?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
When hydrophobically modified chitosan (HM-CHI) comes into contact with red blood cells (RBCs), it can cause agglomeration. This property leads HM-CHI to be a potential agent to form a rapid haemostatic plug to stop bleeding at wound sites. In this study, we investigated the properties of the HM-CHI biopolymer to act as an agent that rapidly clots blood. We have examined the synthesis and structural characteristics of HM-CHI – blood substances using X-ray diffraction, infrared spectroscopy and elemental analysis. To understand the rheological behaviour of RBCs within HM-CHI, we have studied the effects of HM-CHI on RBC aggregation and morphology using a rheometer and scanning electron microscopy (SEM), respectively. When mixed with sodium citrate, HM-CHI rapidly transformed human blood into an elastic gel. In contrast, un-modified chitosan (i.e. without hydrophobes) was unable to clot blood. The hydrophobias within HM-CHI entered into the RBC membranes and connected to the cells by a sample-spanning network, which subsequently led to the formation of an elastic gel. The gelling ability of HM-CHI is similar to that of fibrin-based sealants, but at a much lower cost and greater availability.
Introduction
The blood coagulation cascade is an exquisite example of a responsive self-assembly process in biology [Citation1,Citation2]. When there is a wound, a cascade of self-assembly events correspondingly occur in the blood at the site of the wound [Citation3–6]. The net outcome of these events is globular protein and fibrinogen, which are catalysed by a second protein and thrombin to yield chains [Citation7]. A network of insoluble fibrin chains forms a haemostatic “plug” or clot, which creates a physical barrier preventing loss of blood from the wound site [Citation8,Citation9]. The coagulation cascade is a series of delicately balanced events to stop loss of blood. If it occurs too easily, blood clots could form at improper sites, possibly leading to strokes or other complications [Citation10].
For long time, scientists have been using the advantages of the fibrin coagulation process to produce haemostatic dressings and bandages [Citation11–13]. Fibrin-based haemostatic sealants were first developed in the 1940s and have been proven to be effective on preventing bleeding [Citation14–16]. For example, haemostatic sealants containing dry human fibrinogen and thrombin powder have been widely used in solid bandage backings to efficiently stop bleeding. A strong fibrin seal is formed to stop the bleeding when the bandage is tightly pressed onto the site of injury. However, human fibrinogen and thrombin are very expensive proteins and have very limited supplies [Citation17], which largely restricts the application of the fibrin bandages as an effective first aid device to a large number of general population.
Therefore, an inexpensive haemostatic agent that possesses equivalent blood-clotting properties to the fibrin is needed. Various advanced haemostats have been produced and brought to markets, but currently none of them have been found to have the matched efficacy of fibrin sealants [Citation18,Citation19]. Many of the products mainly function to absorb the blood at a wound site rather than to coagulate it. Ellis-Behnke et al. have recently reported a self-assembly synthetic peptide capable of forming a nanofibrous network to achieve haemostasis independency of the natural coagulation cascade [Citation20,Citation21].
Chitosan (CHI) is a primary candidate potentially developed as a wound-dressing material due to its superior mucoadhesive properties with biological tissues, haemostatic activities, low toxicity, relevant biodegradabilities and anti-infection capabilities [Citation22–24]. CHI is a cationic polysaccharide. The adhesive properties of CHI are derived from the ionic interactions of polysaccharides with tissues and mucous layers [Citation25,Citation26]. Low-molecular-weight CHI is known to enhance the interactions of wound-healing medicines with the epithelial cell surface. Despite these advantages, the rigid crystalline structure of CHI makes it have a low solubility in water. Therefore, its potential in the haemostatic applications is partially retarded. CHI has been used as a haemostatic material due to its cationic and antimicrobial properties. However, its haemostatic efficacy in the treatment of severe wounds is very limited and inadequate. Therefore, there are many researches focusing on improving the haemostatic ability of CHI. By combining carboxymethyl CHI (CMC) with serum albumin (SA) and collagen, Shi et al [Citation27] developed biocompatible microspheres that showed high haemostatic efficiency for quick intra- and post-operative haemostasis. Utilizing electrospinning methods, Gu et al [Citation28] fabricated CHI-gel nanofibre mats with enhanced haemostatic properties absent of any crosslinking agent.
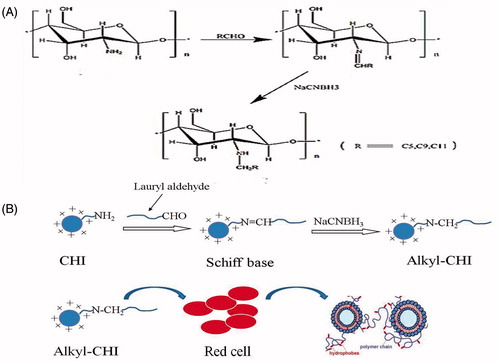
Early studies have reported that hydrophobically modified CHI (HM-CHI) has potential to be an effective haemostatic agent [Citation29] and applications in the pharmaceutical, cosmetics and textile fields [Citation30]. Recent study on the interaction between N-alkyl CHI and blood cells have reported that toxicity against human red blood cells and human epithelial Caco-2 cells was found to be proportional to the length of the alkyl chain [Citation31]. However, there is little investigation in characterizing the coagulated substance produced from HM-CHI with blood. This draws back the further applications of HM-CHI as a haemostatic material. In this study, we synthesized HM-CHI using the method reported previously by other researchers [Citation32]. Our hypothesized mechanism of HM-CHI haemostatic action (Scheme 1(B)) involves the anchoring of hydrophobes from the polymer into the hydrophobic interiors of blood cell membranes. Thereby, blood cells would become connected by biopolymer chains into a sample-spanning gel network, which could potentially halt the flow of blood. We probe this hypothesis in vitro via rheological studies. We investigated the agglomeration product of blood with the HM-CHI including verifying the structure and the degree of substitution of dodecyl HM-CHI with blood using an infrared spectrum and element analyser. We have also evaluated the morphological characteristics, the solubility and degree of hydrophobicity of HM-CHI. To fully understand the effects of HM-CHI on aggregation and morphology of red blood cells (RBCs), we have studied the rheological behaviour of HM-CHI derivatives with RBCs. We observed aggregation, gross distortion and agglomeration of HM-CHI with RBCs, suggesting that HM-CHI caused individual RBCs to clot. The evaluations carried out in this study ascertained the potential of HM-CHI to be used a haemostatic agent.
Materials and methods
Reagents
CHI of medium molecular weight (190–310 K), lauryl aldehyde and sodium cyanoborohydride were purchased from Sigma Aldrich (St. Louis, MO). Acetic acid (0.15 M) was purchased from Aladdin Chemistry Co. Ltd. (Shanghai, China). All other chemicals were of analytical grade and were not modified upon arrival.
Preparation of HM-CHI
The synthetic route used for HM-CHI is shown in Scheme 1(A), which was reported in a previous paper [Citation31]. Briefly, CHI (4 g) was dissolved in acetic acid solution (220 mL, 0.2 mol/L). After stirring at room temperature, 150 mL ethanol was added to the mixture. An appropriate amount of lauryl aldehyde was added to adjust the pH to 5.1. Precipitate formed after dripping sodium cyanoborohydride into the mixture until achieving a pH of 10. Then filtered and washed with 70 wt%, 80 wt% and 100 wt% ethanol/water solution to neutral. Finally, the product was ground into powder after freeze-drying. According to the mole ratio of CHI and lauraldehyde, HM-CHI with various grafting degrees was prepared.
Characterization of the polymer
The chemical structure of CHI and HM-CHI was analysed using a Fourier transform infrared (FT-IR) spectrophotometry (VERTEX 70; Bruker, Bremen, Germany) and 1HNMR spectrophotometry measured in D2O/CD3COOD (3:1, v/v) using a 300 MHz spectrometer (Bruker AVANCE Bremen, Germany).
Elemental analysis
An elemental analyser (Fujifilm, Minato, Tokyo, Japan) was used to evaluate the degree of HM-CHI substitution. Substitution degree was defined as 100 CHI alkyl substitution reactions generated from one unit number. Before and after substitution reactions, the number of nitrogen molecules did not change, but the number of carbon and hydrogen molecules did change. Therefore, based on the baseline nitrogen content, differences in the amount of carbon and hydrogen was used to determine the degree of substitution of N-alkylated CHI. The following equation was used to calculate the degree of substitution:
X-ray diffraction
Diffraction spectra of HM-CHI and CHI were measured using X-ray diffraction, which analysed the crystallization of alkylated CHI. Specific operating parameters included an accelerating current of 20 mA and voltage of 36 kV, a scanning range of 2 Θ (equals =4–40°) and speed of 2°/min, as well as a 0.02° step width (alpha) copper target (Kα) (Bruker Smart X-ray diffractometer; Bruker, Billerica, MA).
Scanning electron microscopy (SEM)
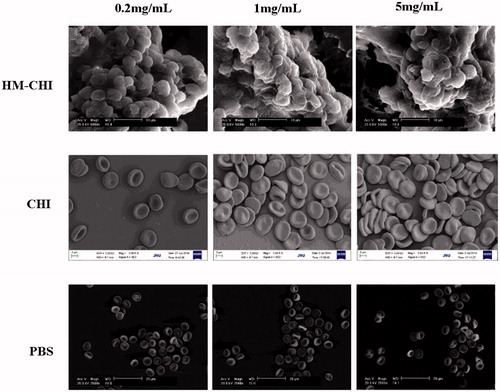
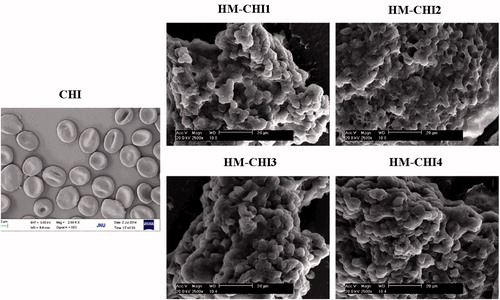
Citrated whole blood was centrifuged at 1000×g for 5 min. After removing the plasma and buffy coat layer, RBCs were washed with phosphate-buffered saline (PBS). Washed RBCs were incubated with solutions of unmodified chitosan and HM-CHI of different substitution degree for 10 min. With improvement of substitution degree, solubility of HM-CHI in the acetic acid solution decreased. Therefore, HM-CHI1∼HM-CHI4, as described in , was chosen for the detection of RBC morphology. Washed with PBS and then fixed with 4% paraformaldehyde overnight. Fixed RBCs were deposited on glass slides, dehydrated with 70%, 85%, 95%, 100% (v/v) ethanol for 10 min and then air-dried. Dried RBCs were coated with gold and visualized using SEM (Philips XL 30 SEM; SEMTech Solutions Inc., Billerica, MA).
Table 1. Data of the element analysis of N-alkylated CHI.
Rheology of a mixture of HM-CHI and blood
To eliminate autologous blood clotting, blood samples were treated with anticoagulation prior to rheology. The rheometer (Malvern Instruments, Malvern, Worcestershire, United Kingdom) was set to a component selection of 20 mm, a 4° angle of vertebral powder and 37 °C. This experiment was separated into two parts. In each case, we adjusted the polymer concentration in the overall mixture to 0.25 wt%. with 0.2 M acetic acid. First, we analysed the linear viscoelastic response under periodic loading. In brief, we quickly mixed 90 μL blood treated with sodium citrate or 90 μL RBC/PBS (1/1v/v) with 90 μL HM-CHI4 solution and following experiments is this degree of substitution. The controls consisted of CHI and PBS only. We placed 80 μL of the mixture on the plate to test for a change in the modulus. Second, we thoroughly investigated the viscosity of the mixture under shear force. Specifically, 90 μL of blood was mixed with HM-CHI or CHI solution and then transferred to the plate for the steady rheometry test.
Results and discussion
Synthesis and characterization of CHI derivatives
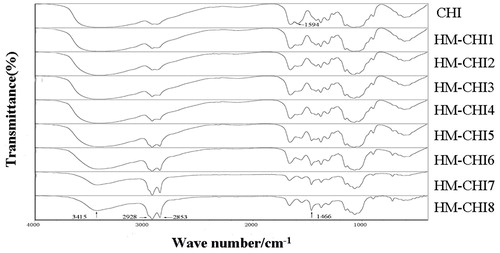
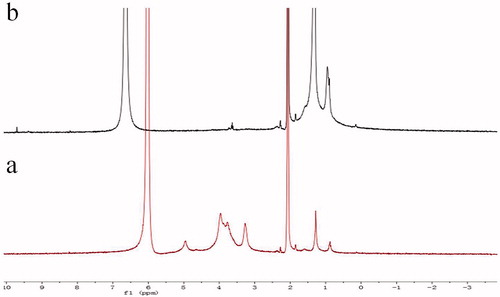
The structure of the synthesized conjugates was determined using FT-IR and 1HNMR. First, the amine groups of the CHI were reacted with the alkyl of lauryl aldehyde, which produced an CHI derivative. In the FT-IR spectra () shows the raw CHI and alkylated HM-CHI with different degrees of substitution. Compared with the raw CHI, the alkylated HM-CHI exhibited some differences on its IR spectrum such as the feeding ratio (aldehyde/CHI). It was clear that the band intensity of the stretching vibration band of CH3 (2928 cm−1) and CH2 (2853 cm−1) enhanced with an increase in the aldehyde/CHI ratio. Moreover, the in-plane bending vibration band (1466 cm−1) of CH3 and CH2 emerged together, indicating that the alkyl substitution reaction did occur. Additionally, the absorbance band of −NH2 (1594 cm−1) disappeared while N-H (1560 cm−1) increased. The IR spectrum illustrates that the substitution reaction occurred at the site of nitrogen atoms (-NH2). Furthermore, no remarkable changes occurred in the hydroxyl stretching vibration band (3415 cm−1). Successful synthesis of CS–DCA copolymers was confirmed with the 1H NMR spectra (). Comparing the spectra of HM-CHI and CHI, we concluded that the alkyl was successfully grafted onto the CHI from the appearance of new peaks at 9.6 ppm corresponded to the protons of CH2 groups of the alkyl.
Elemental analysis
lists the initial monomolecular ratio (NH2/aldehyde) and the relative contents of the elements as determined by elemental analysis. These were used to calculate the corresponding degree of substitution of different chitosan derivatives. In addition to the decreasing NH2/aldehyde ratio, a reduction in nitrogen content occurred as the carbon content and the corresponding degree of substitution for different CHI derivatives increased. Also, the degree of substitution (DS) of HM-CHI7 and HM-CHI8 was greater than 100, indicating that both derivatives had bi-substitution reactions because all of the hydrogen atoms bonded with nitrogen had been displaced by the alkyl residue in CHI.
X-ray diffraction analysis
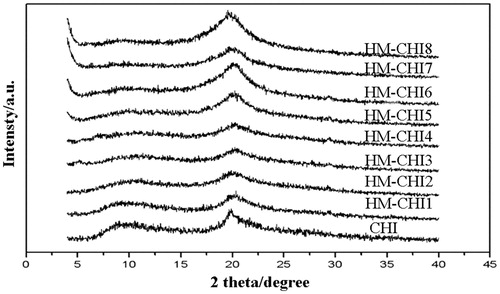
shows distinct diffraction peaks at 9° and 20° within the CHI crystal structure. Subsequently, as the degree of HM-CH substitution increased, the 9° diffraction peak was significantly suppressed, with the HM-CHI7 peak reducing to a straight line. This result indicates that the substitution reaction did occur. The crystal structure of the CHI derivatives changed by grafting the lauryl group; specifically, we observed a decrease in hydrogen bonding within CHI and broken integrity of the crystal structure.
HM-CHI mixture and blood rheology analysis
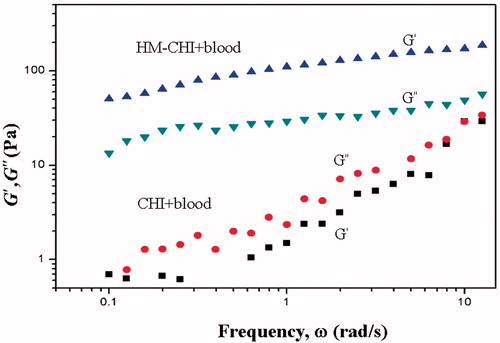
shows the results from the dynamic rheology analysis (oscillatory shear), which investigated the linear viscoelastic response of the samples [Citation33]. The data plotted are for the elastic (G′) and viscous (G″) moduli as a function of the angular frequency (ω). The HM-CHI/blood sample primarily exhibited an elastic response typical of a physical gel. It is important to note that G′ exceeds G″ over the entire frequency range and the moduli are weakly dependent on frequency. Weak frequency dependence on moduli is indicative of a sample-spanning network that is able to store shear deformation. This network relaxes very slowly over long time periods. Conversely, the CHI/blood sample exhibited a viscous response, in which both its moduli decreased sharply with frequency and G″ exceeded G′ over the frequency range.
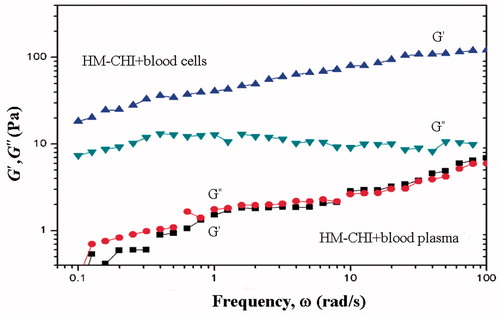
illustrates the differences in the experimental designs of the HM-CHI rheological analyses using blood plasma and RBCs. HM-CHI with RBCs under the dynamic rheological response revealed that gelation within the entire G′ was greater than G″. The graphic images show that HM-CHI with RBCs formed a gel. The viscosity of the CHI and plasma mixture exhibited a very weak response, with G″ within the scope of the angular frequency slightly higher than G′. After combining HM-CHI and RBCs, the dynamic rheological analysis revealed a gelation response, with the whole domain within G′ being greater than G″. The HM-CHI and plasma mixture exhibited a very weak viscous response, with G″ in the corner frequency range slightly higher than G′, indicating that after mixing the resolution is in a viscous state. These results indicate that HM-CHI is capable of clotting RBCs [Citation34–37].
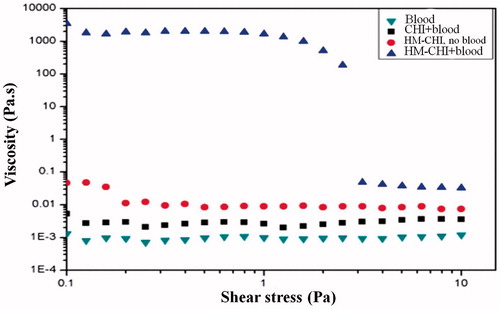
presents data for the same samples via static rheological experiments, where the apparent viscosity is plotted as a function of shear stress. The chitosan/blood sample had a constant viscosity of 0.007 Pa·s, approximately 8–9 times that of blood alone. A 0.25 wt% acetic solution of HM-CHI in water had a viscosity of 0.07 Pa·s in the low-shear limit, indicating that the sample was slightly viscous but far from being a gel. In contrast, the HM-CHI/blood sample had a low-shear viscosity of approximately 7000 Pa·s, which is a millionfold higher than that of blood. Also, in this case, the steep drop in viscosity around a stress of 2 Pa is indicative of a yield stress, meaning that the sample hardly flows at stresses below this value [Citation36–40].
Effects of HM-CHI on RBCs
In this study, the aggregation and morphological changes of RBCs were examined in the presence of the HM-CHI using SEM (). The PBS group did not cause RBCs to aggregate nor did it alter their morphology. In contrast, RBCs aggregated in the presence of HM-CHI and CHI. The most remarkable observations were with HM-CHI: we observed aggregation, gross distortion and agglomeration, indicating that HM-CHI can cause individual RBCs to clot.
We studied RBCs aggregation and morphology when adding HM-CHI and CHI at different concentrations. Next, we observed whether a change in the different degrees of substitution of HM-CHI affected RBC aggregation and morphology (). In terms of the CHI group, most RBCs maintained normal morphology even though some aggregated. HM-CHI did change RBC morphology and formed a clot. Additionally, no marked differences were observed among the effects from different degrees of substitution of the HM-CHI derivatives. From these results, we can deduce that the RBC-HM-CHI interaction is mainly mediated by hydrophobic interactions, because the amine group in CHI did not cause RBC deformation but HM-CHI did. Moreover, the hydrophobic interaction was not affected by a difference in the degree of substitution of HM-CHI.
Conclusions
We have shown that the amphiphilic biopolymer, HM-CHI, can act as an effective haemostatic agent. It has the ability to transform whole blood into a gel, and to quickly stop bleeding from both minor and severe injuries in small and large animals. The gelling mechanism is predicated on the self-assembly of HM-CHI chains, in such a way that their hydrophobes anchor into the RBC membrane, subsequently bridging the cells into a three-dimensional network. Thus, the cells are active components (nodes or junction points) in the network rather than being physically trapped in a polymer mesh. The gelling ability of HM-CHI is similar to that of fibrin-based sealants, but at a much lower cost and greater availability. Our research indicates that HM-CHI could be an effective and safe haemostatic agent for treatment of both external and internal bleeding injuries.
Acknowledgements
This study was supported financially by the Natural Science Foundation of China (51303064 and 31271019), the Ph.D. Programs Foundation of the Ministry of Education of China (20124401120015), the Science and Technology Program of Guangzhou (201601010270, 2017010160489), the Pearl River S&T Nova Program of Guangzhou (201710010155), and the Science and Technology Project of Guangdong province (2011B031300006, 2015A010101313).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Byshevskii A, Chiriat'ev EA, Umutbaeva MK. Isolation of fibrin assembly inhibitor from plasma and tissue extracts. Gematologiia i Transfuziologiia 1985;30:35.
- Macfarlane RG. An enzyme cascade in the blood clotting mechanism, and its function as a biochemical amplifier. Nature. 1964;202:498.
- Ding F, Deng H, Du Y, et al. Emerging chitin and chitosan nanofibrous materials for biomedical applications. Nanoscale. 2014;6:9477.
- Dowling MB, Kumar R, Keibler MA, et al. A self-assembling hydrophobically modified chitosan capable of reversible hemostatic action. Biomaterials. 2011;32:3351.
- Huang R, Li W, Lv X, et al. Nanoparticle-conjugated aptamer targeting hnRNP A2/B1 can recognize multiple tumor cells and inhibit their proliferation. Biomaterials. 2015;53:58.
- Zhou B, Hu Y, Li J, et al. Chitosan/phosvitin antibacterial films fabricated via layer-by-layer deposition. Int J Biol Macromol. 2014;64:402.
- Peerschke EI, Jesty J, Reid KB, et al. The soluble recombinant form of a binding protein/receptor for the globular domain of C1q (gC1qR) enhances blood coagulation. Blood Coagul Fibrinolysis. 1998;9:29.
- Fischer TH, Connolly R, Thatte HS, et al. Comparison of structural and hemostatic properties of the poly-N-acetyl glucosamine Syvek Patch with products containing chitosan. Microsc Res Tech. 2004;63:168.
- Ono K, Saito Y, Yura H, et al. Photocrosslinkable chitosan as a biological adhesive. J Biomed Mater Res. 2000;49:289.
- Lin KY, Lo JH, Consul N, et al. Self-titrating anticoagulant nanocomplexes that restore homeostatic regulation of the coagulation cascade. ACS Nano. 2014;8:8776.
- Arellano-Rodrigo E, Lopez-Vilchez I, Galan AM, et al. Coagulation factor concentrates fail to restore alterations in fibrin formation caused by Rivaroxaban or Dabigatran in studies with flowing blood from treated healthy volunteers. Transfus Med Rev. 2015;29:242.
- Nielsen VG. Effects of PentaLyte and Voluven hemodilution on plasma coagulation kinetics in the rabbit: role of thrombin-fibrinogen and factor XIII-fibrin polymer interactions. Acta Anaesthesiologica Scandinavica. 2005;49:1263.
- Whelihan MF, Kiankhooy A, Brummel-Ziedins KE. Thrombin generation and fibrin clot formation under hypothermic conditions: an in vitro evaluation of tissue factor initiated whole blood coagulation. J Crit Care. 2014;29:24.
- Alston SM, Solen KA, Sukavaneshvar S, et al. In vivo efficacy of a new autologous fibrin sealant. J Surg Res. 2008;146:143.
- Burnouf T, Goubran HA, Chen TM, et al. Blood-derived biomaterials and platelet growth factors in regenerative medicine. Blood Rev. 2013;27:77.
- Saif R, Jacob M, Robinson S, et al. Use of fibrin-based sealants and gelatin-matrix hemostats in laparoscopic liver surgery. Surg Laparosc Endosc Percutan Tech. 2011;21:131.
- Li ZJ, Fu X, Tian P, et al. Fibrin sealant before wound closure in total knee arthroplasty reduced blood loss: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2015;23:2019.
- Ishii K, Kawashima H, Hayama T, et al. Combination of a liquid fibrin sealant with sheet-type hemostatic agents: experimental evaluation in partial nephrectomy animal model. Int J Urol. 2011;18:478.
- Nie W, Yuan X, Zhao J, et al. In situ forming chitosan-based hydrogel as a lung sealant for biological lung volume reduction. Carbohydr Polym. 2013;96:342.
- Smith LA, Liu X, Ma PX. Effect of surfactant types on the biocompatibility of electrospun HAp/PHBV composite nanofibers. Soft Matter. 2008;4:2144.
- Zhang Z, Ma PX. Enhancing performances of solution-processed inverted ternary small-molecule organic solar cells: manipulating the host-guest donors and acceptor interaction. Macromol Rapid Commun. 2015;36:1735.
- Alsarra IA. Chitin and Chitosan as Functional Biopolymers for Industrial Applications. Int J Biol Macromol. 2009;45:16.
- Jayakumar R, Prabaharan M, Kumar PTS, et al. Fabrication of a porous wall and higher interconnectivity scaffold comprising gelatin/chitosan via combination of salt-leaching and lyophilization methods. Biotechnol Adv. 2011;29:322.
- Ueno H, Mori T, Fujinaga T. A novel thermally-activated crosslinking agent for chitosan in aqueous solution: a rheological investigation. Adv Drug Deliv Rev. 2001;52:105.
- Kim IY, Seo SJ, Moon HS, et al. The preparation and cytocompatibility of injectable thermosensitive chitosan/poly(vinyl alcohol) hydrogel. Biotechnol Adv. 2008;26:1.
- Sezer AD, Hatipoglu F, Cevher E, et al. Chitosan film containing fucoidan as a wound dressing for dermal burn healing: Preparation and in vitro/in vivo evaluation. AAPS PharmSciTech. 2007;8:39.
- Shi J, Zhao Y, Wu J, et al. Safety and efficacy of a sucrose-formulated recombinant factor VIII product for the treatment of previously treated patients with haemophilia A in China. Haemophilia. 2007;9:1478.
- Gu BK, Park SJ, Kim MS, et al. Neuropeptide substance-P-conjugated chitosan nanofibers as an active modulator of stem cell recruiting. Int J Biol Macromol. 2016;82:89.
- Dowling MB, Smith W, Balogh P, et al. Comparison of new hemostatic granules/powders with currently deployed hemostatic products in a lethal model of extremity arterial hemorrhage in swine. J Surg Res. 2015;193:316.
- Muzzarelli RAA, Tanfani F, Emanuelli M, et al. The characterization of N-methyl, N-ethyl, N-propyl, N-butyl and N-hexyl chitosans, novel film-forming polymers. J Membr Sci. 1983;16:295–308.
- Sahariah P, Benediktssdottir BE, Hjalmarsdottir MA, et al. Impact of chain length on antibacterial activity and hemocompatibility of quaternary N-alkyl and n,n-dialkyl chitosan derivatives. Biomacromolecules. 2015;16:1449–1460.
- Nguyen VQ, Ishihara M, Mori Y, et al. Development of antimicrobial biomaterials produced from chitin-nanofiber sheet/silver nanoparticle composites. Biomed Mater Eng. 2013;23:473.
- Khunawattanakul W, Puttipipatkhachorn S, Rades T, et al. Polymer–Magnesium Aluminum Silicate composite dispersions for improved physical stability of acetaminophen suspensions. Int J Pharm. 2008;351:227.
- Nordby MH, Kjoniksen AL, Nystrom B, et al. Internal structures of agar-gelatin co-hydrogels by light scattering, small-angle neutron scattering and rheology. Biomacromolecules. 2003;4:337.
- Wang Y, Qiu D, Cosgrove T, et al. The rheology of hydrogels based on chitosan–gelatin (bio)polyelectrolyte complexes. Colloids Surf B Biointerfaces. 2009;70:254.
- Bhise KS, Dhumal RS, Shailesh B, et al. Reduced ulcerogenic potential and antiarthritic effect of Chitosan–Naproxen Sodium complexes. AAPS PharmSciTech. 2010;11:226.
- Brader JM, Cates ME, Fuchs M. Schematic mode coupling theory of glass rheology: single and double step strains. Phys Rev E Stat Nonlin Soft Matter Phys. 2012;86:021403.
- Dos Santos ZM, Pereira MR, Fonseca JL. Removal of As(III) and As(V) from water by chitosan and chitosan derivatives: a review. Carbohydr Polym. 2013;98:321.
- Kempe S, Metz H, Bastrop M, et al. In vitro and in vivo evaluation of thermosensitive chitosan hydrogel for sustained release of insulin. Eur J Pharm Biopharm. 2008;68:26.
- Khare P, Jain SK. Influence of rheology of dispersion media in the preparation of polymeric microspheres through emulsification method. AAPS PharmSciTech. 2009;10:1295.