Abstract
The diagnosis of lung cancer mostly occurs when the cancer is already in an advanced stage. In this situation, there are few options for the treatment and most of them have few chances of success. In this study, we developed and tested etoposide microparticles as a diagnostic agent for imaging lung cancer at early stages of development. We tested etoposide microparticles labeled with technetium 99m in inducted mice. The results demonstrated that over 10% of the total dose used was uptake by the tumor site. Also, the results showed that the microparticles had a good renal clearance and low uptake by liver and spleen. The data suggest that these micro-radiopharmaceuticals may be used for lung cancer imaging exam, especially single-photo emission computed tomography (SPECT).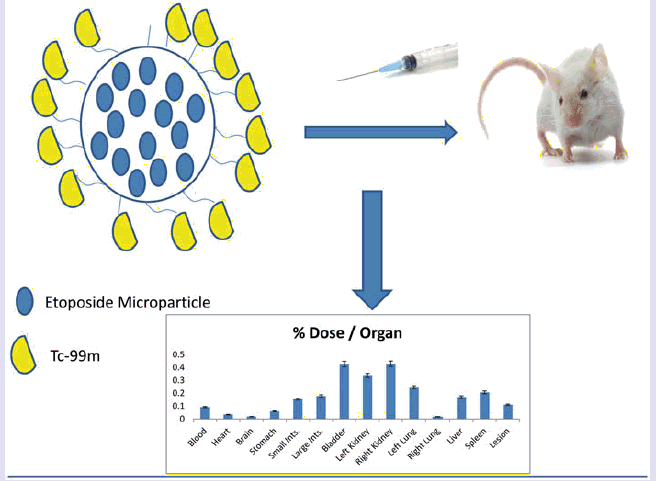
Introduction
Lung cancer is the most deadly and the leading cancer killer in men since the early 1950s and of women since 1980s. The mortality rate is one the highest, raising this cancer to the fourth position in rank of death by cancer worldwide [Citation1,Citation2]. The lung cancer may be classified into: small-cell lung cancer (SCLC) and non-small-cell lung cancer (NSCLC). SCLC arises in the midlevel airway and is a very aggressive, highly metastasizing and lethal cancer type that comprises 15% of all lung cancers. NSCLC is the major type of lung cancer and comprises 85% of all lung cancers. NSCLC includes lung adenocarcinoma, lung squamous cell carcinoma (LSCC), and lung large-cell carcinoma [Citation3].
The statistics on cancer are very discouraging, ∼13 million new cancer cases and 7.6 million cancer deaths occur each year worldwide [Citation4]. In the last 40 years, the volume of financial resources for treatment, research and prevention of cancer was ∼$90 billion [Citation5].
The treatment of lung cancer in USA and Europe remains cisplatin or carboplatin plus etoposide. The etoposide is a chemotherapeutic that works by blocking an enzyme (called topoisomerase II) interacting directly with the ATP-bound enzyme monomer in such a way that each molecule of etoposide stabilizes only a single-stranded break. Depending on the dose of etoposide, single-stranded or double-stranded DNA breaks are generated. Furthermore, the inhibition of topoisomerase II by etoposide is reversible and discontinuation of ternary complex allows quick DNA repair and diminishes the cytotoxicity of the drug, which is necessary for cancer cells to divide and so grow into two new cells. If the enzyme is blocked, the cell’s DNA gets tangled up and the cell cannot divides [Citation6,Citation7]. However, lung cancer still has a poor prognosis, with an overall survival at 5 years ∼15%. Unfortunately, in most patients (80%), the disease is diagnosed at an advanced stage (III–IV) and less in the early stages (I–II), when it would be potentially curable [Citation2].
Imaging is one of most unique approach to visualize tumors in 3D concept. It may be done, normally, by computed tomography (CT), magnetic resonance imaging (MRI), single-photon emission computed tomography (SPECT) and positron emission tomography (PET). However, the first two modalities, i.e. CT and MRI shows a great number of limitations regarding the: (i) minimum detectable tumor (typically >1 mm3 (and often >125 mm3), (ii) usefulness to interrogate tumor microenvironment composition; (iii) limited coverage to a few mm2 and a depth of ∼100 μm before resolution is degraded by light scattering [Citation8–15]. On the other hand, the use of techniques that employ radioactive material as imaging methods, like SPECT and PET may overcome this limitation and obtain images of high quality and specificity [Citation14–17], especially with the use of nano and/or microparticles [Citation18–21]. In this direction, we have developed the etoposide microparticle-labeled with 99mTc as a diagnostic agent for lung cancer in order to develop a new micro-radiopharmaceutical for early, precise and precocious diagnosing of lung cancer.
Materials and methods
Development of etoposide microparticles
The etoposide microparticles were prepared by double emulsion-solvent evaporation method using polycaprolactone (PCL) as polymer. A solution of 50 mg of the drug (etoposide) in 200 μL of 1% polyvinyl alcohol (PVA) aqueous solution was dripped into an organic solution of 50 mg of polymer in 2 mL of dichloromethane under agitation (Ultra-Turrax T10 Basic IKA, Hitachi, Wilmington, NC) for 1 min at 21,200 rpm. This first emulsion was emulsified again with 4 mL of PVA 1.0 wt% solution by homogenization at 21,200 rpm for 2 min to produce a water-in-oil-in-water (W/O/W) emulsion. Then, to form the particles the organic phase was removed by evaporation under reduced pressure during 1.5 h at 25 °C.
Etoposide NPs mean size assessment
Etoposide microparticles size distribution, mean size and polydispersity index (PDI) were determined by dynamic light scattering (DLS) using the equipment Zetasizer Nano ZS (Malvern Instruments, Malvern, UK). Measurements were performed in triplicate at 25 °C and the laser incidence angle in relation to the sample was 173° using a 12 mm2 quartz cuvette. The mean ± standard deviation (SD) was assessed.
Scanning electron microscopy
The morphology of etoposide microparticles were performed by scanning electron microscopy (SEM) (TM 3000; Hitachi). In this study three SEM images were performed, as followed: (i) immediate image (2 h) after the preparation of the microparticle; (ii) 4 h after the preparation of the microparticle and labeled with 99mTc and (iii) 1 month after the preparation of the microparticle after labeling with 99mTc.
Labeling with 99mTc nano-radiopharmaceuticals
The method used was the direct-labeling process as described previously [Citation22–26]. The labeling process used 150 μL of the etoposide microparticles. First, 2 mCi (∼300 μL) solution of pertechnetate (Na99mTcO4–) was incubated with stannous chloride (SnCl2) solution (30 μL/mL) (Sigma-Aldrich, St. Louis, MO) for 20 min at room temperature. Then, this solution was incubated with 150 μL of etoposide microparticles for 10 min, which labeled the microparticles with Tc-99m.
In order to characterize the labeled etoposide microparticles, paper chromatography was made using Whatman paper no 1 (triplicate). The paper chromatography was performed using 2 μl of the labeled nanoparticle in acetone (Sigma-Aldrich, St. Louis, MO) as mobile phase. The radioactivity of the strips were verified in a gamma counter (Perkin Elmer Wizard® 2470, Shelton, CT) after 2 h. In order to confirm the stability of the labeling process of the etoposide microparticles, a lately paper chromatography was performed after 8 h ( and ).
Table 1. Percentage of labeled etoposide microparticles by ascending chromatograms of 99mTc compared with free pertechnetate (Na99mTcO4−) in 2 h.
Table 2. Percentage of labeled etoposide microparticles by ascending chromatograms of 99mTc compared with free pertechnetate (Na99mTcO4−) in 8 h.
In vivo analysis
Tumor xenograft models
A549 cells (American Type Culture Collection, Manassas, VA) were cultured in RPMI (Gibco/Life Technologies Inc., Rockville, MD) supplemented with 10% of fetal bovine serum (Gibco/Life Technologies Inc.) and 50 μg/mL of gentamicin (Gibco/Life Technologies Inc.). Mycoplasma contamination in cultured cells was excluded using Lonza Mycoplasma Detection Kit (Portsmouth, NH).
Tumors were established by subcutaneous (sc) injection of 2 × 106 A-549 cells at the back of seven 6-week-old male Balb/c nude mice. Tumor size was monitored for 3 weeks and measured by a caliper. The tumor size before imaging was ∼2 cm. Mice were observed three times per week for evidence of distress, ascites, paralysis or excessive weight loss.
Biodistribution studies
Evaluation of the biodistribution of etoposide microparticles were made with a Intervention Group using loaded microparticles etoposide labeled with 99mTc (n = 7). Mice were anesthetized with mix solution of 10% ketamine and 2% xylazine in volume of 15 μL and administered intramuscularly (thigh). The micro-radiopharmaceuticals (3.7 MBq in volume of 0.2 mL) were administered by retro-orbital via. Mice were sacrificed by asphyxiation using a carbon dioxide gas chamber after 2 h (120 min) of radio-compound administration. Organs [brain, lungs, kidneys, stomach, small and large intestine, bladder, heart, blood pool and the xenografted tumor (lesion)] were removed, weighted and the activity in each organ, blood and tumor has been counted by a gamma counter (Perkin Elmer Wizard® 2470). The results were expressed as μCi per organ.
SPECT imaging
SPECT were performed to obtain planar images after 120 min retro-orbital injection of the micro-radiopharmaceuticals (3.7 MBq in 0.2 mL), integrating for 5 min the radiation counts centered at 140 KeV, with a Millenium Gamma Camera (GE Healthcare, Cleveland, OH) using a 15% window. The images were done in Balb/c nude mouse inducted with lung cancer as described in xenografted model.
Results and discussion
Etoposide NPs mean size assessment
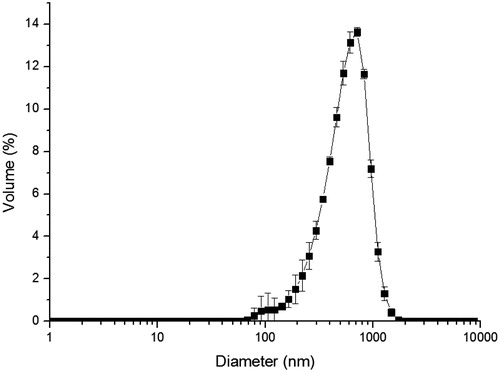
Etoposide microparticles presented a mean size of 430 ± 10.2 nm, with a PDI of 0.23 ± 0.02 and a unimodal and narrow size distribution ().
Scanning electron microscopy
The data from SEM corroborates the data from DLS. Also shows that the etoposide microparticles has a spherical shape and aggregates forming clots with a size range of 450 nm. Is important to notice that although with a higherrate of aggregation in the beging (2 h) it seems that this reach a optimal plateau, and that the aggregation stop trough the time, and after one in one moth is totally stable. Is also important to notice that the presence of 99mTc does not interfere in the stability or in the morphology of the microparticles, as in the aggregation process ().
Labeling with 99mTc micro-radiopharmaceuticals
The direct method used for labeling etoposide microparticles was a sucess and all the microparticles showed a rate over 99% of labeling (). The lately paper chromatography (after 8 h of labeling) also showed a great result and confirmed the stability of the labeling process ().
Biodistribution studies
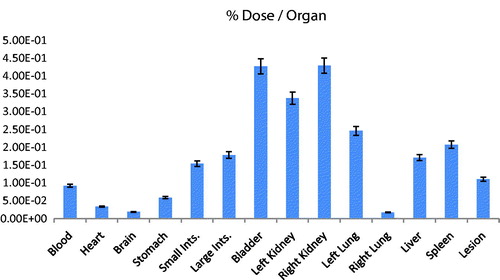
The result of the biodistribution study is expressed in The results from biodistribution may show several information. First of all, is possible to observe that a considerable uptake occurred in the lesion (xenografted lung tumor), a total of Σ0.11 μCi. This is almost 15% of the total dose administrated. In general, for acquisition of an imaging is necessary that at least a value equivalent to 5% of the total dose reach the site. So, this result confirmed that this etoposide microparticle may be used as an imaging agent for lung cancer diagnosing. The etoposide microparticles also showed an expressive value Σ0.33 μCi and Σ0.42 μCi in left and right kidney, respectively. This means that the etoposide microparticles have a good renal clearance. These data are corroborated with the findings in bladder Σ0.42 μCi. The high uptake by the left lung Σ0.24 μCi is due the administration via (retro-orbital via). In this way, the drug reaches the blood through the ocular plexus via the vena cava. Once in the vena cava, the first way is the small circulation, reaching first the left lung and then the right lung, thus, we believe that most is retained in the pulmonary alveoli of the left lung due the size of the etoposide microparticles. The uptake by liver and spleen may be explained by the fact that polymeric microparticles could be retained in organs such as liver and spleen. Also, is possible that etoposide microparticles could activated liver macrophages, increasing the uptake by this organ [Citation27,Citation28]. The uptake by stomach and intestines could be explained due the fact that etoposide is substrate of several ABC transporters, notably ABCB1 (MDR1) and ABCC1 (MRP1), ABCC2 (MRP2), ABCC3 (MRP3) and ABCG2 (BCRP), most of them present in intestine and stomach. Nevertheless, etoposide metabolism principally occurs in the liver, but may also happen in other tissues, like intestinal mucosa, since it is O-demethylated primarily by cytochrome P450 (CYP) 3A4 and to a lesser extent by CYP3A5 [Citation7]. The uptake by the blood means that the etoposide microparticle have a good interaction with blood proteins and may remain linked to it increasing the circulating time. Finally, the uptake by the brain was negligible.
SPECT imaging
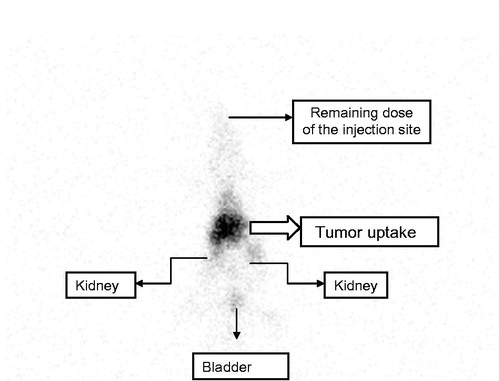
The result () of the SPECT corroborates the findings of the biodistribution, especially the uptake by the tumor and renal clearance due the presence in kidneys and bladder. Is also observed the remaining dose in the top of the image (retro-orbital injection). For this reason, it is not possible to corroborate the negligible uptake by the brain.
Conclusions
The etoposide-microparticle labeled to 99mTc demonstrated to be a micro-radiopharmaceutical that may be used for early diagnosing of lung cancer; nevertheless, the use of a polymeric microparticle showed to be stable and capable to reach the tumor in a high concentration. The biodistribution data also demonstrated that the renal clearance is effective and showed negligible uptake by the brain. The results from SPECT imaging corroborates the biodistribution and also showed the possibility of use of this micro-radiopharmaceutical as imaging agent.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- Abe Y, Tanaka N. The Hedgehog signaling networks in lung cancer: the mechanisms and roles in tumor progression and implications for cancer therapy. Biomed Res Int. 2016;2016:7969286.
- Jimenez-Bonilla JF, Quirce R, Martinez-Rodriguez I, et al. The role of PET/CT molecular imaging in the diagnosis of recurrence and surveillance of patients treated for non-small cell lung cancer. Diagnostics. 2016;6:pii:E36.
- Reddy AT, Lakshmi SP, Reddy RC. PPARγ as a novel therapeutic target in lung cancer. PPAR Res. 2016;2016:8972570.
- Espina C, Porta M, Schuz J, et al. Environmental and occupational interventions for primary prevention of cancer: a cross-sectorial policy framework. Environ Health Perspect. 2013;121:420–426.
- Marshall E. Cancer research and the $90 billion metaphor. Science. 2011;331:1540–1541.
- Pietanza MC, Zimmerman S, Peters S, et al. Seeking new approaches to patients with small cell lung cancer. Am Soc Clin Oncol Educ Book. 2016;35:e477–e482.
- Rezonja R, Knez L, Cufer T, et al. Oral treatment with etoposide in small cell lung cancer - dilemmas and solutions. Radiol Oncol. 2013;47:1–13.
- Schwab KE, Gailloud P, Wyse G, et al. Limitations of magnetic resonance imaging and magnetic resonance angiography in the diagnosis of intracranial aneurysms. Neurosurgery. 2008;63:29–34.
- Cuccarese MF, Dubach JM, Pfirschke C, et al. Heterogeneity of macrophage infiltration and therapeutic response in lung carcinoma revealed by 3D organ imaging. Nat Commun. 2017;8:14293.
- Badea CT, Athreya KK, Espinosa G, et al. Computed tomography imaging of primary lung cancer in mice using a liposomal-iodinated contrast agent. PLoS One. 2012;7:e34496.
- Yang M, Li L, Jiang P, et al. Dual-color fluorescence imaging distinguishes tumor cells from induced host angiogenic vessels and stromal cells. Proc Natl Acad Sci USA. 2003;100:14259–14262.
- Hoffman RM, Yang M. Color-coded fluorescence imaging of tumor-host interactions. Nat Protoc. 2006;1:928–935.
- Yaqoob Z, Psaltis D, Feld MS, et al. Optical phase conjugation for turbidity suppression in biological samples. Nat Photonics. 2008;2:110–115.
- Chi C, Du Y, Ye J, et al. Intraoperative imaging-guided cancer surgery: from current fluorescence molecular imaging methods to future multi-modality imaging technology. Theranostics. 2014;154:1072–1084.
- Rud E, Baco E, Klotz D, et al. Does preoperative magnetic resonance imaging reduce the rate of positive surgical margins at radical prostatectomy in a randomised clinical trial? Eur Urol. 2015;68:487–496.
- Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452:580–589.
- Pysz MA, Gambhir SS, Willmann JK. Molecular imaging: current status and emerging strategies. Clin Radiol. 2010;65:500–516.
- James ML, Gambhir SS. A molecular imaging primer: modalities, imaging agents, and applications. Physiol Rev. 2012;92:897–965.
- Lee D-E, Koo H, Sun I-C, et al. Multifunctional nanoparticles for multimodal imaging and theragnosis. Chem Soc Rev. 2012;41:2656–2672.
- Huang Y, He S, Cao W, et al. Biomedical nanomaterials for imaging-guided cancer therapy. Nanoscale. 2012;4:6135–6149.
- Santos do Carmo F, Ricci-Junior E, Cerqueira-Coutinho C, et al. Anti-MUC1 nano-aptamers for triple-negative breast cancer imaging by single-photon emission computed tomography in inducted animals: initial considerations. Int J Nanomed. 2016;12:53–60.
- Sarcinelli MA, de Souza Albernaz M, Szwed M, et al. Nanoradiopharmaceuticals for breast cancer imaging: development, characterization, and imaging in inducted animals. Onco Targets Ther. 2016;9:5847–5854.
- Cerqueira-Coutinho C, Vidal LP, Pinto SR, et al. Drug metabolism: comparison of biodistribution profile of holmium in three different compositions in healthy Wistar rats. Appl Radiat Isot. 2016;112:27–30.
- Cerqueira-Coutinho CS, De Campo VE, Rossi AL, et al. Comparing in vivo biodistribution with radiolabeling and Franz cell permeation assay to validate the efficacy of both methodologies in the evaluation of nanoemulsions: a safety approach. Nanotechnology. 2016;27:015101.
- Cerqueira-Coutinho C, Missailidis S, Alessandra-Perini J, et al. Comparison of biodistribution profile of monoclonal antibodies nanoparticles and aptamers in rats with breast cancer. Artif Cells Nanomed Biotechnol. 2016;45:598–601.
- Pascual L, Sancenon F, Martinez-Manez R, et al. Mesoporous silica as multiple nanoparticles systems for inflammation imaging as nano-radiopharmaceuticals. Micropor Mesopor Materials. 2017;239:426–431.
- Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33:941–951.
- De Jong WH, Hagens WI, Krystek P, et al. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials. 2008;29:1912–1919.