Abstract
The design of mild and non-toxic synthesis of metallic nanoparticles is a topical subject in the nanotechnology field. The objective of this present study is to screen the bioactivity of biosynthesized nanoparticles by aqueous fruit extract of Chaenomeles sinensis. The reducing and stabilizing ability of C. sinensis to fabricate gold (Cs-AuNps) and silver (Cs-AgNps) nanoparticles was confirmed by UV–visible (UV–Vis) spectroscopy at 562 nm and 477 nm, respectively. The field-emission transmission electron microscopy (FE-TEM) and X-ray diffraction analysis (XRD) verify the nano-scale morphology and crystallinity of Cs-AuNps (20–40 nm) and Cs-AgNps (5–20 nm). Furthermore, we evaluated the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical-scavenging capacity, antimicrobial activity against Staphylococcus aureus and Escherichia coli and cytotoxicity against breast cancer cells. The results showed that Cs-AuNps (IC50: 725.93 μg/mL) displayed superior inhibitory activities on DPPH than Cs-AuNps. The biosynthesized Cs-AuNps successfully inhibited the growth of pathogenic bacteria S. aureus (ATCC 6538) and E. coli (BL21). The cytotoxic effect of Cs-AuNps and Cs-AgNps was evaluated in murine macrophage (RAW264.7) and human breast cancer cell lines (MCF7) by MTT assay. Thus, the present study explores the biomedical applications of gold and silver nanoparticles synthesized by C. sinensis.
Introduction
The studies in nanoparticle field are currently areas of intensive scientific research due to an abundant range of potential applications, including biomedical, chemical, optical and electronic fields [Citation1]. Metal nanoparticles are of a great scientific interest owing to their ability to effectively bind bulk materials, such as drugs, prodrugs, antibodies and phytonutrients, which designate them as good delivery carriers. Moreover, the size, shape, charge and surface characteristics of metal nanoparticles can be easily manipulated, expediting the development of efficient drug nanocarriers. Furthermore, as nanoparticle’s size varies from 1 to 100 nm, it provides them with an ability to invade a particular cell, for instance, tumour cells.
However, the methods to synthesize metallic nanoparticles strive with challenges, such as the usage of toxic solvents, generation of hazardous by-products and excessive energy expenditure. To synthesize metallic nanoparticles in an environmentally benign way, three aspects of nanoparticle synthesis should be evaluated: choice of solvent, reducing and stabilizing agent. In our research, we present a rapid and green alternative of synthesizing of gold and silver nanoparticles using naturally occurring phytonutrients found in the aqueous extract of Chaenomeles sinensis fruit as both reducing and stabilizing agent. The reaction is carried out in distilled water without other chemical agents, such as sodium citrate (Na3C6H5O7•2H2O) or precarious sodium borohydride (NaBH4). Metal salts can be reduced and stabilized into nanoparticles by virtue of organic acids (i.e. malic acid, tartaric acid, limonin, vitamin C, tannin), which are the major phytochemicals of C. sinensis fruit [Citation2–4]. Bioreduction methods based on plants [Citation1,Citation5,Citation6], fungi [Citation7] and bacteria [Citation8] are more benefiting as the usage of harmless solvent, non-toxic reducing agents and eco-friendly materials reduce the risks of biomedical applications.
C. sinensis (Thouin) Koehne, commonly known as Chinese quince or “Guang Pi Mu Gua”, is widespread in Korea, China and Japan. The fruits have long been used in both Traditional Chinese Medicine and Korean traditional medicine to relieve inflammation, vitalize digestion and reduce cholesterol and sugar level [Citation9], alleviate rheumatoid arthritis, cough, common cold and remedy diarrhoea [Citation10]. The fruits of C. sinensis are reported to contain pentacyclic triterpene acids, flavonoids, lignans and simple phenolic compounds. Some of these compounds isolated from the extract of C. sinensis have been shown to possess antioxidant, antitussive, antiflatulent, antipruritic and diuretic activities [Citation3].
In the present study, we aim to synthesize novel metal nanoparticles by the aqueous extract of C. sinensis and evaluate their biological activities as a potential antioxidant, antimicrobial and anticancer agents antagonistic to breast cancer cells. Gold (Cs-AuNps) and silver (Cs-AgNps) nanoparticles were characterized by ultraviolet-visible (UV-Vis) spectrophotometry, field-emission transmission electron microscopy (FE-TEM), energy-dispersive X-ray spectroscopy (EDX), selected area electron diffraction pattern (SAED), X-ray diffraction analysis (XRD), dynamic light scattering particle size analysis (DLS) and Fourier transform infrared analysis (FTIR). Also, the synthesized metal nanoparticles were investigated for their antioxidant activity against 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals. Furthermore, Cs-AgNps were tested for their antimicrobial capacity against S. aureus and E. coli. Lastly, their cytotoxicity against breast cancer cells (MCF7) and murine macrophage cells (RAW264.7) was evaluated.
Methods
Materials
Dried fruits of C. sinensis were obtained from Ginseng Bank, Kyung Hee University, Gyeonggi-do, Republic of Korea. Gold (III) chloride trihydrate (HAuCl4•3H2O) and silver nitrate (AgNO3) was purchased from Sigma-Aldrich Chemicals (St. Louis, MO). The pathogenic bacterial strains used in the present study were Staphylococcus aureus [ATCC 6538] and Escherichia coli [BL21]. All bacterial growth media were purchased from Difco, MB Cell, Seoul, Korea. Standard Neomycin (NEO30) discs were purchased from Oxoid Ltd., England. DPPH and L-ascorbic acid (vitamin C) were purchased from Sigma-Aldrich Chemicals. Methyl alcohol (MeOH) was procured from Samchun Pure Chemical Co. Ltd., Gyeonggi-do, Korea.
Biosynthesis of Cs-AuNps and Cs-AgNps
The dried fruits of C. sinensis (10 g) were grinded to fine powder and dispersed in 100 ml distilled water. The suspension was autoclaved for an hour at 100 °C to obtain an aqueous extract. The resulting suspension was further filtered to remove unwanted solids and diluted with distilled water to reach a concentration of 70%. The diluted extract was stored at 4 °C for further use.
HAuCl4•3H2O (1 mM) was added into the diluted extract (70%) in room temperature for 10 s. Similarly, AgNO3 (1 mM) was mixed with the diluted extract at 80 °C for 65 min to initiate metal ion reduction. The change of colour in each reaction mixtures was continuously observed and indicated the formation of nanoparticles. After the synthesis, the biosynthesized gold and silver nanoparticles were collected by centrifugation (13,500 rpm for 15 min), washed thoroughly with sterile water at least thrice (to remove water-soluble biomolecules) and finally washed with 80% MeOH. The washed nanoparticles were kept overnight for air-drying and obtained in powder form for XRD and FT-IR characterization.
Characterization of Cs-AuNps and Cs-AgNps
The reduction of Ag+ ions to Ag0 and Au3+ ions to Au+ in the respective reaction mixture was monitored colorimetrically by UV-Vis spectroscopy (2100 Pro; Amersham Biosciences Corp., Piscataway, NJ) in the range of 300–750 nm. Images of nanoscale Cs-AuNps and Cs-AgNps and their atomic features were acquired by FE-TEM (JEM-2100 F, JEOL). D8 Advance (Bruker, Billerica, MA), with a set of designated configurations (40 kV; 40 mA; CuKα radiation 1.54 Å; a scanning rate of 6°/min; step size 0.02; over the 2θ range of 20–80°) confirmed the three-dimensional configuration and crystallinity of the nanoparticles. The average crystal size diameter of Cs-AuNps and Cs-AgNps were determined by Debye–Scherrer equation: D= Kλ/β cos θ, where D is the crystallite size in nm, K is dimensionless shape factor (0.9), λ is the X-ray wavelength of Cu-Kα radiation in nm, β is the full width at half maximum (FWHM) in radians, and θ is the half of the Bragg angle in radians. The hydrodynamic size distribution and polydispersity index of nanoparticles in suspension was analysed by DLS method. DLS data were performed at 23 °C with a particle size analyser ELSZ-2000 series (Otsuka Electronics Photal, Osaka, Japan). The functional groups present in the fruit extract of C. sinensis and their coherence with Cs-AuNps Cs-AgNps were determined by the FT-IR analysis. Immobilized nanoparticles and dried fruit extract were coated onto KBr pellet and measured by Perkin Elmer Spectrum One FT-IR spectrometer (Boston, MA) in the range of 4000–450 cm−1. The spectra recorded were plotted as transmittance (%) versus wavenumber (cm−1).
DPPH radical quenching assay of Cs-AuNps and Cs-AgNps
In vitro antioxidant activity of Cs-AuNps and Cs-AgNps was calculated using a modified method based on Brand–William’s method [Citation11]. Methanolic nanoparticle suspension of 20 μL was mixed with 180 μL of 1 mM methanolic DPPH solution. Different concentrations of Cs-AuNps and Cs-AgNps (100, 250, 500 and 1000 μg/mL) were selected. The radical scavenging activity was measured as percentage inhibition: [(Acontrol − Asample)/Acontrol] × 100, where Asample is the absorbance of the DPPH solution with nanoparticles and Acontrol is the absorbance of the DPPH solution without nanoparticles. Vitamin C was used as positive control. The reaction mixture was incubated at room temperature in dark condition for 30 min, and the colour change was measured by an ELISA reader at 517 nm. The experiments were executed in triplicates with a mean ± standard error.
Antimicrobial activity of Cs-AgNps
The antimicrobial activity of Cs-AgNps was examined by antimicrobial susceptibility test against pathogenic bacteria, S. aureus and E. coli, using disc-diffusion method. Hundred microliters of overnight log-culture bacteria of each strain was added into Mueller–Hinton agar (MHA) plates and spread equally, using glass spreader. Sterile paper discs were placed on MHA plates and impregnated by 30 μL (100 mg/L) freshly prepared biosynthesized silver nanoparticles suspensions. A standard antibiotic disc of neomycin was chosen as a positive control and maintained on each plate. After incubation at 37 °C for 24 h, the average diameter of each concentration’s zone of inhibition was measured. The experiments were executed in triplicates with a mean ± standard error calculated.
MTT cell proliferation assay
The cell viability RAW264.7 and MCF7 cell lines treated by Cs-AuNps and Cs-AgNps were evaluated using 3–(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2H tetrazolium bromide (MTT) (Life Technologies, Eugene, OR) assay. Cell lines were cultured in Dulbecco’s modified Eagles medium (DMEM) (Gibco-BRL, Grand Island, NY) supplemented with 10% foetal bovine serum (FBS) and 1% penicillin/streptomycin (P/S) (WelGENE Inc., Daegu, Korea) at 37 °C in a humidified atmosphere containing 5% CO2 and 95% air and were seeded at a density of 1 × 104 per well in a 96-well plate (Corning Costar, Lowell, NY). The wells were treated with different concentrations of Cs-AuNps (0, 1, 10, 25 and 50 μg/mL) and Cs-AgNps (0, 0.01, 0.1, 1 and 10 μg/mL) at 37 °C for 48 h at 90% confluency. After incubation for 48 h, 10 μL of MTT (5 mg/mL) in PBS solution was added to each well. The wells were further incubated at 37 °C for 4 h. Further, 100 μL of dimethyl sulphoxide (DMSO) was added to dissolve the insoluble formazan crystals into the coloured solution. An enzyme-linked immunesorbent assay (ELISA) reader (Bio-Tek Instruments, Inc., Vinooski, VT) was used to measure the absorbance of each reaction mixture. The optical density of formazan formed in untreated cells (negative control) represents 100% cell viability. The experiment was done in triplicates with a mean ± standard error.
Statistical analysis
All experiments were performed in triplicates and means with standard errors were calculated. The statistical significance of differences between values of the treated and untreated (control) groups was evaluated by Student’s t-test. Differences with p < .01 were considered significant.
Results and discussion
Biosynthesis and characterization of nanoparticles
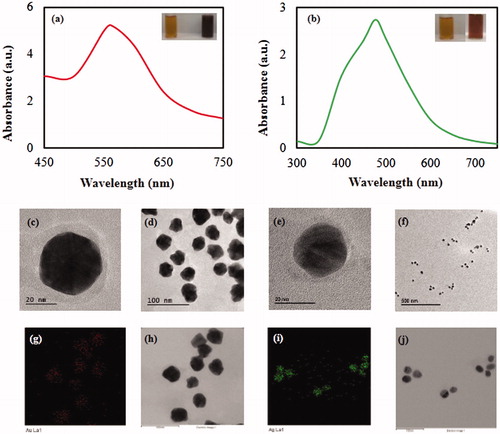
The biosynthesis of Cs-AuNps and Cs-AgNps by dried fruits extract of C. sinensis extract (70%, v/v) was monitored by UV-Vis spectrophotometer as shown in . Reaction mixtures containing gold salt were synthesized at room temperature while those containing silver salts were heated at 80 °C. The UV-Vis spectra showed major absorbance peaks at 562 nm after 10 s of incubation for Cs-AuNps and 477 nm after 65 min of heating for Cs-AuNps. Insets from demonstrate a colour conversion of colloidal gold and silver to dark purple and brown, respectively. Microscopic images captured by FE-TEM demonstrated spherical icosahedral Cs-AuNps with core sizes smaller than 40 nm () and spherical Cs-AgNps with metallic cores around 20 nm (). Spatial distributions of elemental gold and silver were evaluated by elemental mapping; both metallic cores were comprised of elemental gold (i.e. red) and silver (i.e. green), respectively, as demonstrated in ).
Figure 1. UV-Vis spectra of Cs-AuNps (a) and Cs-AgNps (b) with C. sinensis fruit extract. The upper right insets show that the resulting colloid suspensions are brown for Cs-AuNps (a) and purple for Cs-AgNps (b). FE-TEM images of Cs-AuNps (c, d) and Cs-AgNps (e, f). Elemental distribution of Cs-AuNps (g, h) and Cs-AgNps (i, j).
The crystallinity of the biosynthesized nanoparticles was evaluated by SAED and XRD techniques. The multiple electron diffraction patterns correspond to the polycrystalline nature of the nanoparticles () which conform to lattice planes of Bragg’s reflection (111), (200), (220) and (311) planes. Similarly, XRD spectra of Cs-AuNps () and Cs-AgNps () demonstrated identical diffraction peaks. Multiple negligible diffraction peaks in were present indicating the formation of bio-organic impurities in Cs-AgNps [Citation5]. No artificial diffraction peaks due to crystallographic impurities were evident in Cs-AuNps, indicating pure gold metal. Cs-AuNps and Cs-AgNps were face-centred cubic and primarily composed of (111) orientation [Citation12]. From this finding, the average crystallite sizes of nanoparticles have been estimated by Scherrer equation: Cs-AuNps and Cs-AgNps maintained average crystallite sizes of 9.87 and 9.03 nm, respectively. Moreover, the crystal structures of Cs-AuNps and Cs-AgNps have lattice constants of 4.07 Å and 4.08 Å, respectively, calculated experimentally from the most intense peaks (111) of XRD diffraction patterns. The FWHM (full width at half maximum) and particle diameter sizes which correspond to the polycrystalline diffraction peaks of Cs-AuNps and Cs-AgNps were tabulated in Supplementary Tables 1 and 2.
Figure 2. SAED patterns Cs-AuNps (a) and Cs-AgNps (d). XRD spectrum of Cs-AuNps (b) and Cs-AgNps (e). EDX spectrum of Cs-AuNps (c) and Cs-AgNps (f).
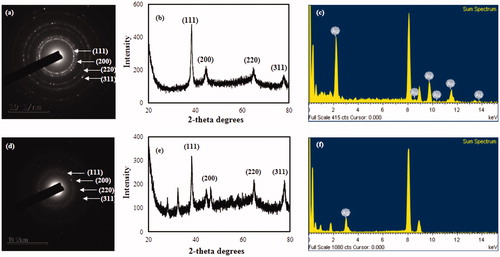
The EDX spectra () demonstrated highest optical absorbance peaks at 2.3 and 3 keV, which correspond to the characteristic peaks of gold and silver, respectively. The peaks recorded at 8 keV correspond to the copper grid used for analysis.
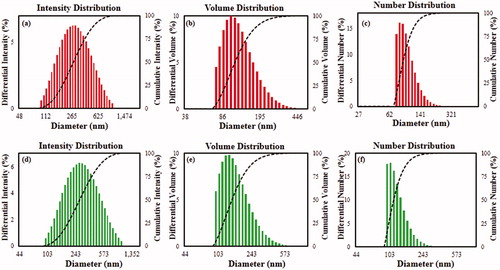
The size distribution profiles of Cs-AuNps and Cs-AgNps was performed using a particle size analyser with respect to intensity, number and volume by DLS method. Based on intensity distribution, DLS analysis revealed a range of Cs-AuNps () with a Z-average value of 195 nm and a PDI of 0.244 and Cs-AgNps () with a Z-average value of 269 nm and a PDI of 0.250. Since the PDI values were larger than 0.1, a critical value for mono-disperse particles, Cs-AuNps and Cs-AgNps were low-range poly-disperse [Citation13]. The thickness of the capping layer accounts for the discrepancy in the nanoparticle sizes analysed by XRD and DLS. DLS method measures the hydrodynamic size of nanoparticles in aqueous suspension, which includes the metallic core and any phytochemicals attached on the particle surface [Citation14].
Figure 3. Particle size distributions of Cs-AuNps (a, b, c) and Cs-AgNps (d, e, f) with respect to intensity, volume, and number.
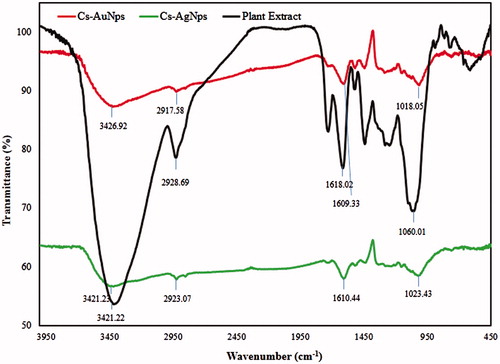
Surface chemical compositions of Cs-AuNps and Cs-AgNps were compared against dried fruits of C. sinensis by FTIR analysis to characterize surface adsorption on nanoparticle surfaces. The surface composition of Cs-AuNps and Cs-AgNps is dependent on the medium in which they are synthesized. As evaluated in , Cs-AuNps (red) and Cs-AgNps (green) acquired similar bands, which were exhibited by dried C. sinensis fruits (black). FTIR spectrum of dried fruits powder (, black) exhibited multiple intense bands at 3421.22 and 2928.69 cm−1 corresponding to the stretching of O–H bond of phenolic groups and C-H bond of alkanes, respectively [Citation15]. The presence of these bands suggests the adsorption of flavonoids and triterpene acids in the capping layer of the nanoparticles. Furthermore, peak at 1618.02 cm−1 and intense band located at 1060.01 cm−1 correspond to N = H bond and to the ether groups (C–O bond stretch), respectively.
DPPH radical quenching assay of Cs-AuNps and Cs-AgNps
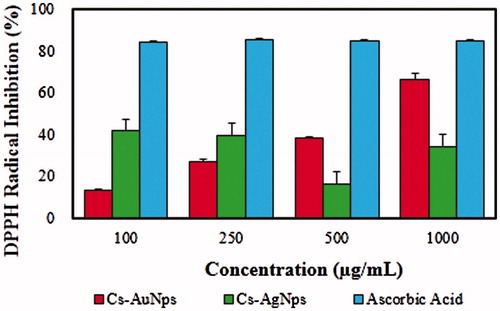
The antioxidant activity of Cs-AuNps and Cs-AgNps was evaluated against free DPPH radicals as shown in . DPPH consists of stable free radical molecules and is readily reduced by accepting hydrogen or electron from nanoparticles [Citation6]. shows the dose-dependent scavenging activity of Cs-AuNps and Cs-AgNps, while vitamin C of the same concentrations was maintained as a positive control. The IC50 values were determined by linear regression; the IC50 value of Cs-AuNps was determined to be 725.93 μg/mL. On the contrary, Cs-AgNps did not exhibit a dose-depending antioxidant activity against DPPH radical; at a concentration of 1 mg/mL only 34.13% of free DPPH radical was scavenged. In short, Cs-AuNps exhibit superior antioxidant activity than Cs-AgNps and demonstrate potential as novel antioxidant agents. The antioxidant activity of biosynthesized nanoparticles can be attributed to the antioxidant property of C. sinensis [Citation16]. These results suggest that the protective capping layer of Cs-AuNps and Cs-AgNps by aqueous fruit extract seems to be the major contributors to the free radical scavenging activity.
Antimicrobial activity of Cs-AgNps
Silver nanoparticles have been extensively reported to possess effective bactericide activity and have experienced a renewed interest as antimicrobial agents due to the emergence of antibiotic-resistant bacteria [Citation1,Citation17]. In this study, the antimicrobial activity of Cs-AgNps was investigated against model Gram-positive S. aureus and Gram-negative E. coli by disc-diffusion assay and compared against standard antibiotic neomycin as a positive control. Zones of inhibition around impregnating paper discs by Cs-AgNps began to form after 24 h of incubation at 37 °C (. The mean diameters of the inhibition zones were measured and tabulated in . Cs-AgNps exhibited dose-dependent activity from 15 to 45 μg/disc. Biosynthesized Cs-AgNps showed wider zones of inhibition against E. coli than S. aureus with the largest activity (14.66 ± 0.33 mm) exhibited at 45 μg/disc. This result suggests that Cs-AgNps possessed a superior cell inhibition against Gram-negative E. coli than Gram-positive S. aureus. The less susceptibility of S. aureus can be attributed to the fact that the peptidoglycan layer in Gram-positive bacteria is thicker than in Gram-negative bacteria [Citation18]. In summary, Cs-AgNps may be considered as a good antimicrobial agent.
Figure 6. Zones of inhibition of purified Cs-AgNps suspensions (15, 30 45 μL) and Neomycin (NEO30) as standard antibiotics as control against S. aureus (a) and E. coli (b).
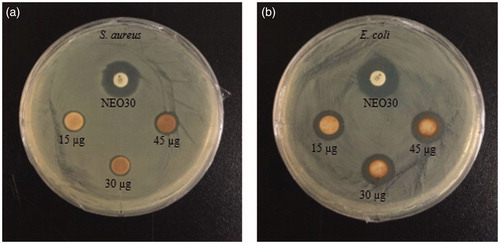
Table 1. Diameter of zone of inhibition (mm) of samples (30μL) containing purified Cs-AgNps.
Past studies have unveiled several possible mechanisms in which silver could inhibit the viability of microorganisms: binding with the thiol groups (S-H) which result in enzyme deactivation and increased cell uptake of Ag+ due to nanoparticle’s attachment onto the cell membrane surface which disrupts the hydrogen bonds in DNA molecules and effectively killing the cells [Citation19].
MTT cell proliferation assay
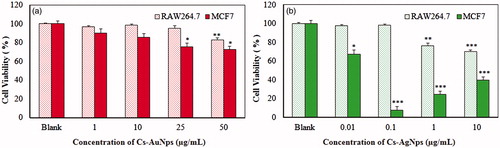
The cytotoxicity of the biosynthesized Cs-AuNps and Cs-AgNps was investigated in RAW264.7 and MCF7 cell lines as in vitro models for normal and cancerous cells. The cytotoxicity of nanoparticles is heavily influenced by the surfactants and medium at which they are synthesized. Based on , treatments of Cs-AuNps and Cs-AgNps on both cell lines resulted in dose-dependent decrease in cell viability; however, both nanoparticles exhibited higher cytotoxicity against MCF7 cells than RAW264.7 cells. When subjected to Cs-AuNps, significant cytotoxicity in MCF7 cells began to be observed at >25 μg/mL, whereas no significant cytotoxicity was observed in RAW264.7 cells at 25 μg/mL. Significant cytotoxicity toward breast cancer cells was amplified when cells were treated with Cs-AgNps; the viability of breast cancer cells was notably reduced at a concentration of 0.01 μg/mL. At a concentration of 0.1 μg/mL, less than 10% of breast cancer cells were observed to be viable, while no cytotoxicity effect was detected in RAW264.7 cells. Based on MTT results, these nanoparticles inherently possess the ability to inhibit the proliferation of breast cancer cells without significantly repressing the growth of healthy cells. Due to these unique biological activities and a more environmentally friendly synthesis, Cs-AuNps and Cs-AgNps offer a better alternative than the existing gold and silver nanoparticles manufactured by physical and chemical methods for medical applications.
Conclusions
The usage of environmentally benign and inexpensive resources provides a broad range of opportunities for further investigation of nanoparticle in the biological field. In the present study, we utilized the dried fruit extract of C. sinensis to reduce and stabilize nanoparticles without adding auxiliary hazardous chemicals. Cs-AuNps and Cs-AgNps have been successfully obtained through a green synthesis and demonstrated icosahedral shape with core sizes smaller than 40 nm and spherical shape with metallic cores around 20 nm, respectively. The bioactivities of the biosynthesized nanoparticles were screened and analysed in vitro for antioxidant, antimicrobial and cytotoxicity activities against breast cancer cells. Cs-AuNps demonstrated superior free radical scavenging activity against DPPH radicals than Cs-AgNps. Cs-AgNps, in its turn, were effective antimicrobial agents against pathogenic E. coli and S. aureus. Finally, these nanoparticles inherently possess the ability to inhibit the proliferation of breast cancer cells without significantly repressing the growth of healthy cells as evaluated by MTT assays. There has been a multitude of researches made in nanobiotechnology field. Consequently, application of biological sources, such as medicinal plants, will create a new standard for the synthesis of various nanoparticles with significant therapeutic and pharmacological effects.
Supplemental_content.zip
Download Zip (20.5 KB)Acknowledgement
This research was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries, Republic of Korea (KIPET NO: 316065–3).
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- Singh P, Kim YJ, Wang C, et al. The development of a green approach for the biosynthesis of silver and gold nanoparticles by using Panax ginseng root extract, and their biological applications. Artif Cells Nanomed Biotechnol. 2016;44:1150–1157.
- Sancheti S, Sancheti S, Bafna M, et al. Antihyperglycemic, antihyperlipidemic, and antioxidant effects of Chaenomeles sinensis fruit extract in streptozotocin-induced diabetic rats. Eur Food Res Technol. 2010;231:415–421.
- Oku H, Ueda Y, Ishiguro K. Antipruritic effects of the fruits of Chaenomeles sinensis. Biol Pharm Bull. 2003;26:1031–1034.
- Dubey SP, Lahtinen M, Sillanpää M. Tansy fruit mediated greener synthesis of silver and gold nanoparticles. Process Biochem. 2010;45:1065–1071.
- Markus J, Wang D, Kim YJ, et al. Biosynthesis, characterization, and bioactivities evaluation of silver and gold nanoparticles mediated by the roots of Chinese herbal Angelica pubescens Maxim. Nanoscale Res Lett. 2017;12:46.
- Pérez ZEJ, Mathiyalagan R, Markus J, et al. Ginseng-berry-mediated gold and silver nanoparticle synthesis and evaluation of their in vitro antioxidant, antimicrobial, and cytotoxicity effects on human dermal fibroblast and murine melanoma skin cell lines. IJN. 2017;12:709–723.
- Silva LP, Bonatto CC, Polez VLP. Green synthesis of metal nanoparticles by fungi: current trends and challenges. Advances and Applications Through Fungal Nanobiotechnology: Springer; 2016. p. 71–89.
- Markus J, Mathiyalagan R, Kim YJ, et al. Intracellular synthesis of gold nanoparticles with antioxidant activity by probiotic Lactobacillus kimchicus DCY51T isolated from Korean kimchi. Enzyme Microb Technol. 2016;95:85–93.
- Zhang LH, Li SF. Effects of micronization on properties of Chaenomeles sinensis (Thouin) Koehne fruit powder. Innov Food Sci Emerg Technol. 2009;10:633–637.
- Sawai R, Kuroda K, Shibata T, et al. Anti-influenza virus activity of Chaenomeles sinensis. J Ethnopharmacol. 2008;118:108–112.
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol. 1995;28:25–30.
- Edison TJI, Sethuraman M. Instant green synthesis of silver nanoparticles using Terminalia chebula fruit extract and evaluation of their catalytic activity on reduction of methylene blue. Process Biochem. 2012;47:1351–1357.
- Kallumadil M, Tada M, Nakagawa T, et al. Suitability of commercial colloids for magnetic hyperthermia. J Magn Magn Mater. 2009;321:1509–1513.
- Singhal G, Bhavesh R, Kasariya K, et al. Biosynthesis of silver nanoparticles using Ocimum sanctum (Tulsi) leaf extract and screening its antimicrobial activity. J Nanopart Res. 2011;13:2981–2988.
- Adina C, Florinela F, Abdelmoumen T, et al. Application of FTIR spectroscopy for a rapid determination of some hydrolytic enzymes activity on sea buckthorn substrate. Rom Biotechnol Lett. 2010;15:5738–5744.
- Hamauzu Y, Yasui H, Inno T, et al. Phenolic profile, antioxidant property, and anti-influenza viral activity of Chinese quince (Pseudocydonia sinensis Schneid.), quince (Cydonia oblonga Mill.), and apple (Malus domestica Mill.) fruits. J Agric Food Chem. 2005;53:928–934.
- Wang C, Singh P, Kim YJ, et al. Characterization and antimicrobial application of biosynthesized gold and silver nanoparticles by using Microbacterium resistens. Artif Cells Nanomed Biotechnol. 2016;44:1714–1721.
- Silhavy TJ, Kahne D, Walker S. The bacterial cell envelope. Cold Spring Harb Perspect Biol. 2010;2:a000414.
- Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009;27:76–83.