Abstract
In this study, we examined the efficacy of liposomal oleic acid-based antibiotic formulations on 32 strains of multidrug-resistant Pseudomonas aeruginosa (MDRPa). The average size of liposomes were 93.12 ± 2.3 nm holding a negative zeta potential at −57.3 ± 0.89. Liposomal antibiotic formulations were tested against 32 MDRPa strains isolated from burn wounds and urine samples, which exhibited an MIC of ≤8 μg/mL, whereas MIC of free antibiotics ranged from 32 to >1024 μg/mL. The results clearly indicate that the liposomes composed of naturally occurring oleic acid, could be used therapeutically either alone or in combination with antibiotics to effectively treat P. aeruginosa infections.
Introduction
Immunocompromised patients and critically ill patients are prone to acquiring infections caused by a wide range of pathogens, Pseudomonas aeruginosa being a major etiologic agent. The increasing resistance of P. aeruginosa to a multitude of currently available antibiotics requires urgent action. The first line of antibiotics, used in the treatment of P. aeruginosa infections are imipenem, piperacillin–tazobactam, ceftazidime, gentamycin and cefepime. The alternative drugs include ciprofloxacin and tobramycin [Citation1]. The resistance of P. aeruginosa to these drugs is a crucial challenge to physicians [Citation2]. The virulence factors of this pathogen may counteract host defenses and can cause damage to host tissues or increase its competitiveness. Extensive research is focused on liposomal-mediated antibiotic therapy to enhance their antimicrobial activity and reduce toxic adverse effects [Citation3]. Liposomes are well-studied as drug carrier molecules for delivering antimicrobial agents because they usually provide a sustained drug release effect, minimize drug toxicity, and increase overall drug efficacy [Citation4]. Antimicrobial treatments containing free fatty acid (FFA) components existing naturally in human skin, breast milk and blood stream in combination with antibiotics, will further improve the bactericidal action and reduce the opportunity for developing resistance [Citation5]. Long-chain unsaturated fatty acids such as oleic acid and linoleic acid show potent antimicrobial activity against numerous pathogenic microorganisms, including methicillin-resistant Staphylococcus aureus [Citation6] than long-chain saturated fatty acids like palmitic acid and stearic acid. Liposomal vesicles holding favourable properties of high drug loading capacity, biodegradability and size with a natural antimicrobial molecule, oleic acid on its lipidic outer layer may fuse rapidly with P. aeruginosa membrane. The currently prescribed antibiotics, together with natural antimicrobial FFAs in a liposomal formulation would produce a synergistic effect by eradicating P. aeruginosa infections. To expand the existing knowledge of liposomal antibiotics, the present work was conducted to design an oleic acid-based heterolipid vehicle with a variety of antibiotics in order to manage infections caused by multidrug-resistant (MDR) P. aeruginosa.
Materials and methods
Selection of resistant strains
Patients with burn wounds and urinary tract infections seeking treatment at Sathyabama University Dental College and Hospitals and Sri Ramachandra Medical College and Hospitals between July 2013 and July 2014 were included in the study. Sample processing, isolation and identification of P. aeruginosa were done using standard bacteriological procedures [Citation7]. Antibiotic susceptibility test was done by the Kirby–Bauer disc diffusion method and results interpreted according to Clinical and Laboratory Standards Institute (CLSI) (2015) [Citation8]. The following antibiotics were tested: ampicillin–sulbactam (10/10 μg/mL), piperacillin–tazobactam (100/10 μg/mL), ceftazidime (30 μg/mL), cefepime (30 μg/mL), ceftriaxone (30 μg/mL), cephalexin (30 μg/mL), imipenem (10 μg/mL), amikacin (30 μg/mL), gentamicin (10 μg/mL), tobramycin (10 μg/mL), nitrofurantoin (200 μg/mL) and ciprofloxacin (5 μg/mL). Pseudomonas aeruginosa ATCC 27853 was used as a quality control strain. Those isolates, which showed resistance to two or more antibiotics were considered MDR [Citation9]. MDRPa strains obtained by the disc diffusion method was confirmed by minimum inhibitory concentration (MIC) through broth dilution method. Antibiotics were serially diluted to obtain concentration ranging from 1 to 1024 μg/mL in Mueller Hinton broth. Culture suspension was adjusted to 0.5 McFarland standards and 20 μL was added to each tube. These tubes were then incubated at 37 °C for 16–20 h. MIC was considered as the highest dilution of antibiotic that inhibited visible growth.
Preparation and characterization of LipoOA and LipoOA antibiotics
Thin-layer evaporation method was adapted for preparing liposomal formulations [Citation10]. LipoOA was prepared using l-α-phosphatidyl choline (Egg PC), cholesterol with and without oleic acid in the ratio 7.5:1.5:6.7 and 13.5:1.5:0 (mol:mol). The lipid mixture was briefly mixed with 5 mL of chloroform and dried. Solvent traces were removed by purging N2 gas for 15 min. The dried lipid film was individually rehydrated with 5 mL of a 10 mg/mL antibiotic solution in phosphate-buffered saline (PBS). The suspension was vortexed for 3 min and warmed in a water bath at room temperature. After incubation, multilamellar vesicles (MLV) were produced by sonicating (Sonics VCX 130) the lipid suspension for duration of 3 min. MLVs are then sonicated with Ti probe for 60 s at 20 W to produce large unilamellar vesicles (LUVs). A polycarbonate membrane (Millipore, NY, USA) with 0.1 μm pore size was used for extruding LUVs to ultimately form the small unilamellar vesicles (SUVs). The cycle was repeated 15 times. The lipid suspensions were protected from light and were analysed for particle size and zeta potential using the Malvern zetasizer ZS, Malvern Instruments, UK, without any form of dilution of the sample. The encapsulation efficiency of oleic acid in LipoOA suspension was determined using an Agilent 1100 Thermo Finnigan LC-MS/MSn LCQ DECA Ion Trap, San Jose, CA. The surface morphology of bare liposome and LipoOA was analysed with a Zeiss Gemini Supra 55 SEM, Oberkochen, Germany. The copper disc was pasted with carbon tape and the liposomal suspension was dispersed over the tape. The disc was coated with gold in ionization chamber before microscopic analysis.
Determination of antibiotic concentration
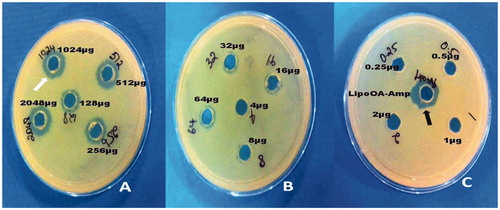
Suspension of P. aeruginosa strain (ATCC 27853) in PBS matching the turbidity of a 0.5 McFarland standard (1.5 × 108 CFU/mL) was used to obtain multiple lawn culture on Mueller–Hinton agar. Small wells were made in the agar using a vacuum hole puncher and 40 μL of serially diluted ampicillin (amp) was added to each well (0.25–2048 μg/mL) and lysed LipoOA-antibiotic (LipoOA-Ab) of unknown concentration was added in single well (1% Triton X-100 was used as lysing agent). The plates were incubated overnight at 37 °C and zone of inhibition around the known concentrations of antibiotics was used as a control to compare the zone size surrounding the LipoOA-Ab of unknown concentration.
Antibacterial activity of LipoOA and LipoOA antibiotics against MDRPa
The antibacterial activity of OA and LipoOA were tested against a fixed amount of culture (1 × 106 CFU in 100 μL) with various concentrations of free OA (2–1024 μg/mL) and lipo-OA (1.68–134.8 μg/mL) at 37 °C for 5 h in a 96-well plate. Later 3 μL of culture suspension from each concentration was spotted onto Mueller–Hinton Agar and incubated overnight at 37 °C. Highest dilution of OA and LipoOA that inhibited the growth was considered as MIC. Further the bactericidal activity of LipoOA-Ab formulations (12 antibiotics) was tested against 32 resistant strains by broth dilution method. Serial dilutions of LipoOA-Ab (1024, 512, 256, 128, 64, 32, 16, 8, 4, 2, 1, 0.5 and 0.25 μg/mL) in 2 mL Mueller–Hinton broth was prepared. Culture suspension was adjusted to 0.5 McFarland standards and 20 μl of culture was added to each dilution. The tubes were incubated at 37 °C for 24 h. Pseudomonas aeruginosa (ATCC 27853) was used as a quality control strain. MIC was considered as the highest dilution of antibiotics that inhibited visible growth.
Results
Selection of resistant strains
Seventy-five isolates were obtained from 125 patients (both pus and urine specimens) and were screened for antimicrobial resistance levels with different antibiotics. Hundred percent resistance was obtained for ampicillin, cephalexin and ceftazidime, while imipenem showed the highest susceptibility of 93.2%. Among the 75 strains, 32 were considered MDR which were further confirmed via the MIC study ().
Table 1. Comparison of resistant strains of P.aeruginosa minimal inhibition concentration (MICs) between free and liposomal antibiotics isolated from pus and urine.
Characteristics of liposomes
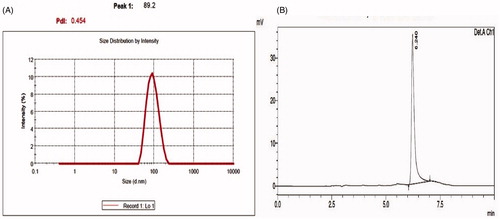
The size of liposomes made from egg PC and cholesterol were 83.4 ± 11.3 nm. The average size of LipoOA-Ab generated in this study was 89.21 ± 2.3 nm with an encapsulation efficiency of 17.3% (). These formulations were heterogeneous with a polydispersity index (PDI) of ∼0.45 ± 0.1. PDI is an estimate of the width of distribution. The surface zeta potential of LipoOA formulated was −57.3 mV in deionized water. In contrast, the zeta potential of the corresponding bare liposomes without OA was −8.2 mV.
Figure 1. Characterization of antibiotics oleic acid-loaded liposomes (LipoOA-Ab) and encapsulation efficiency of oleic acid in LipoOA. (A) Average hydrodynamic size (diameter, nm) of LipoOA antibiotics measured by dynamic light scattering (DLS). (B) Mass spectrum of LipoOA.
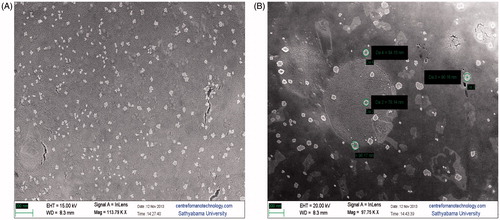
The morphological characteristics of the vesicle preparations were investigated using scanning electron microscope. Micrographs () revealed stable spherical vesicles of sizes between 78 and 96 nm compared with significantly smaller particle size measurements (<50 nm) for bare liposomes, which may be a reflection of the overall population.
Antibacterial activity of LipoOA and LipoOA-Ab against MDRPa
Known concentrations of ampicillin (Amp) were tested from 2048 to 0.25 μg/mL. The unknown concentration of LipoOA-Amp was tested to compare with standard concentrations of Amp. The results showed the zone of LipoOA-Amp was equal to 1024 μg/mL of standard Amp (), hence it was concluded that liposome contained 1024 μg of ampicillin per milliliter. Similar experimental conditions were maintained for all antibiotic formulations and the concentration was determined to be 1000 ± 20 μg/mL. The MIC value for LipoOA against the highly resistant strain PS88 was 67.4 μg/mL, which is significantly lower than free oleic acid (OA) of 1024 μg/mL. Further, the MICs of antibiotics encapsulated in LipoOA against all resistant strains were several folds lower than those of free antibiotics, as shown in . For instance, the MIC of free AMP and CN for resistant strains were >1024 μg/mL, whereas for LipoOA-Amp, LipoOA-CN, it was reduced to 8–16 μg/mL. The MIC for free CAZ is 32–512 μg/mL, whereas for LipoOA-CAZ, the MIC was 2–8 μg/mL. Similar results of reduction in MIC values were obtained for other antibiotics when encapsulated with LipoOA in comparison with free antibiotics.
Discussion
The emergence of P. aeruginosa infection, its increasing antibiotic resistance, and the associated diagnostic challenges leads to an increasing use of a concoction of higher spectrum antibiotics and is associated with increased mortality and morbidity in patients with P. aeruginosa infections [Citation11]. In this study, six different classes of antibiotics, aminoglycosides (tobramycin, amikacin, gentamycin), penicillin (piperacillin/tazobactum, ampicillin), carbapenems (imipenem), nitrofurans (nitrofurantoin), cephalosporins (ceftazidime, cephalexin, cefepime, ceftriaxone) and fluoroquinolones (ciprofloxacin) were ineffective against the clinical strains collected from burn wound site and urine. Encapsulation of antimicrobial molecules in lipid vesicular formulations will enhance the activity of drugs against extracellular pathogens, in particular to overcome bacterial drug resistance [Citation12]. It has been proven that the use of liposomes containing oleic acid not only increases the cytoplasmic delivery of liposome-entrapped compounds [Citation13,Citation14], but also has bactericidal properties of its own [Citation15]. In our study, the average size of liposomes bearing oleic acid and antibiotics were 93.12 ± 2.3 nm with a negative zeta potential (−57.3 mV ±0.89) exhibiting a narrow size distribution (PDI: 0.23 ± 0.02). The enhanced stability of OA in liposomes is due to the unique physiochemical properties of liposomes and the amphiphilic nature of oleic acid [Citation16]. It was observed from the liquid chromatography–mass spectrometry (LC-MS) chromatogram that 34.6 μg/mL (17.3%) of OA can be encapsulated with an initial input of 200 μg/mL. These entrapment rates were sufficient and were limited for loading antibiotics, as higher concentrations of OA may lead to respiratory distress if formulated to test during clinical studies [Citation17]. Initially, we encapsulated oleic acid into liposomes in an attempt to test the antimicrobial activity of oleic acid and liposomal oleic acid against a highly resistant strain PS88. Several fold decrease in resistance was observed in LipoOA than pure OA, this may be due to the fusion of liposomes with the bacterial outer membrane thereby releasing the OA. These findings suggest that P. aeruginosa membrane interactions were not based on surface adsorption or aggregation, but due to fusion. However, the ability of some virulent strains of P. aeruginosa to have thicker cell walls, makes it impermeable to oleic acid, thereby decreasing oleic acid’s antibacterial effects at the cell membrane [Citation18]. Further efforts were undertaken to encapsulate the antibiotics into LipoOA vesicles. A series of 32 bacterial strains were selected based on their multidrug resistance to six classes of antibiotics. These resistant strains when subjected to liposomal antibiotics exhibited the highest rates of susceptibility against ß-lactams (cefepime, piperacillin–tazobactam, ceftazidime, ampicillin), carbapenems (imipenem), aminoglycosides (gentamicin, tobramycin, amikacin), nitrofurans (nitrofurantoin), cephalosporins (cephalexin, ceftriaxone) and fluoroquinolones (ciprofloxacin). In conclusion, our findings suggest that LipoOA formulation could be utilized to encapsulate antibiotics, which can potentially be tapped to develop novel methods to tackle the emerging challenge of multidrug resistance in P. aeruginosa.
Acknowledgements
The authors wish to thank Sri Ramachandra University, Porur, Chennai, and Sathyabama University for providing the opportunity to carry out this research work.
This study was approved by the Institutional Ethics Committee, Sri Ramachandra University, Porur, Chennai, India. Reference number: IEC-NI/13/FEB/32/18.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- McPhee SJ, Papadakis MA, Tierney LM. Current medical diagnosis and treatment. New York: McGraw-Hill Medical; 2010.
- Roberts JA, Abdul-Aziz MH, Lipman J, et al. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis. 2014;14:498–509.
- Zhu X, Radovic-Moreno AF, Wu J, et al. Nanomedicine in the management of microbial infection - overview and perspectives. Nano Today. 2014;9:478–498.
- Garg T. Current nanotechnological approaches for an effective delivery of bio-active drug molecules in the treatment of acne. Artif Cells Nanomed Biotechnol. 2016;44:98–105.
- Obonyo M, Zhang L, Thamphiwatana S, et al. Antibacterial activities of liposomal linolenic acids against antibiotic-resistant Helicobacter pylori. Mol Pharmaceutics. 2012;9:2677–2685.
- Farrington M, Brenwald N, Haines D, et al. Resistance to desiccation and skin fatty acids in outbreak strains of methicillin-resistant Staphylococcus aureus. J Med Microbiol. 1992;32:56–60.
- Tille P. Bailey & Scott’s diagnostic microbiology. London: Elsevier Health Sciences; 2013.
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. Twenty-Fifth Informational Supplement. Wayne (PA): CLSI; 2015. Document M100-S25.
- Bessa LJ, Fazii P, Di Giulio M, et al. Bacterial isolates from infected wounds and their antibiotic susceptibility pattern: some remarks about wound infection. Int Wound J. 2015;12:47–52.
- Dua JS, Rana AC, Bhandari AK. Liposome: methods of preparation and applications. Int J Pharm Stud Res. 2012;3:14–20.
- Hirsch EB, Tam VH. Impact of multidrug-resistant Pseudomonas aeruginosa infection on patient outcomes. Expert Rev Pharmacoecon Outcomes Res. 2010;10:441–451.
- Abed N, Couvreur P. Nanocarriers for antibiotics: a promising solution to treat intracellular bacterial infections. Int J Antimicrob Agents. 2014;43:485–496.
- Collins D, Norley ST, Rouse B, et al. Liposomes as carriers for antitumor and antiviral drugs: pH- sensitive immunoliposomes and sustained release immunoliposomes. In: Ciregoriadis G, editor. Liposomes as drug carriers. New York: Wiley; 1988. p. 761–770.
- Daraee H, Etamadi A, Kouhi M, et al. Application of liposomes in medicine and drug delivery. Artif Cells Nanomed Biotechnol. 2016;44:381–391.
- Huang CM, Chen CH, Pornpattananangkul D, et al. Eradication of drug resistant Staphylococcus aureus by liposomal oleic acids. Biomaterials. 2011;32:214–221.
- Srisuk P, Thongnopnua P, Raktanonchai U, et al. Physico-chemical characteristics of methotrexate-entrapped oleic acid-containing deformable liposomes for in vitro transepidermal delivery targeting psoriasis treatment. Int J Pharm. 2012;427:426–434.
- Broccard AF, Shapiro RS, Schmitz LL, et al. Influence of prone position on the extent and distribution of lung injury in a high tidal volume oleic acid model of acute respiratory distress syndrome. Crit Care Med. 1997;25:16–27.
- Kenny JG, Ward D, Josefsson E, et al. The Staphylococcus aureus response to unsaturated long chain free fatty acids: survival mechanisms and virulence implications. PLoS One. 2009;4:e4344.