Abstract
RNA interference (RNAi)-based therapeutic approaches are under vibrant scrutinisation to seek cancer cure. siRNA suppress expression of the carcinogenic genes by targeting the mRNA expression. However, in vivo systemic siRNA therapy is hampered by the barriers such as poor cellular uptake, instability under physiological conditions, off-target effects and possible immunogenicity. To overcome these challenges, systemic siRNA therapy warrants the development of clinically suitable, safe, and effective drug delivery systems. Herein, we review the barriers, potential siRNA drug delivery systems, and application of siRNA in clinical trials for cancer therapy. Further research is required to harness the full potential of siRNA as a cancer therapeutic.
Introduction
Cancer is a group of diseases that sequences from more than one genetic changes and involving abnormal cell boom with the capability to invade or spread to other parts of the body [Citation1]. Cancer is one of the major targets of RNAi-primarily based therapy, as oncogenes, mutated tumour suppressor genes and numerous different genes contributing to tumour development are potentially important for gene silencing by RNAi. The delivery of nucleic acid therapeutics (e.g. DNA, siRNA, shRNA, and antisense oligonucleotides) to down-regulate mutated genes, and to silence unwanted gene expression; is turning into an exceptionally appealing method to suppressing tumour cell growth and invasion. Optimal combinations of potent anticancer siRNAs and effective delivery systems can also pave the way for the successful clinical application of siRNAs.
The history of RNAi
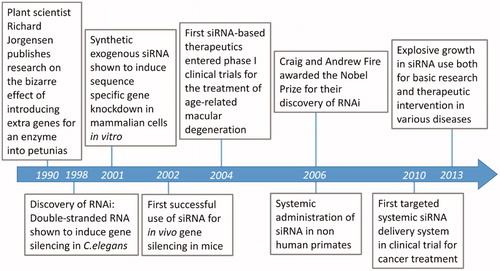
In the 1990s, a surprising observation on gene-silencing phenomenon was made in Petunias as shown in . Plant scientist Richard Jorgensen and colleagues published research on the bizarre effect of introducing extra genes for an enzyme into Petunias [Citation2]. Fire and Mello, were first to report that the double-stranded RNAs (dsRNAs) can trigger gene silencing of complementary messenger RNA sequences in the nematode worm Caenorhabditis elegans [Citation3] and the term “RNA interference” (RNAi) was coined. Elbashir et al. [Citation4] reported that synthetic exogenous siRNA induce sequence specific gene knock down in mammalian cells in vitro [Citation4]. The first observation of sequence specific gene silencing in mice using siRNA was achieved for hepatitis C virus [Citation5]. In 2004, only six years after the discovery of RNAi, the first siRNA-based human therapeutic was developed as treatment for wet age-related macular degeneration, and entered phase I clinical trials [Citation6]. In 2006, Fire and Mello were awarded the Nobel Prize for their discovery of RNAi. Davis et al. [Citation7] reported the first targeted siRNA delivery of nanoparticles in humans via systemic injection. The field of RNAi is moving forward at a remarkable pace as shown in . Currently the explosive growth in siRNA is used both for basic research and therapeutic intervention in various diseases including cancer.
Figure 1. Example of petunia plants wherein genes for pigmentation are silenced by RNAi [Citation82].
![Figure 1. Example of petunia plants wherein genes for pigmentation are silenced by RNAi [Citation82].](/cms/asset/c51ba90c-5984-47c6-9537-5d98f00731e8/ianb_a_1307210_f0001_c.jpg)
Mechanism of RNA interference
Therapeutic gene silencing by siRNA
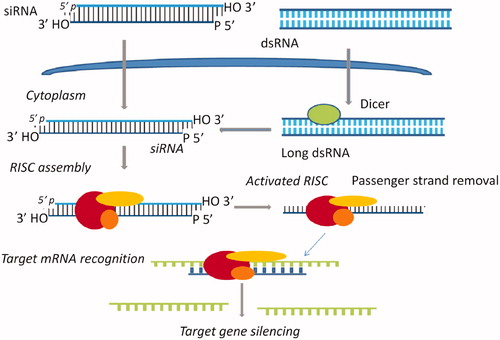
RNAi is a fundamental pathway found in many eukaryotes, including animals. As demonstrated in , RNAi is triggered by the presence of long double-stranded RNA, which is cleaved into the fragments known as siRNA (21–23 nucleotides long) by the endonuclease dicer. siRNA are loaded onto RNA-induced silencing complex (RISC). RISC contains Argonaute protein (Ago-2) capable of cleaving and removing the passenger strand of the siRNA duplex. The single stranded guide RNA, in association with protein of the RISC complex directs the specificity of the target mRNA recognition through complementary base pairing [Citation8,Citation9]. Argonaute-2 degrades the mRNA complementary to the antisense strand [Citation10], and endonucleolytic cleavage occurs between bases 10 and 11 relative to the 5′ end of the antisense siRNA strand [Citation11–14], thereby causing gene silencing and mRNA degradation.
Figure 3. The mechanism of RNA interference. Long dsRNA is introduced into the cytoplasm, where it is cleaved into siRNA by Dicer. Alternatively, siRNA can be introduced directly into the cell. The siRNA incorporated into RISC assembly, and the sense (passenger) strand is degraded by the protein Argo-2 in the RISC. The remaining antisense strand serves as a guide to recognise the corresponding mRNA. The activated RISC–siRNA complex bind to and degrades the target mRNA, which leads to the silencing of the target gene.
Barriers to siRNA delivery
siRNA hold promise as therapeutic gene silencing, however several barriers still exist in order to achieve effective and controlled in vivo delivery and limit the use of RNAi in the clinic [Citation15]. siRNA formulations for systemic application face a chain of hurdles in vivo before reaching the cytoplasm of the target cell. Post-injection, the siRNA complex must navigate the circulatory system of the body while avoiding kidney filtration, uptake by phagocytes, aggregation with serum proteins and enzymatic degradation by endogenous nucleases [Citation16]. One of the first biological barriers encountered with the aid of administered siRNA is the nuclease activity in plasma and tissues. The major nuclease in plasma is a 3′exonuclease however cleavage of internucleotide bonds can also take place. The reported half-life for unmodified siRNA in serum ranges from several minutes to 1 h [Citation17]. In addition, the kidney plays a key role in siRNA clearance and several studies in animals claimed that the biodistribution of siRNA shows the highest uptake in the kidney. In addition to circulating nuclease degradation and renal clearance, a major barrier to in vivo delivery of siRNA is uptake by the reticuloendothelial system (RES). The RES is composed of phagocytic cells, including circulating monocytes and tissue macrophages, the physiological function of which is to clear foreign pathogens and to remove cellular debris and apoptotic cells [Citation18]. Tissue macrophages are most abundant in the liver and the spleen; tissues that also receive high blood flow and exhibit a fenestrated vasculature. Thus, it is not surprising that these organs accumulate high concentrations of siRNA following systemic administration [Citation19].
Free siRNA, which is a type of anionic and hydrophilic double-stranded small RNA, is not readily taken up by cell. Moreover, the hydrophilicity and negative charge of siRNA molecules prevents them from readily crossing biological membranes. This suggests that siRNA needs to be packaged in vesicles in order to enter cells [Citation20].
The most important challenge that needs to be overcome is the potential for “off-target” effects. A gene that shares a sturdy homology to the target gene has the potential to be inadvertently knocked down, leading to possibly severe unintended side effects. siRNA shows miRNA-like off-target silencing of a large number of unintended transcripts with partial identity to its sequence [Citation21,Citation22]. This mechanism has unpredictable cellular consequences, with important toxic phenotypic effects. Another challenge to siRNA therapy is “immune stimulation”, which is recognition of a siRNA duplex by the innate immune system. Introduction of too much siRNA is known to result in the activation of innate immune responses. The immune system is probably activated via the dsRNA sensor; protein kinase R, inflammatory cytokines and interferon were found to be induced by activation of NF-kB and interferon regulatory factors following the recognition of siRNA by toll-like receptor7 (TLR7), TLR8 and TLR9 [Citation23–25]. To overcome these difficulties, the development of safe and effective in vivo delivery system is essential ().
Table 1. Problems with naked siRNA for clinical applications.
Potential systemic siRNA drug delivery system for cancer therapy
The major problem facing siRNA-based therapeutics for cancer and different diseases is delivering siRNA to the target cell population in vivo. The efficacy of siRNA-based drugs in combating cancer requires potent and effective gene silencing in the tumour cells. To achieve efficient delivery of siRNA, an ideal systemic siRNA delivery system should have the following characteristics: (i) be biocompatible, biodegradable and non-immunogenic, (ii) must guard siRNA from serum nucleases during transit through the circulation and on release into endosomes, (iii) avoid rapid hepatic or renal clearances, and (iv) mediate siRNA delivery into target cells while sparing normal tissues. Thus, the design criteria of an in vivo, systemic siRNA delivery system should involve methods for increasing the serum half-life of the siRNA, its distribution to target tissue, its cellular uptake with subsequent intracytoplasmic release without degradation, and avoiding off-target gene silencing activity.
The currently developed siRNA delivery systems for cancer therapy mainly include: (i) chemical modifications of siRNA, (ii) lipid based siRNA delivery system, (iii) polymer based siRNA delivery system, (iv) conjugate siRNA delivery systems, (v) co-delivery of siRNA and anticancer drugs, and (vi) inorganic nanoparticles (quantum dots, carbon nanotubes, and gold nanoparticles as explained in ).
Figure 4. Various types of siRNA cancer therapeutics: (a) siRNA strand chemical modification, (b) Lipoplexes, (c) stable nucleic acid-lipid particles (SNALPs), (d) polymer-based delivery, (e) dendrimer-siRNA conjugate, (f) conjugate siRNA delivery, (g) aptamer-siRNA chimaeras, (h) quantum dot complexed with siRNA, (i) carbon nanotube-siRNA conjugate, (j) gold nanoparticles.
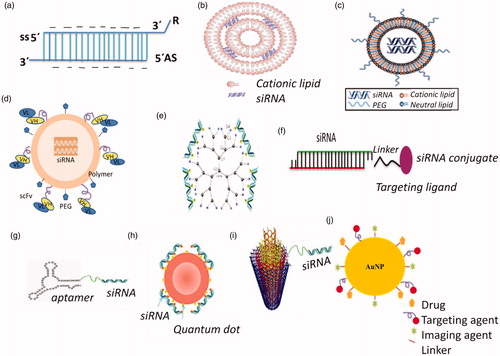
These modifications help to address the problems in naked siRNA related to (1) serum stability, (2) clearance of large molecular mass material, (3) high toxicity (cytotoxicity), (4) ligand–receptor interaction, (5) vascular permeability to reach cancer tissues, and (6) renal clearance. These benefits are difficult to obtain from a single modification. The siRNA delivery interact with various components, therefore, if these modifications are not designed well, they may also lead to problems. For example, nano-modification may leave high positive charge on the surface of nanoparticles causing unfavourable aggregation with erythrocytes. Another issue with using nanoparticles is the tendency of absorption of serum opsonin proteins, clearing it from the blood and hence barring it from reaching targets. On the other hand, if not well engineered, synthetic or polymer modification may cause issues related to large molecular mass and non-biodegradability leading to toxicity.
Chemical modifications of siRNA for cancer therapy
For anticancer siRNAs to exert their activity in cancer tissues, chemical modifications of naked siRNA have been used to generate nuclease-resistant siRNA to avoid degradation, enhance the stability of siRNA, and improve circulation time as well as tumour uptake in vivo. The performance of siRNA is considerably improved after chemical modification to siRNA strands; the sites include sugar, base, phosphorous acid, strand end or backbone of each sense and antisense strands (). A common modification is the replacement of the phosphodiester (PO4) group with phosphorothioate (PS) at the 3′end [Citation26]. The introduction of PS backbone linkages at the 3′ end of the RNA strands can inhibit enzymatic degradation by reducing siRNAs susceptibility to exonucleases. The introduction of an O-methyl group (2′-O-Me), a fluoro (2′-F) group or a 2-methoxyethyl (2′-O-MOE) group resulted in prolonged half-lives and RNAi activities in cultured cells and plasma [Citation27]. The modification of siRNA with 2,4-dinitrophenol (DNP) leads to improved nuclease resistance along with an increase in membrane permeability of the modified siRNA [Citation28]. The basic requirement of successful modifications is enhancing siRNA serum stability without negative effects on its gene silencing activity. Indeed boranophosphonate siRNA showed a better resistance to nuclease degradation in spite of the fact that it reduces RNAi activity [Citation29]. The degradation of modified siRNA into non-natural molecules results into reduced RNAi activity and production of toxic metabolites. As a result the delivery systems in which unmodified siRNA will be loaded as a cargo for targeted systemic delivery, have gained prominence.
Lipid-based siRNA delivery system for cancer therapy
Lipid-based systems for delivering anticancer siRNAs embody varied lipid nanoparticles, including liposomes, micelles, emulsions, and solid lipid nanoparticles [Citation20]. Cationic lipid components in the carriers are essential elements for interacting with negatively charged siRNAs, they also play a pivotal role in the delivery efficiencies of siRNAs [Citation30].
Liposomes/lipoplexes
Lipoplexes are one of the most attractive nonviral vectors for plasmid and siRNA delivery [Citation31]. The transfection mechanism of liposomes involves static interactions between negatively charged nucleic acids and cationic lipids as shown in . Once mixed along, they spontaneously form lipoplexes [Citation32,Citation33]. Cationic lipids (100–300 nm in size) can protect siRNA from enzymatic degradation and increase the circulating half-life and uptake by cells. Cationic lipids such as 1,2-dioleoyl-3-trimethylammonium propane (DOTAP) and N-{1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium methyl sulfate (DOTMA), along with helper lipids such as DOPE, are often used to form cationic liposomes and complex with negatively charged deoxyribonucleic acid and siRNA, resulting in high in vitro transfection efficiency [Citation34]. Commercially available formulations like lipofectamine 2000 are used for in vitro transfection [Citation35]. Cationic liposomes have limited success in vivo, they show dose-dependent toxicity and pulmonary inflammation can arise as a result of reactive oxygen intermediates [Citation36–38]. In a recent study, chemotherapeutics and MCL1-specific siRNA co-delivered using trilysine-derived cationic lipid-based liposomes was found to decrease the expression of MCL1 in the tumour tissues of keratin-forming human epidermal carcinoma (KB) cell-xenografted mice [Citation39]. In one study of anticancer siRNA was co-formulated with a diagnostic agent in cationic liposomes for theranostic purposes [Citation40].
Neutral nanoliposomes
The development of neutral 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine (DOPC) based nanoliposomes (∼mean size 65 nm), which encapsulate siRNA can deliver siRNA in vivo into tumour cells more efficiently than cationic liposomes and naked siRNA, respectively [Citation41]. DOPC-encapsulated siRNA liposomes, which target genes, e.g. EphA2 [Citation42], FAK [Citation43], neuropilin-2 [Citation44] demonstrated significantly inhibited tumour growth in an orthotropic model of ovarian carcinoma and colorectal cancer xenografts in mice.
Stable nucleic acid lipid particles (SNALP)
Stable nucleic acid lipid particles (SNALPs) are a major advance in lipid-based siRNA delivery. The first non-human primate study on siRNA delivery was carried out with SNALPs [Citation45]. SNALPs are microscopic particles approximately 120 nm in diameter, which have been used to deliver siRNAs therapeutically to mammals in vivo. In SNALPs, the siRNA is surrounded by a lipid bilayer containing a mixture of cationic and fusogenic lipids, coated with diffusible polyethylene glycol [Citation46] (). The siRNA-lipid complexes showed considerably enhanced cellular internalization and endosomal escape of siRNA. Morrissey et al. suggested that HBV replication was inhibited through the delivery of a siRNA-SNALP complex that targeted HBV RNA. Three daily intravenous injections of 3 mg/kg/day reduced serum HBV levels by at least one order of magnitude, and the effect was specific, dose-dependent and lasted for up to seven days after dosing [Citation47]. Zimmerman et al. demonstrated the ability of SNALPs to enable knockdown of ApoB in the liver of cynomolgus monkeys [Citation48]. Alnylam Pharmaceuticals (Cambridge, MA, USA) has developed the first dual-targeted siRNA drug, SNALP-FORMULATED siRNAs targeting vascular endothelial growth factor (VEGF) and KSP in ALN-VSP02. Tekmira (Barnaby, Canada) started in 2015 a Phase II clinical trial to evaluate the efficacy of siRNA containing SNALP for the treatment of Ebola virus (TKM-100201) [Citation49].
Other lipid-like delivery systems are lipidoid nanoparticles, which are comprised of cholesterol and PEG-modified lipids specific for siRNA delivery [Citation50]. To improve SNALP-mediated delivery, Akinc et al. developed a new chemical method to allow rapid synthesis of a large library of lipidoids and tested their efficacy in siRNA delivery [Citation51]. The 98N12–5 lipidoid-based siRNA formulation, showed 75–90% reduction in ApoB or FVII factor expression in hepatocytes in nonhuman primates and mice [Citation51].
Polymer-based siRNA delivery system for cancer therapy
Cationic polymer-based delivery systems have been investigated for the non-viral delivery of siRNAs. In polymer-based delivery, the siRNA is condensed within different kinds of cationic polymers such as chitosan, cyclodextrin, PEI that form nanoparticles, and the surface of the nanoparticles is decorated with PEG and targeting moieties as shown in . Examples of cationic polymers used for the delivery of anticancer siRNA are enlisted in .
Table 2. Examples of siRNA delivery systems for cancer therapeutics.
Chitosan
Chitosan is one of the most widely investigated nonviral, naturally derived polymeric gene delivery vectors. It is a cationic polysaccharide composed of β-(1–4)-linked d-glucosamine and N-acetyl-d-glucosamine units. Because of its cationic property, chitosan and its derivatives have been extensively studied for the delivery of plasmid DNA and siRNA in vitro and in vivo [Citation59]. Chitosan-coated polyisohexylcyanoacrylate nanoparticles have been studied for the delivery of RhoA-specific siRNA, efficiently inhibited the growth of aggressive xenografted breast cancer in mice [Citation52]. Self-assembling α-tocopherol oligochitosan nanoparticles have been formulated for the delivery of siRNA against MCL1 [Citation53].
Cyclodextrins
Cyclodextrins are natural polymers, which can form water soluble inclusion complexes with small and large molecules [Citation60]. The cyclodextrin-containing polycation system for the targeted delivery of siRNA was developed [Citation61] . This system consisted of a cyclodextrin – containing polymer, PEG for stability, and human transferrin as the targeting ligand for binding to transferrin receptors, which are often overexpressed on cancer cells. This targeted nanoparticle system, called CALLA-01, was developed for the first siRNA Phase I trial by Calando Pharmaceuticals (Pasadena, CA, USA) [Citation7]. The siRNA in CALLA-01 is designed to inhibit tumour growth via a mechanism to reduce expression of the M2 subunit of ribonucleotide reductase (R2).
Polyethylenimine
Polyethylenimine (PEI), a commonly used synthetic cationic polymer for the delivery of anticancer siRNAs [Citation30]. PEI is available as branched or linear form in various molecular weights. Because of its high cationic charge density, PEI forms small and compact nanoparticles with nucleic acids, and provides silencing of target gene expression after siRNA delivery in vitro and in vivo. Polyplexes of PEI and HER-2 receptor-specific siRNA were shown to produce gene silencing and exert anti-tumour effects in mice [Citation54]. A lipid-linked PEI was reported to improve the siRNA delivery function of PEI [Citation55].
Dendrimers
Dendrimers are synthetic, highly branched macromolecules with three-dimensional nanometric structure (). The unique structural properties such as tunable size, accessible terminal functional groups and cargo encapsulation in a nanometer size add to their potential as drug carriers [Citation62]. Cationic dendrimers have proven useful in masking the charge of siRNA long enough for in vivo delivery. Polycationic dendrimers have found applications as non-viral siRNA delivery vectors. In this approach polycationic dendrimers, conjugated with targeted lipid moieties, complex with 2-modified siRNA. Polycationic dendrimers such as poly(amidoamine) (PAMAM) and poly(propylenimine) (PPI) dendrimers have been studied for siRNA delivery in recent years. PAMAM dendrimers have become the most used dendrimer-based carriers for gene delivery. However, PAMAMs were demonstrated to be cytotoxic, predominately related to apoptosis mediated by mitochondrial dysfunction [Citation63]. Surface-modified and cationic PAMAM dendrimers show very low cytotoxicity, even at high concentrations and efficiently penetrated cancer cells in vitro [Citation64]. Finlay et al. [Citation56] targeted the in vivo breast cancer cell metastasis by downregulating TWIST1, a transcription factor activates the EMT, using third generation amphiphilic PAMAM dendrimer YTZ3–15 complexed with TWIST1 siRNA. PPI dendrimers were also used to formulate siRNA nanoparticles, and these nanoparticles showed efficient gene silencing [Citation65].
Conjugate siRNA delivery system for cancer therapy
A common approach for targeted delivery of siRNA to specific cells or tissues is conjugation to deliver materials such as functional peptides, antibodies, aptamers, oleophilic molecules or PEG to improve stability, prolong circulation time, and facilitate cellular uptake of siRNAs on in vivo administration as shown in [Citation66].
Lipophile-siRNA conjugates, which were the first conjugate delivery systems to indicate efficacy in vivo, consist of siRNA conjugated to cholesterol [Citation45]. Cholesterol was conjugated to the 3′-terminus of the sense strand of siRNA via a pyrrolidone linkage. To further optimise cholesterol-siRNA, high density lipoprotein was bound, which increased gene silencing efficacy by 8–15 fold in vivo [Citation67]. For enhanced intracellular delivery, siRNA may be conjugated with cell-penetrating peptides or protein transduction domains [Citation68]. Penetratin, transportan and trans-activator of transcription (TAT) protein enable the cellular uptake of hypophilic macromolecules like peptides and nucleic acids [Citation69,Citation70]. However, conjugation of cationic peptides to anionic siRNA may neutralise and reduce the penetrating efficacy of these peptides [Citation68]. In addition, CPP-siRNA conjugates may exhibit cytotoxicity caused by cell membrane perturbation or immunogenicity [Citation71]. Aptamers are oligonucleotide or peptide molecules that bind to specific target molecules. Aptamers are explored for targeted siRNA delivery as an alternative to antibodies because of their chemical versatility, stability, and low immunogenicity [Citation72]. For targeted delivery of siRNA, aptamer-siRNA chimeric () RNAs have been developed [Citation57] and employed to target prostate-specific membrane antigen (PSMA), which is over expressed in prostate cancer cells. The chimeric RNA was demonstrated to bind only PSMA-expressing cells, resulting in depletion of siRNA target proteins and cell death. According to a recent study, gene knockdown by EpCAMaptamer-siRNA chimeras suppresses epithelial breast cancers and their tumour -initiating cells [Citation58].
Co-delivery of siRNA and anticancer drugs
Various types of nanocarriers have been investigated for the co-administration of siRNA and antineoplastic drugs in an effort to enhance anticancer effects by overcoming multidrug resistance or inducing different caspase-mediated cell death pathways. Judiciously designed multifunctional drug/siRNA co-delivery nanocarriers will considerably increase their in vivo tumour accumulation via both passive and active tumour-targeting abilities. Thus, multifunctional nanomedicines offer great promise in overcoming the drawbacks of current treatment modalities, including chemotherapy [Citation73].
Inorganic nanoparticles
A number of inorganic nanoparticles have been emerging as potential siRNA delivery systems devised for therapeutic purposes. They include quantum dots (QDs), carbon nanotubes (CNTs), and gold nanoparticles.
Quantum dots
Quantum dots are semiconducting inorganic crystals with superior photo stability and tunable optical properties for an extensive selection of nonoverlapping colours () [Citation74]. They have bioactivity, and in certain cases, depending on the bulk material used, have no toxicity but a low payload capacity. Quantum dots are a powerful tool in cancer targeting, imaging living animals and investigation of pathophysiology in tumour tissue [Citation75].
Carbon nanotubes
Carbon nanotubes have recently emerged as a replacement choice for cancer treatment, as a carrier for siRNA delivery (). CNTs with their nanoneedle structure have been able to independently translocate into cytoplasm without inducing necrobiosis [Citation76,Citation77]. Carbon nanotubes can be divided into single-walled and multiwalled categories. Single walled CNTs functionalised with –CONH–(CH2)6–NH3 + Cl− act as siRNA carriers; siRNA is free from the nanotube side-wall to silence the expression of enzyme polymerase. This action inhibits the synthesis of enzyme and prevents cancer cells from getting replicative immortality thus suppressing tumour growth [Citation78,Citation79]. A number of excellent articles have been published that highlight the use of carbon nanotubes for delivery of small molecule drugs and nucleic acids [Citation80,Citation81].
Gold nanoparticles
Gold nanoparticles (Au NPs) (usually 10–20 nm) () are used as a promising siRNA delivery carrier due to their excellent biocompatibility, ease of synthesis, high surface-to-volume ratio, and facile surface functionalization [Citation82]. For the delivery of nucleic acids, gold nanoparticles functionalised with positively charged quaternary ammonium or branched PEI [Citation83], or coated with a cationic lipid bilayer have been reported [Citation83]. The attachment of oligonucleotides to the surface of gold nanoparticles has also been reported. Ding et al. [Citation84] compiled a review on gold nanoparticles for nucleic acid delivery.
Conclusion and future perspectives
Post-transcriptional gene silencing with the aid of RNAi represents a promising new gene therapy approach. An abnormally high number of oncogenes are one of characteristic of cancer. siRNA has a potential to target any cancer-related genes and represents a new class of cancer therapeutics. The engineering of siRNA carriers has gained a lot of interest in recent years, as a result of development of effective delivery systems that can carry siRNA into tumours tissues and the cytoplasm of tumour cells. Several clinical trials of siRNA-based anticancer therapies are summarised in . The momentous progress in siRNA-based formulation development shall continue to enlighten/apprehend its possible therapeutic application. The ideal nanocarrier system should protect the RNAi therapeutic agent from the circulatory environment and efficiently deliver the therapeutic agent to tumour cells. The future research has to focus on achieving well-organised delivery of siRNA to the desired cells (with no off-target effects), increasing resistance to nuclease, and avoiding immune responses. Great potential exists for nano-siRNA drugs in cancer treatment; further interdisciplinary research is needed in this developing and promising field of nano-siRNA drugs. The siRNA based drug delivery systems hold promise provided their safety and efficacy are adjudged as “satisfactory” by the regulatory agencies.
Table 3. siRNA Cancer therapeutics in clinical trials.
Disclosure statement
The authors report no conflict of interest.
References
- Stratton MR, Campbell PJ, Futreal PA. The Cancer genome. Nature. 2009;458:719–724.
- Jorgensen RA, Cluster PD, English J, et al. Chalcone synthase co suppression phenotypes in petunia flowers: comparison of sense vs. antisense constructs and single-copy vs. complex T-DNA sequences. Plant Mol Biol. 1996;31:957–973.
- Fire A, Xu S, Montgomery MK, et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811.
- Elbashir SM, Harborth J, Lendeckel W, et al. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498.
- McCaffrey AP, Meuse L, Pham TT, et al. Gene expression: RNA interference in adult mice. Nature. 2002;418:38–39.
- Whelan J. First clinical data on RNAi. Drug Discov Today. 2005;10:1014–1015.
- Davis ME, Zuckerman JE, Choi CH, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067–1070.
- Matranga C, Tomari Y, Shin C, et al. Passenger-strand cleavage facilitates assembly of siRNA into Ago2-contaning RNAi enzyme complexes. Cell. 2005;123:607–620.
- Tolia NH, Joshua-Tor L. Slicer and the Argonautes. Nat Chem Biol. 2007;3:36–43.
- Ameres SL, Martinez J, Schroeder R. Molecular basis for target RNA recognition and cleavage by human RISC. Cell. 2007;130:101–112.
- Rand TA, Petersen S, Du F, et al. Argonaute2 cleaves the anti-guide strand of siRNA during RISC activation. Cell. 2005;123:621–629.
- Dykxhoorn DM, Lieberman J. Knocking down disease with siRNAs. Cell. 2006;126:231–235.
- Novina CD, Sharp PA. The RNAi revolution. Nature. 2004;430:161–164.
- Lingel A, Sattler M. Novel modes of protein-RNA recognition in the RNAi pathway. Curr Opin Struct Biol. 2005;15:107–115.
- Peocot CV, Calin GA, Coleman RL, et al. RNA interference in the clinic: challenges and future directions. Nat Rev Cancer. 2011;11:59–67.
- Alexis F, Pridgen E, Molnar LK, et al. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm. 2008;5:505–515.
- Layzer JM, McCaffrey AP, Tanner AK, et al. In vivo activity of nuclease-resistant siRNAs. RNA. 2004;10:766–771.
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969.
- Jackson AL, Bartz SR, Schelter J, et al. Expression profiling reveals off-target gene regulation by RNAi. Nat Biotechnol. 2003;21:635–637.
- Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8:129–138.
- Jackson AL, Burchard J, Schelter J, et al. Widespread siRNA ‘off-target’ transcript silencing mediated by seed region sequence complementarily. RNA. 2006;12:1179–1187.
- Hornung V, Guenthner-Biller M, Bourquin C, et al. Sequence-specific potent induction of IFN-alpha by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat Med. 2005;11:263–270.
- Marques JT, Williams BR. Activation of the mammalian immune system by siRNAs. Nat Biotechnol. 2005;23:1399–1405.
- Kariko K, Bhuyan P, Capodici J, et al. Small interfering RNAs mediate sequence-independent gene suppression and induce immune activation by signaling through toll-like receptor 3. J Immunol. 2004;172:6545–6549.
- Harborth J, Elbashir SM, Vandenburgh K, et al. Sequence, chemical, and structural variation of small interfering RNAs and short hairpin RNAs and the effect on mammalian gene silencing. Antisense Nucleic Acid Drug Dev. 2003;13:83–10.
- Czauderna F, Fechtner M, Dames S, et al. Structural variations and stabilizing modifications of synthetic siRNAs in mammalian cells. Nucleic Acids Res. 2003;31:2705–2716.
- Liao H, Wang JH. Biomembrane-permeable and ribonuclease-resistant siRNA with enhanced activity. Oligonucleotides . 2005;15:196–205.
- Hall AHS, Wan J, Shaughnessy EE, et al. RNA interference using boranophosphate siRNAs: structure-activity relationships. Nucleic Acids Res. 2004;32:5991–6000.
- Li L, Shen Y. Overcoming obstacles to develop effective and safe siRNA therapeutics. Expert Opin Biol Ther. 2009;9:609–619.
- Zhang S, Zhao B, Jiang H, et al. Cationic lipids and polymers mediated vectors for delivery of siRNA. J Control Release. 2007;123:1–10.
- Fenske DB, Cullis PR. Liposomal nanomedicines. Expert Opin Drug Deliv. 2008;5:25–144.
- Elouahabi A, Ruysshaert JM. Formulation and intracellular trafficking of lipoplexes and polyplexes. Mol Ther. 2005;11:336–347.
- Sarisozen C, Salzano G, Torchilin VP. Recent advances in siRNA delivery. Biomol Concepts. 2015;6:321–341.
- Kim HS, Song IH, Kim JC, et al. In vitro and in vivo gene transferring characteristics of novel cationic lipids, DMKD (O, O′-dimyristyl-N-lysyl aspartate) and DMKE (O,O′-dimyristyl-N-lysyl glutamate). J Control Release. 2006;115:234–241.
- Santel A, Aleku M, Keil O, et al. A novel siRNA-lipoplex technology for RNA interference in the mouse vascular endothelium. Gene Ther. 2006;1:1222–1234.
- Dokka S, Toledo D, Shi X, et al. Oxygen radical-mediated pulmonary toxicity induced by some cationic liposomes. Pharm Res. 2000;17:521–525.
- Spagnou S, Miller AD, Keller M. Lipidic carriers of siRNA: differences in the formulation, cellular uptake, and delivery with plasmid DNA. Biochemistry . 2004;43:13348–13356.
- Lv H, Zhang S, Wang B, et al. Toxicity of cationic lipids and cationic polymers in gene delivery. J Control Release. 2006;114:100–109.
- Shim G, Han SE, Yu YH, et al. Trilysinoyl oleylamide-based cationic liposomes for systemic co-delivery of siRNA and an anticancer drug. J Control Release. 2011;155:60–66.
- Kenny GD, Kamaly N, Kalber TL, et al. Novel multifunctional nanoparticle mediates siRNA tumour delivery, visualisation and therapeutic tumour reduction in vivo. J Control Release. 2011;149:111–116.
- Gewirtz AM. On future's doorstep: future’s doorstep: RNA interference and the pharmacopeia of tomorrow. J Clin Invest. 2007;117:3612–3614.
- Landen CN Jr, Chavez-Reyes A, Bucana C, et al. Therapeutics EphA2 gene targeting in vivo using neutral liposomal siRNA delivery. Cancer Res. 2005;65:6910–6918.
- Halder J, Kamat AA, Landen CN Jr, et al. Focal adhesion kinase targeting using in vivo short interfering RNA delivery in neutral liposomes for ovarian carcinoma therapy. Clin Cancer Res. 2006;12:4916–4924.
- Gray MJ, Van Buren G, Dallas NA, et al. Therapeutic targeting of neuropilin-2 on colorectal carcinoma cells implanted in the murine liver. J Nat Cancer Inst. 2008;100:109–120.
- Soutschek J, Akinc A, Bramlage B, et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432:173–178.
- Rossi JJ. RNAi therapeutics: SNALP siRNAs in vivo. Gene Ther. 2006;13:583–584.
- Morrissey DV, Lockridge JA, Shaw L, et al. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat Biotechnol. 2005;23:1002–1007.
- Zimmermann TS, Lee HC, Akinc A, et al. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441:111–114.
- Xu CF, Wang J. Delivery systems for siRNA drug development in cancer therapy. Asian J Pharm Sci. 2015;10:1–12.
- Shen H, Sun T, Ferrari M. Nanovector delivery of siRNA for cancer therapy. Cancer Gene Ther. 2012;19:367–373.
- Akinc A, Zumbuehl A, Goldberg M, et al. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat Biotechnol. 2008;26:561–569.
- Pille JY, Li H, Blot E, et al. Intravenous delivery of anti-RhoA small interfering RNA loaded in nanoparticles of chitosan in mice: safety and efficacy in xenografted aggressive breast cancer. Hum Gene Ther. 2006;17:1019–1026.
- Noh SM, Han SE, Shim G, et al. Tocopheryloligochitosan-based self-assembling oligomersomes for siRNA delivery. Biomaterials. 2011;32:849–857.
- Urban-Klein B, Werth S, Abuharbeid S, et al. RNAi-mediated gene targeting through systemic application of polyethylenimine (PEI)-complexed siRNA in vivo. Gene Ther. 2005;12:461–466.
- Alshamsan A, Hamdy S, Samuel J, et al. The induction of tumor apoptosis in B16 melanoma following STAT3 siRNA delivery with a lipid-substituted polyethlenimine. Biomaterials. 2010;31:1420–1428.
- Finlay J, Roberts CM, Lowe G, et al. RNA-based TWIST1 inhibition via dendrimer complex to reduce breast cancer cell metastasis. Biomed Res Int. 2015;2015:382745.
- McNamara JO, II, Andrechek ER, Wang Y. Cell type-specific delivery of siRNAs with aptamer-siRNA chimeras. Nat Biotechnol. 2006;24:1005–1015.
- Gilboa-Geffen A, Hamar P, Le MT, et al. Gene knockdown by EpCAMaptamer-siRNA chimeras suppresses epithelial breast cancers and their tumor-initiating cells. Mol Cancer Ther. 2015;14:2279–2291.
- Mao S, Sun W, Kissel T. Chitosan-based formulations for delivery of DNA and siRNA. Adv Drug Deliv Rev. 2010;62:12–27.
- Davis ME, Brewster ME. Cyclodextrin-based pharmaceutics: past, present and future. Nat Rev Drug Discov. 2004;3:1023–1035.
- Lieskovan S, Heidel JD, Bartlett DW, et al Sequence-specific knockdown of EWS-FLI1 by targeted, nonviral delivery of small interfering RNA inhibits tumor growth in a murine model of metastatic Ewing's sarcoma. Cancer Res. 2005;65:8984–8992.
- Kesharwani P, Tekade RK, Gajbhiye V, et al. Cancer targeting potential of some ligand-anchored poly(propylene imine) dendrimers: a comparison. Nanomedicine. 2011;7:295–304.
- Lee JH, Cha KE, Kim MS, et al. Nanosizedpolyamidoamine (PAMAM) dendrimer-induced apoptosis mediated by mitochondrial dysfunction. Toxicol Lett. 2009;190:202–207.
- Patil ML, Zhang M, Taratula O, et al. Internally cationic polyamidoamine PAMAM-OH dendrimers for siRNA delivery: effect of the degree of quaternization and cancer targeting. Biomacromolecules. 2009;10:258–266.
- Taratula O, Garbuzenko OB, Kirkpatrick P, et al. Surface engineered targeted PPI dendrimer for efficient intracellular and intratumoral siRNA delivery. J Control Release. 2009;140:284–293.
- Jeong JH, Mok H, Oh YK, et al. siRNA conjugate delivery systems. Bioconjug Chem. 2009;20:5–14.
- Wolfrum C, Shi S, Jayaprakash KN, et al. Mechanism and optimization of in vivo delivery of lipophilic siRNAs. Nat Biotechnol. 2007;25:1149–1157.
- Meade BR, Dowdy SF. Exogenous siRNA delivery using peptide transduction domains/cell penetrating peptides. Adv Drug Deliv Reviews. 2007;59:134–140.
- Muratovska A, Eccles MR. Conjugate for efficient delivery of short interfering RNA (siRNA) into mammalian cells. FEBS Lett. 2004;558:63–68.
- Chiu YL, Ali A, Chu CY, et al. Visualizing a correlation between siRNA localization, cellular uptake, and RNAi in living cells. Chem Bio. 2004;11:1165–1175.
- Moschos SA, Jones SW, Perry MM, et al. Lung delivery studies using siRNA conjugated to TAT (48–60) and penetratin reveal peptide induced reduction in gene expression and induction of innate immunity. Bioconjugate Chem. 2007;18:1450–1459.
- Guo P, Coban O, Snead NM, et al. Engineering RNA for targeted siRNA delivery and medical application. Adv Drug Deliv Rev. 2010;62:650–666.
- Saraswathy M, Gong S. Recent development in the co-delivery of siRNA and small molecule anticancer drugs for cancer treatment. Mater Today. 2014;327:1–9.
- Chen AA, Derfus AM, Khetani SR, et al. Quantum dots to monitor RNAi delivery and improving gene silencing. Nucleic Acids Res. 2005;33:190–198.
- Gao X, Cui Y, Levenson RM, et al. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotechnol. 2004;22:969–976.
- Lu Q, Moore JM, Huang G, et al. RNA polymer translocation with single-walled carbon nanotubes. Nano Lett. 2004;4:2473–2477.
- Bhatnagar I, Venkatesan J, Kim SK. Polymer functionalized single walled carbon nanotubes mediated drug delivery of gliotoxin in cancer cells. J Biomed Nanotechnol. 2014;10:120–130.
- Lee JM, Yoon T-J, Cho YS. Recent developments in nanoparticle-based siRNA delivery for cancer therapy. Biomed Res Int. 2013;2013:1–10.
- Kam NW, Liu Z, Dai H. Functionalization of carbon nanotubes via cleavable disulfide bonds for efficient intracellular delivery of siRNA and potent gene silencing. J Am Chem Soc. 2005;127:12492–12493.
- Kirkpatrick DL, Weiss M, Naumov A, et al. Carbon nanotubes: solution for the therapeutic delivery of siRNA. Materials. 2012;5:278–301.
- Liu Z, Tabakman S, Welsher K, et al. Carbon nanotubes in biology and medicine: in vitro and in vivo detection, imaging and drug delivery. Nano Res. 2009;2:85–120.
- Ghosh P, Han G, De M, et al. Gold nanoparticles in delivery applications. Adv Drug Deliv Rev. 2008;60:1307–1315.
- Song WJ, Du JZ, Sun TM, et al. Gold nanoparticles capped with polyethyleneimine for enhanced siRNA delivery. Small. 2010;6:239–246.
- Ding Y, Jiang Z, Saha K, et al. Gold Nanoparticles for Nucleic Acid Delivery. Mol Ther. 2014;22:1075–1083.
- Matzke MA, Matzke AJ. Planting the Seeds of a New Paradigm. PLoS Biol. 2004;2:e133.