nanoparticles in dermal and transdermal drug delivery via the follicular route. Eur J Pharm Biopharm. 2015;97:152–163.
- Kakadia PG, Conway BR. Lipid nanoparticles for dermal drug delivery. Curr Pharm Des. 2015;21:2823–2829.
- Jenning V, Gysler A, Schäfer-Korting M, et al. Vitamin A loaded solid lipid nanoparticles for topical use: occlusive properties and drug targeting to the upper skin. Eur J Pharm Biopharm. 2000;49:211–218.
- Liu J, Hu W, Chen H, et al. Isotretinoin-loaded solid lipid nanoparticles with skin targeting for topical delivery. Int J Pharm. 2007;328:191–195.
- Lv Q, Yu A, Xi Y, et al. Development and evaluation of penciclovir-loaded solid lipid nanoparticles for topical delivery. Int J Pharm. 2009;372:191–198.
- Gupta M, Vyas SP. Development, characterization and in vivo assessment of effective lipidic nanoparticles for dermal delivery of fluconazole against cutaneous candidiasis. Chem Phys Lipids. 2012;165:454–461.
- Pradhan M, Singh D, Singh MR. Development characterization and skin permeating potential of lipid based novel delivery system for topical treatment of psoriasis. Chem Phys Lipids. 2015;186:9–16.
- Zhu W, Yu A, Wang W, et al. Formulation design of microemulsion for dermal delivery of penciclovir. Int J Pharm. 2008;360:184–190.
- Mei Z, Chen H, Weng T, et al. Solid lipid nanoparticle and microemulsion for topical delivery of triptolide. Eur J Pharm Biopharm. 2003;56:189–196.
- Chakraborty S, Shukla D, Mishra B, et al. Lipid–an emerging platform for oral delivery of drugs with poor bioavailability. Eur J Pharm Biopharm. 2009;73:1–15.
- Kohli AK, Alpar HO. Potential use of nanoparticles for transcutaneous vaccine delivery: effect of particle size and charge. Int J Pharm. 2004;275:13–17.
- Mitri K, Shegokar R, Gohla S, et al. Lipid nanocarriers for dermal delivery of lutein: preparation, characterization, stability and performance. Int J Pharm. 2011;414:267–275.
- Kalhapure RS, Sonawane SJ, Sikwal DR, et al. Solid lipid nanoparticles of clotrimazole silver complex: an efficient nano antibacterial against Staphylococcus aureus and MRSA. Colloids Surf B Biointerfaces. 2015;136:651–658.
- Gonzalez-Mira E, Egea MA, Garcia ML, et al. Design and ocular tolerance of flurbiprofen loaded ultrasound-engineered NLC. Colloids Surf B Biointerfaces. 2010;81:412–421.
- Honary S, Zahir F. Effect of zeta potential on the properties of nano-drug delivery dystems – a review (part 1). Trop J Pharm Res. 2013;12:265–273.
- Gomes MJ, Martins S, Ferreira D, et al. Lipid nanoparticles for topical and transdermal application for alopecia treatment: development, physicochemical characterization, and in vitro release and penetration studies. Int J Nanomedicine 2013;9:1231–1242.
- Ghanbarzadeh S, Hariri R, Kouhsoltani M, et al. Enhanced stability and dermal delivery of hydroquinone using solid lipid nanoparticles. Colloids Surf B Biointerfaces. 2015;136:1004–1010.
- Nnamani PO, Hansen S, Windbergs M, et al. Development of artemether-loaded nanostructured lipid carrier (NLC) formulation for topical application. Int J Pharm. 2014;477:208–217.
- Jose S, Anju SS, Cinu TA, et al. In vivo pharmacokinetics and biodistribution of resveratrol-loaded solid lipid nanoparticles for brain delivery. Int J Pharm. 2014;474:6–13.
- Müller RH, Radtke M, Wissing SA. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev. 2002;54 Suppl 1:S131–S155.
- Sun J, Bi C, Chan HM, et al. Curcumin-loaded solid lipid nanoparticles have prolonged in vitro antitumour activity, cellular uptake and improved in vivo bioavailability. Colloids Surf B Biointerfaces. 2013;111:367–375.
- zur Mühlen A, Schwarz C, Mehnert W. Solid lipid nanoparticles (SLN) for controlled drug delivery–drug release and release mechanism. Eur J Pharm Biopharm. 1998;45:149–155.
- Gokhale R, Schmidt C, Alcorn L, et al. Transdermal drug delivery systems of albuterol: in vitro and in vivo studies. J Pharm Sci. 1992;81:996–999.
- Gönüllü Ü, Üner M, Yener G, et al. Formulation and characterization of solid lipid nanoparticles, nanostructured lipid carriers and nanoemulsion of lornoxicam for transdermal delivery. Acta Pharm. 2015;65:1–13.