Abstract
Green synthesis of silver nanoparticles (AgNPs) provides the alternative method with cost effectiveness and the eco-friendly process by using natural biomolecules as reducing and stabilizing agents. Alternative to the most studies of plant extracts, this work demonstrated a use of egg extract of apple snail (Pomacea canaliculata) for an eco-friendly production of AgNPs. The extract contained at least six proteins with the molecular weight in a range of 24–65 kDa that exhibited the reducing activity. The dispersive AgNPs were produced in the reaction containing only the extract and silver nitrate, as determined by the characteristic surface plasmon resonance peak of silver at 412 nm. The synthesized AgNPs were spherical with the average diameter of 9.0 ± 5.9 nm. The X-ray diffraction pattern and selected area electron diffraction (SAED) analyses confirmed the face-cubic centre (fcc) unit cell structure of AgNPs. The synthesized AgNPs exhibited the antibacterial activity against both Gram-positive Staphylococcus aureus and Gram-negative Escherichia coli. Results of this work clearly showed the potential use of the egg extract of apple snail for a green synthesis of small size AgNPs exhibiting the antibacterial activity.
Introduction
Metallic nanoparticles have drawn many research attentions due to their various applications in the fields of catalysis, optical biosensors, removal of heavy metal ions, and medical diagnosis and therapy [Citation1]. It was estimated that nanotechnology’s industrial value would reach $3 trillion by 2020 and trended to increase yearly [Citation2]. Among these nanoparticles, silver nanoparticle (AgNP) is one receiving extensive studies because of its interesting properties of the higher surface to volume ratio, optical properties, and antibacterial activity, which differ from the properties of its bulk size [Citation3]. Based on the antibacterial activity, AgNPs have been added to more than 300 commercial products ranging from textiles, food storage containers, plastics, biomedical devices, soaps and cosmetics [Citation2].
With a high demand, many synthesis approaches of AgNPs have been reported. The most common methods for producing AgNPs are chemical and physical methods, such as evaporation-condensation, laser ablation, chemical reduction, electrochemical techniques, photoinduced reduction, UV-initiated photoreduction, irradiation methods and microemulsion techniques [Citation4]. In general, conventional physical methods usually require high energy and often produce a low yield. Although a high production of nanoparticles can be obtained by chemical methods, they often use hazard chemicals and produce toxic by-products [Citation5]. For these reasons, a biosynthesis of AgNPs has received increasing interest due to their simple, eco-friendly, non-toxic, and cost-effective processes. The biosynthesis of AgNPs can be mainly divided into two groups; in vivo and in vitro syntheses. For in vivo synthesis, living organisms are used as biofactories to produce metallic nanoparticles by enzymatic processes inside the cells; fungus (Aspergillus flavus) [Citation6], bacteria (Corynebacterium strain SH09) [Citation7]. For in vitro synthesis, a formation of AgNPs is facilitated via a use of biomolecules and other substances extracted from bacteria [Citation8], fungi [Citation9], yeasts [Citation10], plants [Citation11], and algae [Citation12] as reducing and stabilizing agents. In addition, the secreted proteins and enzymes in the cultures of Bacillus amyloliquefaciens, Bacillus subtilis, Aeromonas sp. THG-FG1.2, and Weissella oryzae DC6 were also reported to facilitate the formation of AgNPs [Citation13–15]. Among in vitro syntheses, most reports focused on using plant extracts to facilitate a production of AgNPs. The extracts derived from various species and parts of plants were used for green syntheses of AgNPs, such as arabica coffee seeds (Coffea Arabica) [Citation16], papaya fruit (Carica papaya) [Citation17], Asian bushbeech leaves (Gmelina asiatica) [Citation18], and ginseng leaves (Panax ginseng) [Citation19]. The main components in plant extracts that might function as reducing agents to reduce Ag+ to Ag0 are proteins, amino acids, organic acid, vitamins, flavonoids, alkaloids, polyphenols, terpenoids, and polysaccharides [Citation20]. As compared with plant extracts, the extracts of crustacean eggs are one of the interesting sources of reducing and stabilizing agents due to their abundant availability, low cost, high protein content, and available chromophores [Citation21], potentially function as reducing and stabilizing agents. However, there has been no report on using the extracts of crustacean eggs for a green synthesis of AgNPs.
With an exceptional adaptation of drought climate in the tropical region, apple snails (Pomacea canaliculata) are considered the worst invasive pest species that widely spread in several natural waterways and rice fields [Citation22]. Adult females can lay their red egg clusters, each containing 30–300 eggs, above the water [Citation23]. Their eggs contain perivitelline fluid (PVF) filling with high contents of polysaccharides (34.8%), proteins (13.0%), and carotenoids (72 nmol/g) [Citation21,Citation24], which are potentially served as reducing and stabilizing agents for a green synthesis of AgNPs. The use of the egg extract of apple snail for a production of AgNPs is not only the alternative eco-friendly synthesis approach but also the value-added means to apple snails. Thus, in this study, we are interested in investigating a possibility to use the aqueous extract of apple snail eggs to facilitate a formation of AgNPs.
Materials and methods
Materials
Fresh apple snail eggs were collected from Huai Yang reservoir, Nakhon Ratchasima, Thailand (GPS 14.906295, 101.995610). Silver nitrate was purchased from QReC (Auckland, New Zealand). D(+)-glucose anhydrous was obtained from VWR International (Gavere, Belgium). Muller-Hinton (MH) broth was purchased from Merck (Darmstadt, Germany). All chemicals used were of analytical grade.
Preparation of the extract of apple snail eggs
Apple snail eggs (5 g) were ground to powder in liquid nitrogen before suspending in 10 ml distilled water. The suspended solution was centrifuged at 6000×g for 5 min and the supernatant was collected as the egg extract of apple snail. The protein concentration of the extract was determined by Bradford protein assay (Bio-Rad, Hercules, CA). The proteins in the extract were visualized on a 4–15% gradient sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) gel stained with Coomassie brilliant blue R-250 dye. The gel image was analysed by a FireReader V4 1 D software of a gel documentation system (UVItec, Cambridge, UK).
Determination of reducing power
The reducing power of the egg extract of apple snail was determined using the method adapted from the previous publication [Citation25]. The extract (2.5 ml) of various concentrations (0.125–4 mg/ml) was mixed with 200 mM sodium phosphate buffer, pH 6.6 (2.5 ml), and 1% potassium ferricyanide (2.5 ml). After incubating at 50 °C for 20 min, 10% trichloroacetic acid (2.5 ml) was mixed. The solution was centrifuged at 3000×g for 10 min. The upper layer of the solution (1.25 ml) was removed to a new tube before mixing with distilled water (1.25 ml) and a freshly prepared 0.1% ferric chloride (0.25 ml). The reducing power of the tested samples was determined by the colour changes, which were measured at the absorbance of 700 nm using a UV–Vis spectrophotometer (Analytik Jena Specord® 250 Plus, Jena, Germany). All determinations were performed in five replications.
Synthesis of AgNPs using the egg extract of apple snail
To study whether a reducing agent, glucose, is required for a synthesis of AgNPs, the extract of apple snail eggs at different final concentrations (2, 4, and 8 mg/ml) was mixed with 1 M AgNO3 (0.25 ml) in a presence or absence of 2 M glucose (0.5 ml), before adjusting the reaction volume to 5.5 ml with deionized water. The solutions were continuously stirred at 60 °C for 48 h in the dark and the formation of AgNPs was monitored at the wavelength of 300–700 nm. To monitor the formation of AgNPs in a time course of 48 h, the reactions (5.5 ml) containing the extract and AgNO3 in the conditions with and without glucose were incubated at 60 °C with light protection. The reactions were sampling at 0, 6, 12, 24, and 48 h to measure the absorbance at 300–700 nm.
Characterization of the synthesized AgNPs
The morphology and size of the synthesized AgNPs were analysed from the images taken by a transmission electron microscope (TEM, FEI Tecnai G2 20, FEI, Hillsboro, OR) operating at accelerating voltage 200 kV. The suspension of AgNPs was dropped on a carbon-coated copper grid and dried at room temperature before analysing by TEM. The crystalline structure of AgNPs was characterized by selected area electron diffraction (SAED) equipped with TEM (FEI, Hillsboro, OR) operated at 200 kV with a LaB6 filament. The images were recorded with a Gatan Orius 200 CCD Camera (Gatan, Houston, TX). The elemental composition of AgNPs was determined by an energy dispersive X-ray (EDX) spectroscopy equipped with TEM using an EDAX r-TEM SUTW detector (FEI, Hillsboro, OR) operating at an accelerating voltage of 10 kV. The crystalline structure of AgNPs was characterized by an X-ray diffraction (XRD) pattern using the D8 Advance spectrometer (Bruker, Coventry, UK). The operation was carried out using a Cu kα radiation (λ = 1.5418 Å) with a step size 0.02° within the 2θ range of the 30–80 radians. The X-ray tube of voltage and current were set at 40 kV and 40 mA, respectively. The suspension of AgNPs was dropped on a glass coverslip and dried at room temperature before analysing.
Antimicrobial activity of the synthesized AgNPs
The antimicrobial activity of the synthesized AgNPs against two common human pathogens is tested by the standard broth dilution method [Citation26]; the Gram-negative Escherichia coli (ATCC25922) and Gram-positive Staphylococcus aureus (ATCC25923). A single colony of each bacteria was initially cultured in 5 ml MH broth in an incubator shaker at 37 °C and 200 rpm for 4 h. The turbidity of the bacterial culture was adjusted equal to 0.5 McFarland standard or 1 × 108 colony-forming units/ml (CFU/ml). The suspension of AgNPs (200 mg/ml) was serially two-fold diluted by MH broth. Different concentrations of AgNPs (100 μl) was mixed with the bacterial culture at the concentration of 5 × 105 CFU/ml (100 μl) in a 96-well culture plate. The cultures were incubated at 37 °C and shaken at 80 rpm, which the bacterial growth was monitored by measuring the optical density at 600 nm. The minimum inhibitory concentration (MIC) was determined by the lowest concentration of AgNPs with no observation of bacterial growth. To determine the minimal bactericidal concentration (MBC), the aliquot of 100 μl was taken from the bacterial cultures at MIC value and two higher concentrations and spread on MH agar plates. The culture plates were incubated at 37 °C for 24 h before the numbers of growing bacterial colony were determined. The MBC was determined by the lowest concentration of AgNPs that killed 100% of the initial bacterial population.
Statistical analysis
All results were presented as means ± standard deviations and the statistical comparisons were determined according to the one-way ANOVA using the SPSS 11.5 for Windows software (SPSS Inc., Chicago, IL). Statistical significance was accepted when the p values were less than .05.
Results and discussion
The egg extract of apple snail
Pink clusters of apple snail eggs were collected from a natural habitat and cleaned with distilled water before extracting. The protein content of the water extraction of apple snail eggs was 25.6 mg per 1 g wet weight of eggs as determined by a Bradford protein assay. The proteins of the extraction were separated on a 4–15% gradient SDS-PAGE gel and six protein bands at the molecular weight of 24, 26, 28, 29, 3, and 67 kDa were clearly observed (). The molecular weight of these proteins were similar to the major proteins in egg PVF of apple snails; perivitelline 1 (PV 1) or ovorubin (35, 32, and 28 kDa), PV 2 (67 and 31 kDa), and PV 3 (34, 29, and 21 kDa) [Citation27].
Figure 1. The egg extract of apple snail (a) visualized on a 4–15% gradient SDS-PAGE gel and (b) its reducing activity at various concentrations.
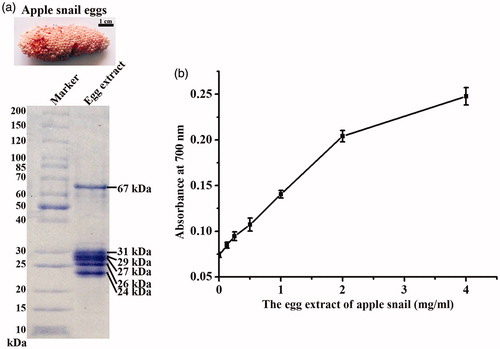
The reducing activity of the egg extract of apple snail egg was determined by the colorimetric reducing assay, which the reduction of the Fe3+/ferricyanide complex to the ferrous form by the reducers of the egg extract could be determined at the absorbance of 700 nm. The level of reducing power was determined from a change of yellow color to various shades of green and blue depending on the reducing power of each compound [Citation25]. The reducing activity of the egg extract of apple snail is shown in , which the reducing activity was increased according to the increased concentrations of the egg extract. This result suggested that the egg extract of apple snail could serve as a good electron donor, thus potentially reduce Ag+ to metallic AgNPs.
The synthesis of AgNPs
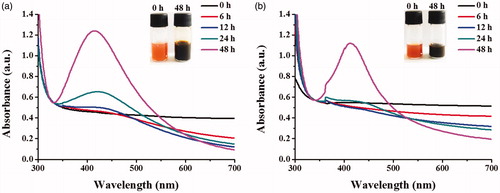
To investigate the optimal conditions to synthesize AgNPs, the egg extract of apple snail at different concentrations were incubated with AgNO3 in a presence or absence of glucose, the adding reducing agent. The maximal UV–Vis absorption in a range of 400–500 nm, the unique localized surface plasmon resonance property, was used to indicate the formation of AgNPs. The results show in , which the formation of AgNPs, as indicated by the SPR peaks at 412 nm, was observed in reactions with and without glucose, suggesting that the extract of apple snail eggs could be used as good reducing and stabilizing agents. The addition of glucose as the reducing agent could induce the greater formation of AgNPs. Through increasing concentrations of the extract, the increasing formation of AgNPs was observed in the reaction containing glucose. Although the intensity of SPR peak of the reaction using 8 mg/ml the extract was dropped, this reduction was due to the aggregation of AgNPs (). In the reaction containing no glucose, only the condition using the egg extract at 8 mg/ml was sufficient to induce a formation of AgNPs as indicated by the detected SPR peak (). The narrow peak widths of SPR peak represented the presence of small size and narrow size distribution of the produced AgNPs [Citation28]. It was likely that the composition of aspartic acid (11.6%), glutamic acid (11.6%), serine (7.5%), tyrosine (4.1%), and phenylalanine (3.9%) of egg proteins [Citation29] and carotenoid pigments of apple snail eggs could serve as good reducing agents [Citation21]. In addition, biomolecules with high molecular weight such as proteins and carbohydrates of the apple snail eggs could stabilize the formed AgNPs by electrostatic interaction of their functional groups with the nanoparticles [Citation30].
Figure 2. UV–Vis spectra of the synthesized AgNPs using different concentrations of the egg extract (2, 4, and 8 mg/ml) in the reactions (a) with glucose and (b) without glucose.
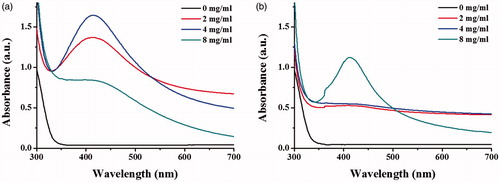
shows the UV–Vis spectra of the produced AgNPs in a time course of 48 h in the condition with and without glucose. With glucose, the formation of AgNPs, as indicated by the SPR peak, was detected at 12 h and increasing SPR intensities were observed as the reaction times were increased (). In the condition without glucose, the SPR peak was first observed as 24 h and the clear SPR peak was detected at the reaction of 48 h (). The formation of AgNPs was also observed from the reaction colour changed from yellow, the colour of the extract, to dark brown. The yellow colour of the extract was likely due to the dissolved carotenoproteins, the carotenoid-protein complex [Citation31].
Characterization of the synthesized AgNPs
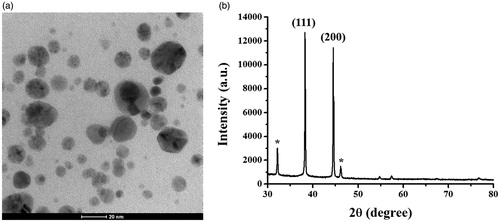
The morphology and size of the synthesized AgNPs were determined from the taken TEM images. shows the good dispersion of spherical AgNPs. Their measured sizes were ranged from 1–30 nm and their average diameter was 9.0 ± 5.9 nm. This size range is similar to the reported size ranges of AgNPs synthesized from algae, bryophytes, pteridophytes, gymnosperms, which generally below 50 nm, whereas AgNPs synthesized from angiosperms, algae, and bacterial sources were larger in sizes between 100 nm and more [Citation32]. The identity of the produced particles was further confirmed by SAED, EDX, and XRD analyses. The XRD pattern of the synthesized AgNPs is shown in , which was used to confirm the crystalline nature of particles. Two X-ray diffraction peaks were detected at the 2θ values of 38.3° and 44.3°, corresponding to the planes (1 1 1) and (2 0 0) of the face-centred cubic (fcc) lattice of silver, suggesting that the produced AgNPs was of metallic silver and was crystalline with an fcc structure. This XRD pattern was matched with the database Joint Committee on Powder Diffraction Standards (JCPDS) file no. 01–087–0718 [Citation26]. Furthermore, the XRD patterns of the produced AgNPs showed some unassigned peaks, indicating that the crystallization of a bio-organic phase on the surfaces of AgNPs [Citation33].
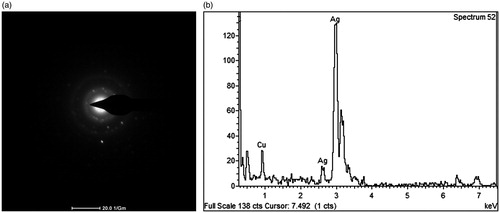
The SAED pattern is shown in , which the bright circular spots along with their crystal orientations appear within the diffraction rings. The SAED pattern revealed the presence of (1 1 1) and (2 0 0) cubic planes which corresponded to the fcc structure of elemental silver (JCPDS file no. 04–0783) [Citation34], thus confirming the crystalline nature of AgNPs. The result of SAED pattern confirmed the fcc structure of AgNPs in a good agreement with the XRD result. The EDX profile of the produced AgNPs in revealed a major peak around 3 keV, indicating the presence of elemental silver. The detection of Cu signals was likely contributed from the carbon supported copper grid using for the sample preparation [Citation35].
Antibacterial activity
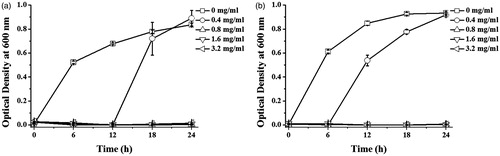
The antibacterial activity of the synthesized AgNPs was determined by the growth inhibition curve of Gram-negative (E. coli) and Gram-positive (S. aureus) bacteria in response to the synthesized AgNPs. In addition, the minimal inhibition concentration (MIC) and MBC values of the produced AgNPs against both bacteria were determined. shows the growth of E. coli and S. aureus incubating with various concentrations of AgNPs in a time course of 24 h. As the concentrations of AgNPs were increased, the relative numbers of the bacteria were reduced as determined by the optical density at 600 nm. The MIC of AgNPs against E. coli and S. aureus were equally at 0.8 mg/ml. The MBC of AgNPs against E. coli and S. aureus were 1.6 and 3.2 mg/ml, respectively. Similar to other reports, the antibacterial activity of the produced AgNPs against Gram-negative bacteria was high than that against Gram-positive bacteria, which was possibly due to the different thickness of peptidoglycan layer of their cell wall [Citation36]. The antibacterial action of AgNPs was proposed to mediate through the binding of the particles to the bacterial cell wall, resulting in the altered structural integrity and increased permeability of the membrane, induction of free radicals and radical-induced membrane damage, disruption of respiratory chain and energy production, and induction of cellular leakage [Citation37]. The released Ag+ of AgNPs can also interact with thiol-containing proteins in the cell wall and disrupt their functions [Citation38]. With their nanometer size, AgNPs also possibly penetrated inside the bacterial cell, causing the disrupted functions of some enzymes and protein, the cellular damage by inducing free radicles, the oxidative damage, and degradation of DNA, and the induction of cell death [Citation39].
Conclusions
This work demonstrated the use of the extract of apple snail eggs, the alternative to most reported plant extracts, as the natural reducing and stabilizing agents to facilitate a formation of AgNPs, which was considered as the eco-friendly and cost-effective approach. The extract of apple snail eggs contained proteins and other biomolecules possessing reducing activity that could reduce Ag+ to Ag0. In addition, the complex structure of proteins and other biomolecules in the extract also stabilized the synthesized AgNPs as dispersive particles in an aqueous condition. The synthesized AgNPs of 9.0 nm in diameter were obtained, which exhibited the potent antibacterial activity against both S. aureus and E. coli, the Gram-positive and Gram-negative bacteria, respectively.
Acknowledgements
This work is supported by the Suranaree University of Technology [SUT1–104–58–24–11].
Disclosure statement
The authors report that they have no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Additional information
Funding
References
- Schrofel A, Kratosova G, Safarik I, et al. Applications of biosynthesized metallic nanoparticles – a review. Acta Biomater. 2014;10:4023–4042.
- Quang Huy T, Van Quy N, Anh-Tuan L. Silver nanoparticles: synthesis, properties, toxicology, applications and perspectives. Adv Nat Sci Nanosci Nanotechnol. 2013;4:033001.
- Wei L, Lu J, Xu H, et al. Silver nanoparticles: synthesis, properties, and therapeutic applications. Drug Discov Today. 2015;20:595–601.
- Iravani S, Korbekandi H, Mirmohammadi SV, et al. Synthesis of silver nanoparticles: chemical, physical and biological methods. Res Pharm Sci. 2014;9:385–406.
- Gurunathan S. Biologically synthesized silver nanoparticles enhances antibiotic activity against Gram-negative bacteria. J Ind Eng Chem. 2015;29:217–226.
- Vigneshwaran N, Ashtaputre NM, Varadarajan PV, et al. Biological synthesis of silver nanoparticles using the fungus Aspergillus flavus. Mater Lett. 2007;61:1413–1418.
- Zhang H, Li Q, Lu Y, et al. Biosorption and bioreduction of diamine silver complex by Corynebacterium. J Chem Technol Biotechnol. 2005;80:285–290.
- Gurunathan S, Kalishwaralal K, Vaidyanathan R, et al. Biosynthesis, purification and characterization of silver nanoparticles using Escherichia coli. Colloids Surf B Biointerfaces. 2009;74:328–335.
- Shaligram NS, Bule M, Bhambure R, et al. Biosynthesis of silver nanoparticles using aqueous extract from the compactin producing fungal strain. Process Biochem. 2009;44:939–943.
- Korbekandi H, Mohseni S, Mardani Jouneghani R, et al. Biosynthesis of silver nanoparticles using Saccharomyces cerevisiae. Artif Cells Nanomed Biotechnol. 2016;44:235–239.
- Priya RS, Geetha D, Ramesh PS. Antioxidant activity of chemically synthesized AgNPs and biosynthesized Pongamia pinnata leaf extract mediated AgNPs – a comparative study. Ecotoxicol Environ Saf. 2016;134:308–318.
- El-Rafie HM, El-Rafie MH, Zahran MK. Green synthesis of silver nanoparticles using polysaccharides extracted from marine macro algae. Carbohydr Polym. 2013;96:403–410.
- Fouad H, Hongjie L, Yanmei D, et al. Synthesis and characterization of silver nanoparticles using Bacillus amyloliquefaciens and Bacillus subtilis to control filarial vector Culex pipiens pallens and its antimicrobial activity. Artif Cells Nanomed Biotechnol. 2016. [Epub ahead of print]. doi: 10.1080/21691401.2016.1241793.
- Singh P, Kim YJ, Wang C, et al. Weissella oryzae DC6-facilitated green synthesis of silver nanoparticles and their antimicrobial potential. Artif Cells Nanomed Biotechnol. 2016;44:1569–1575.
- Singh H, Du J, Yi T-H. Biosynthesis of silver nanoparticles using Aeromonas sp. THG-FG1.2 and its antibacterial activity against pathogenic microbes. Artif Cells Nanomed Biotechnol. 2017;45:584–590.
- Dhand V, Soumya L, Bharadwaj S, et al. Green synthesis of silver nanoparticles using Coffea arabica seed extract and its antibacterial activity. Mater Sci Eng C. 2016;58:36–43.
- Jain D, Daima HK, Kachhwaha S, et al. Synthesis of plant-mediated silver nanoparticles using papaya fruit extract and evaluation of their anti microbial activities. Dig J Nanomater Biostruct 2009;4:557–563.
- Muthukumaran U, Govindarajan M, Rajeswary M, et al. Synthesis and characterization of silver nanoparticles using Gmelina asiatica leaf extract against filariasis, dengue, and malaria vector mosquitoes. Parasitol Res. 2015;114:1817–1827.
- Castro-Aceituno V, Ahn S, Simu SY, et al. Anticancer activity of silver nanoparticles from Panax ginseng fresh leaves in human cancer cells. Biomed Pharmacother. 2016;84:158–165.
- Singh P, Kim Y-J, Zhang D, et al. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 2016;34:588–599.
- Dreon MS, Schinella G, Heras H, et al. Antioxidant defense system in the apple snail eggs, the role of ovorubin. Arch Biochem Biophys. 2004;422:1–8.
- Salleh NHM, Arbain D, Daud MZM, et al. Distribution and management of Pomacea canaliculata in the northern region of malaysia: mini review. APCBEE Procedia. 2012;2:129–134.
- Dreon MS, Fernández PE, Gimeno EJ, et al. Insights into embryo defenses of the invasive apple snail Pomacea canaliculata: egg mass ingestion affects rat intestine morphology and growth. PLoS Negl Trop Dis. 2014;8:e2961.
- Heras H, Garin CF, Pollero RJ. Biochemical composition and energy sources during embryo development and in early juveniles of the snail Pomacea canaliculata (Mollusca: Gastropoda). J Exp Zool. 1998;280:375–383.
- Ferreira ICFR, Baptista P, Vilas-Boas M, et al. Free-radical scavenging capacity and reducing power of wild edible mushrooms from northeast Portugal: individual cap and stipe activity. Food Chem. 2007;100:1511–1516.
- Khamhaengpol A, Siri S. Fluorescent light mediated a green synthesis of silver nanoparticles using the protein extract of weaver ant larvae. J Photochem Photobiol B Biol. 2016;163:337–344.
- Garin CF, Heras H, Pollero RJ. Lipoproteins of the egg perivitelline fluid of Pomacea canaliculata snails (Mollusca: Gastropoda). J Exp Zool. 1996;276:307–314.
- Dong C, Zhang X, Cai H, et al. Green synthesis of biocompatible silver nanoparticles mediated by Osmanthus fragrans extract in aqueous solution. Optik. 2016;127:10378–10388.
- Zagalsky PF. Comparative studies on the amino acid compositions of some carotenoid-containing lipoglycoproteins and a glycoprotein from the eggs and ovaries of certain aquatic invertebrates. Comp Biochem Physiol. 1972;41:385–395.
- Ahmed KBA, Kalla D, Uppuluri KB, et al. Green synthesis of silver and gold nanoparticles employing levan, a biopolymer from Acetobacter xylinum NCIM 2526, as a reducing agent and capping agent. Carbohydr Polym. 2014;112:539–545.
- Pasquevich MY, Dreon MS, Heras H. The major egg reserve protein from the invasive apple snail Pomacea maculata is a complex carotenoprotein related to those of Pomacea canaliculata and Pomacea scalaris. Comp Biochem Physiol B Biochem Mol Biol. 2014;169:63–71.
- Srikar SK, Giri DD, Pal DB, et al. Green synthesis of silver nanoparticles: a review. GSC. 2016;6:34–56.
- Basavegowda N, Rok Lee Y. Synthesis of silver nanoparticles using Satsuma mandarin (Citrus unshiu) peel extract: a novel approach towards waste utilization. Mater Lett. 2013;109:31–33.
- Lee J-H, Lim J-M, Velmurugan P, et al. Photobiologic-mediated fabrication of silver nanoparticles with antibacterial activity. J Photochem Photobiol B Biol. 2016;162:93–99.
- Jyoti K, Baunthiyal M, Singh A. Characterization of silver nanoparticles synthesized using Urtica dioica Linn. leaves and their synergistic effects with antibiotics. J Radiat Res Appl Sci. 2016;9:217–227.
- Wang L, Liu C-C, Wang Y-Y, et al. Antibacterial activities of the novel silver nanoparticles biosynthesized using Cordyceps militaris extract. Curr Appl Phys. 2016;16:969–973.
- Soman S, Ray JG. Silver nanoparticles synthesized using aqueous leaf extract of Ziziphus oenoplia (L.) Mill: characterization and assessment of antibacterial activity. J Photochem Photobiol B Biol. 2016;163:391–402.
- Durán N, Durán M, de Jesus MB, et al. Silver nanoparticles: a new view on mechanistic aspects on antimicrobial activity. Nanomedicine. 2016;12:789–799.
- Salari Z, Danafar F, Dabaghi S, et al. Sustainable synthesis of silver nanoparticles using macroalgae Spirogyra varians and analysis of their antibacterial activity. J Saudi Chem Soc. 2016;20:459–464.