Abstract
Uncontrolled haemorrhage is one of the leading causes of death. This issue is present in controlled environments, such as hospitals, as well as pre-hospital and remote locations. Treatment is more challenging in remote locations where there is a lack of effective products to deliver oxygen and control coagulation. Poorly treated haemorrhage can lead to rapidly deteriorating bodily conditions that can result in organ failure and tissue death. Thus, the availability of products to support oxygen delivery to tissues and coagulation processes within the body is essential for the effective treatment of severe haemorrhage, particularly in out-of-the-hospital settings. The presence of such products would fill the gap that is currently present in emergency treatment. Promising results of an ex-vivo study on a novel haemoglobin-based oxygen carrier OxyVita®C with coagulation capacity (OVCCC) are presented in this article. The proprietary protein protection technology allows for the powderization of protein components without changes in their characteristics and physiological activity. This technology was applied to the oxygen carrier OxyVita®C, to plasma and to platelets. The functionality of all tested components, as well as a mixture of OxyVita®C and platelets, was studied. The results suggest future clinical trials investigating the powderization of OVCCC, plasma and platelets are warranted. The development of this powderization method offers a huge advancement into a field in which no viable products exist.
Introduction
Treatment of extensive blood loss/uncontrolled haemorrhage continues to be a challenging issue in medical emergencies, particularly those that occur outside fully equipped medical facilities. Various methods/protocols of treatment have developed and been refined recently as to be applied in these situations [Citation1–3]. Initially, a first-responder’s (medic) role was to apply physical pressure to initiate coagulation and/or replace blood with an IV fluid. Developments of this treatment method included the maintenance of sufficient blood pressure for transport to a medical facility. Blood replacement typically involved the use of a crystalloid or colloid resuscitation fluid (i.e. Ringer’s solution or Hextend). These developments have markedly shown improvements in treatment success, but did not offer a solution to the total satisfaction of medical help providers [Citation4,Citation5]. Unfortunately, the clinical capacity of treatments is here limited by acting only to replace lost blood volume, and not oxygen delivery and coagulation abilities of lost blood, which become significantly impaired due to the blood loss associated with haemorrhage. Persistent low haemoglobin oxygen saturation resulting in the loss of blood oxygen carrying capacity and correspondingly reduced tissue oxygenation is a recognized indicator of poor patient outcome [Citation6,Citation7]. Traditional blood volume replacement approaches dilute coagulation factors and platelets present in blood causing the reduced ability of body coagulation functions, simply by depletion [Citation6]. In many cases, the overuse of simple blood volume replacement fluids may also lead to patient hypothermia. The interplay between hypothermia, dilutional coagulopathy and concomitant acidosis, often referred to as the “lethal triad”, has been linked to high mortality [Citation1,Citation2].
The recognition of the substantial impact of reduced tissue oxygenation and low haemoglobin oxygen saturation, as well as the problematic depletion of coagulation factors in severe haemorrhage, has prompted the implementation, within definitive care facilities (DCF), of an Advanced Trauma Life Support (ATLS) protocol in conjunction with a massive transfusion protocol (MTP). This MTP utilizes a transfusion of RBCs and plasma in a 1:1 ratio, with decrease in patient mortality. A similar decrease was evident with an MTP that involved a combined treatment of RBC, plasma and platelets in a 1:1:1 ratio [Citation4]. Unfortunately, this refined method of treatment is not available in remote/combat situations, and sometimes, even in a hospital environment, the platelets’ short stability makes its usage tricky and not always effective. The problems with the usage and the lack of whole blood, packed RBC, liquid platelets/frozen plasma in remote/combat conditions is due to many factors, such as short stability time, absence of refrigeration, blood typing/matching issues, unpredictable and variable environmental conditions, and the unavailability of adequate infusion product and media [Citation3–5].
Over the last several years, scientists at OXYVITA, Inc., have developed a red blood cell alternative for the in-vivo oxygen delivery (OxyVita® haemoglobin). This product is applicable when whole blood or RBCs are not available. OxyVita®C, a new-generation haemoglobin-based oxygen carrier (HBOC) [Citation8,Citation9], is produced using a modified zero-linked polymerization process that employs chemical activators to incorporate cross-linked bovine haemoglobin tetramers into “super-polymeric” macromolecules. The liquid form of this HBOC is currently being studied in advanced pre-clinical studies [Citation10]. Due to the unique physiochemical properties of this polymeric haemoglobin, a powder form of this oxygen carrier can be produced through a proprietary modified lyophilization process developed by OXYVITA, Inc. The powder form of OxyVita® can be easily reconstituted into an injectable (IV) liquid state (reconstituted within 10 s in IV water). The structural and functional characteristics of the reconstituted form of this HBOC are identical to the original liquid product () [Citation11,Citation12].
Table 1. Physicochemical properties of OxyVita®C liquid and powder preparations.
Scientists at OXYVITA, Inc., have applied a unique protection process of the proteins with improved and modified lyophilization/spray-drying process to the product, in order to powderized OxyVita®C. The process was proven to allow for molecular preservation of the HBOC and unchanged physiological activity of the product. This motivated us to apply the same technology in the lyophilization of other blood components (plasma and platelets) which are directly involved in coagulatory function [Citation9].
This paper presents introductory results and efforts in working towards the development of a viable mixture of the above-mentioned products that includes components that are mutually compatible without alteration of their individual functions, exist in a powder form for ease of storage and transportation within a remote or combat environment and are able to be reconstituted in a physiological injectable (IV) fluid for immediate use. This approach is consistent with current treatment protocols (meets the MTP requirements) that are based on blood component replacement therapy.
Experimental methods
Preparation of OxyVita®C
OxyVita®C (carbonyl form) was manufactured and made available for these studies by OXYVITA, Inc. (New Windsor, NY). This polymeric haemoglobin is generated by the formation of pseudopeptide bonds between carboxyl groups and amino groups on the surface of bovine cross-linked tetrameric Hb molecules using a chemical activation process [Citation7,Citation8,Citation13–15]. OxyVita®C was engineered to its final formulation by scientists in OXYVITA, Inc., over the past several years.
Preparation of OxyVita® haemoglobin powder
The powder form of OxyVita®C is produced by the lyophilization of the liquid form of this protein, using a lyophilization instrument (Virtis, Inc., Sacramento, California). The instrument allows for the production of this product under specific control parameters and has the capabilities of monitoring (CFR/211 compliant software) the essential steps associated with the freeze-drying process as well as automatic sealing of the product, thus reducing the chances of any endotoxin introduction during the course of the operation.
Before lyophilization, the HBOC is treated with a protective agent that will provide a molecular environment that allows to maintain structural integrity and enhance the thereafter reconstitution (solubility) within IV fluids. In creating the powder form of OxyVita®C consideration has been given to a number of different formulations that include the need for proper buffering, essential electrolytes and final osmolarity critical for infusion applications (IV).
Characterization of the physiochemical properties of OxyVita®C liquid and powder forms
Determination of the physiochemical properties of OxyVita®C before and after lyophilization included: analysis of these samples by size exclusion chromatography (SEC using Fractogel 20–40 μm packing), dynamic light scattering (DLS, DynaPro 801, Protein Solutions, Wyatt Technology, Santa Barbara, CA), UV/visible spectroscopy from 700 to 240 nm (HP 8552 A, diode array recording spectrophotometer), oxygenation behaviour, pH of solutions and osmotic pressure (osmolality) measurements (Advanced Osmometer Model 3D, Advanced Instruments, Inc., Norwood, MA).
Preparations of plasma and platelets in powder form
Platelets and plasma were collected from the volunteer and prepared according to the standard preparation method. The only change in the standard preparation method was that the washed buffer was enriched by adding 1.5% (w/v) of mixture of sucrose and trehalose for the platelets and that the plasma was stored with the addition of 1.5% (w/v) of mixture of sucrose and trehalose.
Lyophilization of plasma and platelets was carried out using our Virtis instrument (Virtis, Inc.). The Virtis instrument has the capabilities of monitoring the essential process steps of the freeze-drying operation as well as automatically sealing of the product, which reduces the chances of introducing any endotoxin during the course of plasma and platelet lyophilization. Prior to the initiation of lyophilization, each of the materials to be lyophilized is treated with proprietary molecular protectant (“protectant OxA” – composition of it to be patented) for a specified incubation time allowing for proper diffusion of the protectant across the solution and the necessary intermolecular interactions to exposed protein components. The procedure is currently part of a patent application. The concentration of the proprietary protectant employed during the lyophilization process is carefully prepared, as it plays an important role in preserving the structural and functional integrity of the components undergoing the freeze-drying process. This method of preparation provides protection for the structural integrity of the proteins/particles and/or its cell membrane components, which allows for the reconstitution thereafter to products of equivalent functionalities. This protective treatment also enhances the solubility of these materials upon reconstitution.
Coagulation measurements of individual components and mixtures
Evaluation of the viability of lyophilized platelets was carried out on a PAP-8 (Bio-Data) light transmission aggregometer using adenosine-5′-diphosphate (ADP) and collagen as the agonists. Standard procedures were followed as outlined by Bio-Data’s protocol. All determinations were carried out at room temperature. Fresh platelets, which were obtained from the volunteer and prepared, were used as controls for these experiments. Once the individual components were produced via lyophilization, various combinations of these components (HBOC, plasma and/or platelets) were mixed to establish mutual compatibility, integrity and functionality.
Results and discussion
Scientists at OXYVITA, Inc., have developed a proprietary technology, which allows lyophilization/spray-drying of its HBOC as well as biological coagulation components (plasma and platelets) without altering the specific properties of these components. The MW (17MDa) and size (36 nm) of the OxyVita®C polymers after reconstitution are similar to the solution form from which the powder is produced, indicating maintenance of protein structural integrity during lyophilization/spray-drying (). A summary results of the physiochemical properties of OxyVita®C () show the similarities of the original liquid form of this HBOC and the powder form after its reconstitution back into a water solution. Reconstitution (solubility) time of these powder forms of OxyVita®C is 10–30 s in water (). The liquid form of this polymer is currently in advanced pre-clinical studies at a number of independent laboratories as a dedicated oxygen delivery protein [Citation9,Citation10,Citation13–15].
The proprietary process which affords specific molecular protection to OxyVita®C has now been applied to protect and next lyophilize human coagulation components (plasma and/or platelets). After the powderization and followed up reconstitution, the functionality of powderized plasma and platelets were observed using microscopy after the addition to the blood samples ().
Human platelets obtained within hospital/blood bank settings have a shelf life limited to 5 days of storage. Through implementation of our proprietary technology, we have determined that it is possible to extend the storage life of these freeze-dried platelets to at least 30 days, maintaining functionality as evident from repeated coagulation studies using a PAP-8 analyzer (Bio-Data) ( and ). These ex-vitro studies were carried out using adenosine-5′-diphosphate (ADP) and collagen as the agonists for coagulation initiation. Several examples of the coagulation behaviour profiles of the stored lyophilized platelets are shown in and comparing these to the control platelets (marked as: Freshly Prepared Platelets, Day 0). All samples tested for their activity are from powderized platelets that have been stored at room temperature for 30 days.
Figure 1. Lyophilized platelet activity versus time in the presence of collagen, a natural agonist, analyzed on a PAP-8 light scattering aggregometer. 30 Days – Dried Platelets sample analyzed were prepared from lyophilized platelets stored at room temperature for 30 days. FPPLA–Day 0 (Fresh Prepared Platelets – control) was a sample of fresh obtained from patience platelets. Day 0–Dried Platelets, reconstituted platelets reconstituted in the same day of preparation. The sample named: OVCD:PLTD (1:1) Day 0 was prepared from the powders of OxyVita®C dried product and powderized platelets. The same amounts of powders were mixed together, dissolved in IV water and afterwards analyzed. This particular sample was prepared at 50% concentration of platelets in the sample.
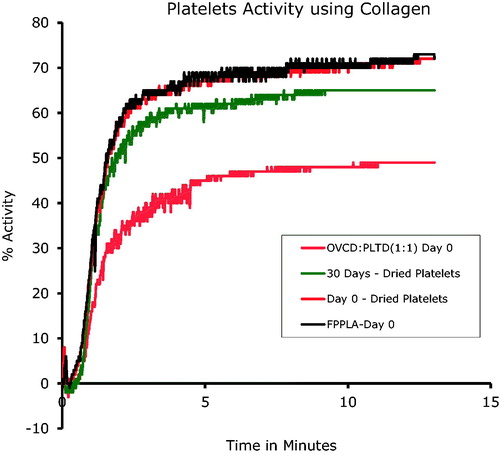
Figure 2. Lyophilized platelet activity versus time in the presence of ADP, a natural agonist, analyzed on a PAP-8 light scattering aggregometer. 30 Days – Dried Platelets sample analyzed were prepared from lyophilized platelets stored at room temperature for 30 days. FPPLA–Day 0 (Fresh Prepared Platelets – control) was a sample of fresh obtained from patience platelets. Day 0 – Dried Platelets, reconstituted platelets reconstituted in the same day of preparation.
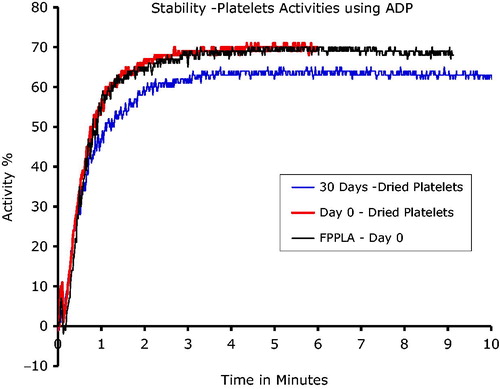
These studies demonstrate the similar coagulation capacity of lyophilized platelets compared to fresh platelets (∼78%), using a PAP-8 analyzer. After 30 days of powder storage, only a 7% decrease in activity was observed (). Reconstitution time of the powder platelets into appropriate media was ∼15 s.
Additional work is needed to prove the ruggedness of the method. The most interesting is application of the methodology to the platelets’ protection and powderization in order to extend their storage time, as the very short shelf life of platelets (5 days) poses a lot of challenges in their application in patient treatment.
In order to evaluate the feasibility of creating a useful reconstituted fluid that would incorporate both oxygen delivery capacity and coagulation components into a single unit for combat situations, various combinations of OxyVita®C, lyophilized plasma and/or platelets were studied to establish their mutual solubility within several different IV fluids. To establish whether each component maintained its individual function, a series of studies were carried out to evaluate the oxygen delivery capacity and the coagulatory behaviour of each of these mixtures. All studies included a determination of mutual solubility, UV/visible spectroscopy to examine potential molecular interactions between components and coagulation behaviour of the various combinations of components using the PAP-8 instrument. We provide data from the PAP-8 instrument () with OxyVita C: platelets in a ratio of 1:1. In order to maintain the same sample volumes for the analyses on PAP-8, this particular sample was prepared at 50% concentration of platelets in the sample, considering all other samples to be at 100% platelets’ concentration ().
Conclusion
The goal of this work was to develop a method for protein protection in order to allow for the powderization of some blood components involved in a coagulation process, as well as an attempt for the initial characterization of the combined product proposed for treatment for haemorrhagic hospital/combat/emergency casualties that would provide oxygen delivery and/or the enhancement of coagulation activity, as proposed for a treatment mode by current MTP. These studies have provided significant evidence of mutual compatibility for the potential application and use of an oxygen delivery protein, OxyVita®C, in conjunction with the addition of essential coagulatory components, plasma and/or platelets, to address haemorrhagic combat situations. The unique contribution of this work is the novel way and these components have been prepared for lyophilization/spray-drying (powderization), using a molecular protectant process developed by OXYVITA, Inc., that allows for retainment of structure and functionality and enhances the reconstitution (solubility) back into a liquid state for IV treatments. The overall results of this effort clearly provide the following conclusions: (1) the powder form of the protected components produced via proprietary lyophilization/spray-drying is mutually soluble and this lyophilization/spray-drying process preserves individual component physiological functions (), (2) the powder form is ideal for this mode of treatment, as it allows for the storage easily, without refrigeration, in any climatic environment and for the transport within field operations with greater ease, and (3) the powder forms can be reconstituted in different infusion media, as needed, and the presented products can be used by the medic as an individual or combined “cocktail” treatment, depending on the medics’ assessment of needs.
Figure 3. Example of RBC aggregation – (a) and (c) in saline. The same sample (a) of RBC in the presence of lyophilized plasma (b) and same sample of RBC (c) in the presence of lyophilized platelets (d). The picture was made after 5 minutes of interaction with either plasma or platelets, classical rouleaux formation occurred. These samples were viewed under standard light microscopy conditions.
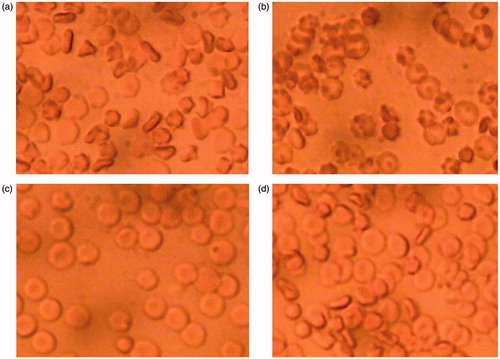
Additionally, the positive results in application of the new method of protection and lyophilization of the proteins encourage further experimentation in application to the other physiologically important proteins, to possible extend the shelf life and afford the storage in powder form for some of them.
Acknowledgements
The authors thank prof. Wojciech Maksymowicz for initiation of mutual cooperation to allow for the research of a novel OxyVita®C product in the Department of Pharmacology and Toxicology, Faculty of Medical Science, University of Warmia and Mazury in Olsztyn.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Lynn M, Jeroukhimov I, Klein Y, et al. Updates in the management of severe coagulopathy in trauma patients. Int Care Med. 2002;28:S241–S247.
- Hoyt DB, Bulger EM, Knudson MM. Death in the operating room: an analysis of a multi-center experience. J Trauma. 1994;37:426–432.
- Moore FA, Nelson T, McKinley BA, et al. Massive transfusion in trauma patients: tissue hemoglobin oxygen saturation predicts poor outcomes. J Trauma. 2008;64:1010–1023.
- Shaz BH, Dente CJ, Harris RS, et al. Transfusion management of trauma patients. Anesth Analg. 2009;108:1760–1768.
- Holcomb JB, Wade CE, Michalek JE, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248:447–458.
- Spahn DR, Rossaint R. Coagulopathy and blood component transfusion in trauma. Br J Anaesth. 2005;95:130–139.
- Razynska A, Bucci E. Zero-link polymerization: a new class of polymeric hemoglobins. In: Tsuchida E, editor. Blood substitutes: present and future perspectives. Lausanne (Switzerland): Elsevier; 1998. p. 265–279.
- Harrington JP, Wollocko H. Molecular design properties of OxyVita hemoglobin, a new generation therapeutic oxygen carrier: a review. J Funct Biomater. 2011;2:414–424.
- Jahr JS, Weeks DL, Desai P, et al. Does OxyVita, a new-generation hemoglobin-based oxygen carrier, or Oxyglobin acutely interfere with coagulation compared with normal saline or 6% hetastarch? An ex vivo thromboelastography study. J Cardiothorac Vasc Anesth. 2008;22:34–39.
- Reynolds PS, Barbee RW, Skaflen MD, et al. Low-volume resuscitation cocktail extends survival after severe hemorrhagic shock. Shock. 2007;28:45–52.
- Harrington JP, Wollocko H, Wollocko A, et al. Next generation hemoglobin based oxygen carrier, OxyVita C, with coagulation capacity using a modified lyophilization process: protection of components [Internet]. 3542-Pos B697, 29 January 2013. [cited 2013 Feb 06] Available from: http://doi.org/10.1016/j.bpj.2012.11.3803
- Harringrton1 JP, Wollocko J, Kostecki E, et al. Development of a multifunctional resuscitation fluid (MRF) incorporating coagulation components (Resusix™), (Stasix.) with a new generation hemoglobin‐based‐oxygen carrier (OxyVita®C): initial compatibility studies. Combat Casualty Care Research Program, U.S. Army Medical Research and Material Command Conference, August 2012.
- Matheson B, Kwansa HE, Bucci E, et al. Vascular response to infusions of a nonextravasating hemoglobin polymer. J Appl Physiol. 2002;93:1479–1486.
- Rebel A, Ulatowski JA, Kwansa H, et al. Cerebrovascular response to decreased hematocrit: effect of cell free hemoglobin, plasma viscosity, and CO2. Am J Physiol Heart Circ Physiol. 2003;285:1600–1608.
- Mito T, Nemoto M, Kwansa H, et al. Decreased damage from transient focal cerebral ischemia by transfusion of zero-linked hemoglobin polymers in mouse. Stroke. 2009;40:278–284.
