Abstract
This study was aimed to prepare, characterize and evaluate in situ gel formulations based on a blend of chitosan (CS), polyvinyl alcohol (PVA) and gellan gum (Gelrite™) for a sustained ocular delivery of besifloxacin (BSF). The developed formulations were evaluated for physicochemical properties, gelation time (Tsol-gel), rheological behaviour, antimicrobial efficacy, pharmacokinetic assessment, gamma scintigraphy study and ocular irritation. The results showed BSF sol-gel system were found to be sensitive enough which underwent instantaneous phase transition upon getting physiological stimulation. The ex vivo permeation experiments indicated that the developed formulation was able to enhance the retention of BSF at corneal surface. The HET-CAM confirmed the non-irritancy of developed formulation and also demonstrated the ability of ocular protection against strongly irritant substances. The results of gamma scintigraphy study revealed the higher concentration of drug retains at the corneal surface. In addition, optimized BSF sol-gel system showed enhanced anti-bacterial activity compared to BSF suspension.
Introduction
Besifloxacin (BSF) is a topical synthetic fluoroquinolone and represents the first chlorofluoroquinolone developed specifically for the topical treatment of ophthalmic infections [Citation1,Citation2]. The broad spectrum activity of BSF includes potent in vitro activity against drug-resistant strains such as ciprofloxacin-resistant, methicillin-resistant S. aureus and methicillin-resistant coagulase-negative Staphylococcus species [Citation3–6]. Besifloxacin ophthalmic suspension 0.6% (Besivance®; Bausch & Lomb, Tampa, FL) is approved by the US FDA for the treatment of bacterial conjunctivitis. Extraocular bacterial infections require an antibiotic treatment regimen to reduce the complications associated with chronic conditions [Citation7]. The action of antibacterial agents depends upon the achieving and maintaining the therapeutic concentration of drug at the site of infection for a prolonged time [Citation8] without compromising the intraocular region [Citation9]. The eye drop is the most common delivery system but have shown rapid pre-corneal drainage, requires frequent instillation, high drug concentration required, which usually leads to a pulse kinetics pattern of drug concentration [Citation10]. The major limitation is the attainment and retention of optimum drug concentration at the site of action within the eye. Most of the systems are applied as solutions or suspensions and the rapid pre-corneal elimination observed with conventional ocular formulations ends in poor drug bioavailability. To overcome the problem of conventional dosage form, there is a need to designing a delivery system that gives a good retention and sustained ocular delivery of the drug. So, these may be overcome by formulating the formulation that undergoes instantaneous in situ gel formation upon ophthalmic administration. They undergo gelation after instillation due to physicochemical changes occurring in the eye. It increases the pre-corneal residence time and better bioavailability of the drug can be achieved by formulating in situ gel [Citation11–14]. Extensive investigations have been done for the development of new systems of ophthalmic drug delivery with prolonged retention time on the eye surface, reduced dosing frequency and improving permeation through trans-corneal layers. Among the novel formulations tried for ocular delivery, in situ gel is a promising approach that is comparable in the ease with available marketed formulations and longer retention at the corneal surface due to its unique property of sol to gel conversion upon physiological stimulation like temperature, pH or ionic interaction and easy to development with low cost. In situ gel system for ocular drug delivery is one of the novel approaches, investigated thoroughly with a number of combinations of available polymer [Citation15–17]. The current work discussed an in situ gel system composed of chitosan, PVA and gellan gum. Chitosan (CS) and PVA-based in situ gel system have been evaluated successfully for the surface property of the blend [Citation16], rheological characterization [Citation18] and its practical utility in drug delivery [Citation19]. Each component present in the system has its unique property, which ultimately fortifies the system efficacy. CS is a cationic polymer that acts as mucoadhesive and penetration enhancer and it also shows phase conversion upon the change in pH and temperature [Citation20,Citation21]. PVA is a polymer provides gel strength [Citation16], while gellan gum shows ion-activated phase transition behaviour.
The present work describes the formulation and evaluation of BSF sol–gel system. The phase transition takes place on the surface by a combination of the triple mechanism (temperature, pH and ion activation) at the cornea. At the time of instillation, dosage form is in solution phase and soon later upon coming in contact with STF, it turns into transparent gel depot. The prepared sol–gel systems were characterized for in vitro gelation, drug release, antibacterial activity, pharmacokinetic study and ocular retention study.
Material and methods
Materials
Besifloxacin was obtained as a generous gift from Avanscure Life Sciences Private Limited, Gurgaon, Haryana. Chitosan (Degree of deacetylation 85–90%, MW 150 kDa) and PVA (MW 30,000–70,000 Da) was purchased from Fluka Co. Ltd., Switzerland, and Sigma Chem. Co. (St. Louis, MO), respectively. Gellan gum (Gelrite® CP Kelco, Atlanta, GA) was obtained as a gift sample from Applied Biosciences, Mumbai, India. All other chemicals and solvents used were of analytical grade.
Selection of polymer
There are many polymers alone or in the blend were screened for the selection of the optimized polymer composition. The preliminary selection of the best composition of polymers was done on the basis of their consistency and gelling capacity into STF. The different polymers used were methylcellulose, ethyl cellulose, hydroxy propyl methyl cellulose (different grades), sodium alginate, chitosan, gelrite, poloxamer and hydoxy propyl cellulose.
Solubility study
Solubility study was performed by dissolving one unit of BSF in one unit of solvent (Buffer). The solubility of BSF was evaluated in different buffer solution viz., phosphate buffer USP (pH 6.5 and 7.5), acetate buffer I.P. (pH 4.6, pH 4.8), boric acid buffer (pH 4.8) and citrophosphate buffer B.P. (pH 6.0 and 6.2). Initially, 1 mg of BSF was dissolved in 1 mL of the buffer after complete solubility more amount of drug was added. The solution was then kept for 24 h to check re-precipitation [Citation22].
Formulation of BSF sol–gel system
The different concentration of chitosan solution was prepared by dissolving weighed amount of chitosan in 1% v/v acetic acid in the boric acid buffer. PVA and gellan gum solution were prepared separately by dissolving the weighed amount in preheated ultrapure water (80 °C) with continuous stirring [Citation23]. The calculated amount of besifloxacin (0.6%) was dissolved in boric acid buffer (pH 4.8) and both the solution mix together get a homogenous in situ gel formulation with BSF concentration (0.3% w/v) in the final formulation ().
Table 1. Formulation table of besifloxacin sol–gel system with observed gelling capacity.
Characterization of sol–gel system
Gelling capacity
Aqueous solutions of varying concentrations of gelrite and chitosan were prepared and evaluated for viscosity and gelling capacity in order to identify the compositions suitable for use as in situ gelling systems. Gelation pH is the point at which physiological change from sol to gel occur upon getting the change in pH. The polymer solution was thoroughly mixed with simulated tear fluid in a ratio of 90:10 by volume. Thus, junction zones that were due to spontaneous nucleation would not be formed in the gel. The measurements were performed in the presence of ions simulating the physiological situation in the eye [Citation16].
Rheological studies
The rheological studies of the developed formulation were carried out on a cone and plate geometry viscometer (Brookfield RVCP DV-III). The viscosity and shear stress of the sample solutions were measured at various shear rates at 25 °C and 37 °C, respectively. The samples were equilibrated on the plate for 5 min. to reach the running temperature prior to each measurement. A typical run comprised of changing the shear rate from 0–200 s−1 at a controlled speed, a 0.1 min wait at 200 s−1, and finally, a decrease in shear rate to 0 s at the same controlled ramp speed.
Clarity, pH and osmolarity
Clarity of the developed formulations was checked by visual examination of both sol and gel form against a white and black background. The pH of the formulations was determined by digital pH meter. Osmolarity is the prerequisite for any developed ophthalmic product, which was confirmed for the developed in situ gel formulation by Osmometer (Fiske Associate, USA).
Drug content
The drug content was determined by diluting 1 mL of the besifloxacin in situ gel formulation with 10 mL with freshly prepared simulated tear fluid having pH 7.4. The dilution was filtered and further diluted with same to get an appropriate concentration. The BSF concentration was determined using a UV-Visible spectrophotometer at 290 nm (Shimadzu 1700, Japan) using simulated tear fluid as blank.
Ex vivo transcorneal permeation
The study was performed with goat eyeballs that were procured from a local slaughterhouse and were maintained in ice-cold normal saline during transportation to the laboratory. The corneas along with 4–5 mm of surrounding sclera tissue were removed cautiously and stored in freshly prepared simulated tear fluid (STF) (pH 7.4) until further experimentation. The goat cornea was placed in diffusion cell chamber (area of 1 cm2) having donor and receiver compartment. The simulated tear fluid (20 mL) was placed in receiver compartment and 1 mL of both BSF suspension and BSF in situ gel was kept in donor compartment. The study was performed at 37 ± 0.5 °C using the thermostat with continuous stirring. The fixed volume (2 mL) of the sample was withdrawn from receiver compartment at the predetermined time interval and the same volume of was replaced to maintain sink condition. The sample was filtered and amount of drug permeation was analysed using HPLC [Citation24].
Histopathology study
The histopathological study of BSF in situ gel formulation was done on excised goat cornea followed the same procedure as ex vivo transcorneal permeation study for 6 h. The treated cornea was removed, washed and immediately fixed with 8% (w/w) formalin solution for stability. The tissue was dehydrated with an alcohol gradient, put in melted paraffin and solidified in block form. Cross sections were cut, stained with haematoxylin and eosin, and microscopically examined by electronic microscope (MOTIC, Japan) and compared with control (untreated cornea).
Antimicrobial efficacy study
The comparative agar diffusion test method was used to assess the antimicrobial efficacy of the BSF in situ gel with BSF suspension. The sterile agar petri plates were incubated with test organisms Pseudomonas aeruginosa and S. aureus to check both samples. Both sterile sample of BSF in situ gel and BSF suspension (1 mL) were poured into the cups and allowed to incubate at 37 °C ± 0.5 °C up to 24 h. The comparison of the BSF in situ gel was assessed over BSF suspension in terms of measuring zone of inhibition (ZOI).
Ocular irritation studies
The HET-CAM test was performed as an alternative to the Draize rabbit eye test to check the ocular tolerance using fertilized hen egg. The fertilized hen eggs were collected and incubated in a humidified incubator at 37 ± 0.5 °C and 55 ± 7% RH for a period of 9 days with manual rotation every day. On the 10th day of incubation with the use of a fine and blunt forceps, the egg shell was scratched around the air cell. After careful removal of the inner egg membranes, the vascular CAM was exposed and (0.5 mL) of BSF sol gel, 0.1 M NaOH and normal saline (0.9%) were applied to the membrane at room temperature as test formulation, positive and negative control respectively. At specific time interval (0, 0.5, 2, 5 min) the irritant effect was observed in the membrane, blood vessels and albumin (haemorrhage, vasoconstriction and coagulation) were observed [Citation20,Citation21,Citation25,Citation26].
Ocular pharmacokinetic study
The ocular pharmacokinetic study was performed by instillation of both BSF suspension and optimized BSF sol-gel system (50 μL) into rabbit eyes. Approximately, 50 μL of aqueous humour was collected at 0, 0.5, 1, 2, 4, 6 and 12 from one rabbit before pre-treatment and after post-treatment. The aqueous humour samples were collected in Eppendorf tubes, sealed and stored at −20°C until HPLC analysis. Pharmacokinetic parameters were calculated by non-compartmental analysis, also called as model-independent analysis using Win Non-Lin version 4.0 (Pharsight Corp., Mountain View, CA). Peak aqueous humour concentration (Cmax) and time of its occurrence (Tmax) were read directly from the individual drug concentration–time profiles. The area under the concentration–time curve (AUC0–t) was calculated according to the linear trapezoidal method.
In vivo ocular retention study
Scintigraphy study of BSF sol–gel system was assessed by the gamma camera (Millenium VG) using radioactive compound Technetium (99mTc) on rabbit eye. Besifloxacin sol–gel was radiolabelled using 99mTc as radioactive agent and stannous chloride as the reducing agent, by direct labelling method. Ketamine HCl injection (i.m. 15 mg/kg) was used to anaesthetize rabbits. Radiolabelled formulation (20 μL) was dropped into the left eye and positioned 5 cm in front of the probe. The recording was started immediately after the administration and continued for 60 min using 128 × 128 pixel matrix. The rate of drainage of radioactivity from the eye was calculated by plotting the time activity curve. A whole body static image was also taken after 2 h of administration of drug/formulation.
Stability study
The stability of the besifloxacin-loaded in situ gel was done as per ICH guidelines [Citation27]. The BSF sol–gel (10 mL) was stored in closed amber colour glass vials at different accelerated condition, that is, 25 ± 2 °C, 60%, 40 ± 2 °C, 75% RH and 5 ± 2 °C (refrigerated condition). The sample was withdrawn at predetermine time interval, that is, 1, 3 and 6 month and analysed the clarity, pH, viscosity and drug content.
Result and discussion
Selection of polymer
In the present study, a combination of CS, PVA and gellan gum (Gelrite®) were selected for the development of novel sol–gel carrier in ocular drug delivery. The number of reports is available regarding safety and use of chitosan in an ocular formulation [Citation16,Citation19]. The CS polymer having bioadhesive as well as and its ability to convert into hydrogel upon on physiological stimulated tear fluid (at pH >6.5) [Citation28,Citation29]. PVA has also been reported for its biocompatibility and unique gelling characteristics that provide adhesive characteristic to this polymer [Citation30]. Gellan gum (Gelrite®) solution also show unique property of ion-induced gelation and has been used successfully for ocular delivery [Citation16,Citation31]. Thus, the present combination of used polymer was supposed to be activated by a number of mechanisms, which may also result in fortifying activity in terms of quick response and better gel strength.
Solubility study
For the development of any ophthalmic preparation, proper selection of vehicle is very important because of eye is very delicate organ of body and vehicle must be nontoxic, isotonic and sterile. In that condition, mostly buffer solution used which having pressure (tonic) to the physiological fluid of eye. The boric acid buffer (pH 4.7) was selected for the development of the formulation because it is isotonic to physiological fluid of eye and antimicrobial activity.
Formulation of sol–gel system
Different batches of the in situ gel system with varying concentration of polymer were developed and optimum concentration of optimized formulation was selected on the basis of gelling capacity as shown in .
Characterization
Clarity, pH and drug content and osmolarity
BSF sol–gel system was evaluated for clarity test and was evaluated at the black and white background and all formulations were found to be clear (). The all formulations were liquid at room temperature with pH range 4.7–5.2 and it converted into gel phase at pH 7.4, that is, tear fluid with isotonic to physiological eye fluid (tear fluid). The drug content of all developed formulations was found within the range of 99.31 ± 3.5 to 100.25 ± 2.5%. The osmolarity of all formulations was determined by Osmometer and found to 280.51 ± 2.43 to 334.23 ± 3.21, respectively. The osmometer of optimized formulation was found to be 305.43 ± 1.31, that is, closer to osmotic pressure of tear fluid ().
Table 2. Physicochemical evaluation parameters of besifloxacin sol–gel system.
Table 3. Comparative antimicrobial assessment study of optimized besifloxacin sol–gel system (CG2) and besifloxacin suspension.
Gelation study
The gelation study of sol–gel system was determined in vitro by using simulated tear fluid which are shown in . All formulations showed different gelling capacity with varying strength. The strength of gel is depends upon the polymers concentration and its ratio. The gelling strength increases on increasing the concentration of chitosan and gellan gum in respective manner.
Table 4. Comparative pharmacokinetic parameter of optimized besifloxacin sol–gel system (CG2) and besifloxacin suspension.
Rheological studies
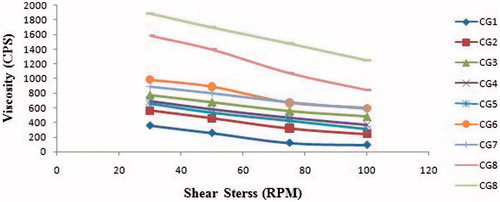
The rheological behaviours of all formulations in the gel form were evaluated at 25 °C and shown in . The viscosities of the formulations diluted by artificial tear fluid were significantly increased by increasing the concentration of chitosan and Gelrite at fixed angular viscosity (50 RPM). The viscosity of all formulation decreases on increased the shear stress, It indicated that formulation exhibit pseudo-plastic behaviour and shear thinning system due to interconversion gel phase to sol phase. The formulation CG2 was selected as optimized formulation having the combination of chitosan: Gelrite, (0.5:0.25%) because it immediately converted into the gel on an application of tear fluid. The administration of ophthalmic preparations should have as little effect as possible on the pseudo-plastic character of the precorneal film [Citation32]. Since the ocular shear rate is very high, ranging from 0.03 s−1. During blinking [Citation33], viscoelastic fluids with a viscosity that is high under low shear rate conditions and low under the high shear rate conditions are often preferred ().
Ex vivo transcorneal permeation study
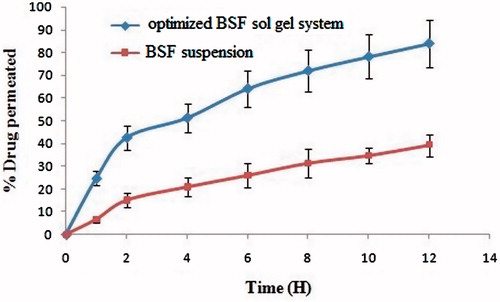
The result obtained for trans-corneal permeation was also in accordance with the hypothesis in which a tri-system was proposed and supposed to have fortified activity due to its unique combination. The trans-corneal permeation of the besifloxacin-loaded sol–gel was found to be 83.95%, which were significantly 2.5 times higher than (p < .01) besifloxacin drug suspension, which has shown 39.26%. The higher permeation of sol–gel system is due to bioadhesive gelling and penetration enhancing the property of chitosan and Gelrite. A possible reason for the observed behaviour may be of penetration enhancement by dual mechanism, that is, CS and hydrogel formation. Hydrogels are supposed to absorb water from the mucosa, which subsequently results in transient widening of tight junction [Citation34] ().
Histopathology study
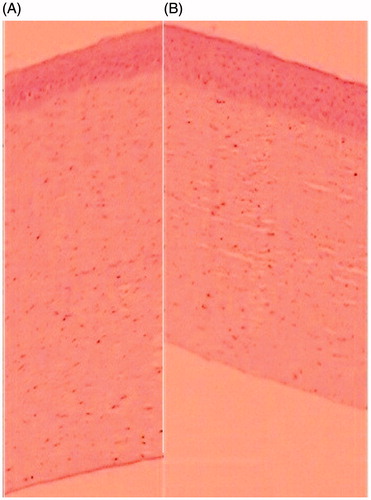
Histopathological study of the formulation was performed for the determination of ocular compatibility or ocular tolerance. It was found that the image showed there is no destruction of the layer of the cornea after application sol–gel system and compared (). The results showed the normal structure and morphology in both control and treated of excised cornea and did not exhibit any histopathological changes. The cells in the corneal membrane were found intact, same in position and no any rupture in the corneal section compared to the control. Hence, it can be concluded that optimized BSF sol–gel system was found to be safe for ocular application and free from any ocular irritation effect.
Antimicrobial study
The results obtained from antimicrobial efficacy were also found to be satisfactory in terms of retention of antimicrobial efficacy of the BSF sol gel. The results of the study are shown in . The optimized BSF sol gel showed prolonged effect than BSF suspension. The significant difference was achieved at 24 h, the developed in situ formulation (5.2 cm against P. aeruginosa and 4.8 cm against S. aureus in 24 h) able to show the effect at 24 h, whereas the drug suspension effect was decreased (1.5 cm against P. aeruginosa and 1.8 cm against S. aureus).
Ocular irritation study
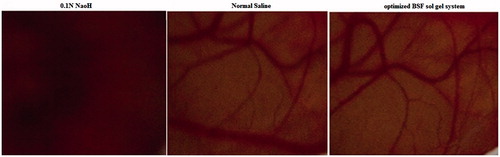
This method of irritation study is a rapid, sensitive and inexpensive method for checking the irritation potential of ophthalmic formulations. The chorioallantoic membrane of the chick embryo have complete tissue including arteries, veins and capillaries and technically responds to injury with a complete inflammatory process, similar to that induced in the conjunctival tissue of rabbit eyes (). The optimized BSF sol–gel was tested for ocular irritation assessment and it was compared with the 0.1 N NaOH and normal saline. The score was measured up to 5 min and the results showed that optimized BSF sol gel and normal saline were practically non-irritating. The images showed that there is no visible corneal damage was observed, which further prove the safety of the presented system. The 0.1%N NaOH solution showed coagulation implied that the solution produced irritating effects. From the study, it was concluded that optimized BSF sol–gel system is non-irritant and well tolerated for ocular administration.
Pharmacokinetic study
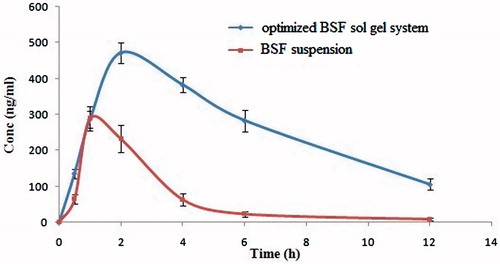
The pharmacokinetic parameters in aqueous humour such as Cmax, Tmax, t1/2, AUC0–t, AUC0–∞, were obtained from the pooled concentration–time; data are shown in . It was found that in drug solution; the drug was detectable in aqueous humour after 12 h in very low concentration, due to rapid pre-corneal drainage of solutions. In contrast, optimized BSF sol–gel system the drug (BSF) was detected in aqueous humour for at least 12 h (105.28 ± 3.35 ng/mL) indicated sustained release profile (). The AUC0–t of optimized BSF sol–gel system and BSF suspension were 3204.36 ± 14.53 ng/mL. h and 839.25 ± 23.43 ng/ml. h. Cmax of sol–gel system was approximately 1.63-fold (471.54 ± 10.23 ng/mL) higher than BSF suspension (289.29 ± 14.72 ng/mL). Higher Cmax of sol gel system due to the positively charged mucoadhesive chitosan and gel strength property of gelrite, thus maintaining prolonged corneal drug concentration gradient. The results also indicated that sol–gel system are able to deliver optimum levels of drug into aqueous humour and show promise for ocular delivery for the management of surface inflammatory disease.
Ocular retention study
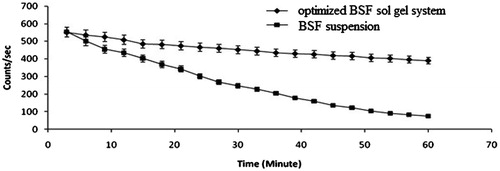
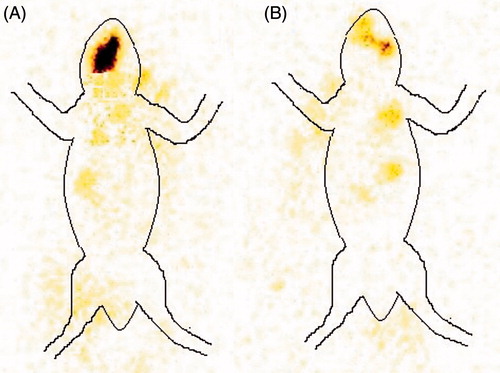
BSF was radiolabeled using Tc-99 m which was selected based on its moderate half-life (6 h) and its ability to radiate low energy γ radiation in comparison to α and β rays, which is not supposed to cause any serious hazard. Instant thin-layer chromatography (ITLC) was used to check the labelling efficiency and acetone was used as mobile phase. The principal of determining labelling efficiency is based on the difference in molecular weight. Due to high-molecular-weight drug–Tc complex remain at the base of the ITLC strip, while Rf value for free Tc reaches to 0.9 approximately. Thus, the labelling efficiency was determined by measuring the difference in top and bottom counts. Stannous chloride in the concentration of 100 μg/mL and pH 6.5 was optimized as reducing agent. Maximum labelling efficiency up to 98.4% with minimum colloids 1.1% were produced in optimized conditions with the stability of the complex up to 24 h. A very good retention was observed in the case of optimized BSF sol–gel system in comparison to BSF suspension which was observed by the absence of radioactivity in systemic circulation, while the distribution of radioactivity was observed in the case of drug solution (). The better retention shown by optimized BSF sol–gel system may be due to dual mechanism, that is, bioadhesive nature of chitosan and sol–gel conversion in biological milieu. Time–activity curve was also plotted to measure the remaining activity on the corneal surface as a function of time (). The results obtained by time activity curve further confirms that the developed optimized BSF sol–gel system shows better retention in comparison to BSF suspension.
Stability study
The stability study of the optimized BSF sol–gel system was done as per ICH guideline. The formulation was placed at 25 °C/60%RH and 40 °C/75% RH. It was found that there is no significant change in the viscosity, pH, clarity and drug content. The degradation rate constant of the formulation was found to be (1.3821 × 10−4 at 25 °C). The shelf-life was calculated by Arrhenius equation and found to be 2.34 years.
Conclusions
The developed in situ gel was novel and sensitive enough to the instantaneous sol–gel stimulation. This instantaneous conversion of sol to gel was supposed to be due to the combination of mechanisms like temperature, pH and ionic interaction. The formulations were less viscous before instillation and formed the strong gel after instillation it into the cul-de-sac. The developed formulation was found to be non-irritant and showed prolonged antimicrobial efficacy. The formulation also exhibited enhanced corneal retention at the corneal surface, which was further confirmed by gamma scintigraphy images. The optimized BSF sol–gel was stable, provided sustained release of drug over a period of 12 h, so it minimizes the frequency of administration. Therefore, the combined mechanism of sol–gel system can be used for ophthalmic drug delivery.
Acknowledgements
Authors are thankful to INMASS for providing the facility to conduct gamma scintigraphy study.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Chang MH, Fung HB. Besifloxacin: a topical fluoroquinolone for the treatment of bacterial conjunctivitis. Clin Ther. 2010;32:454–471.
- O’Brien TP. Besifloxacin ophthalmic suspension, 0.6%: a novel topical fluoroquinolone for bacterial conjunctivitis. Adv Ther. 2012;29:473–490.
- Haas W, Pillar CM, Zurenko GE, et al. Besifloxacin, a novel fluoroquinolone, has broad-spectrum in vitro activity against aerobic and anaerobic bacteria. Antimicrob Agents Chemother. 2009;53:3552–3560.
- Haas W, Gearinger LS, Usner DW, et al. Integrated analysis of three bacterial conjunctivitis trials of besifloxacin ophthalmic suspension, 0.6%: etiology of bacterial conjunctivitis and antibacterial susceptibility profile. Clin Ophthalmol. 2011;5:1369–1379.
- Haas W, Pillar CM, Torres M, et al. Monitoring antibiotic resistance in ocular microorganisms: results from the antibiotic resistance monitoring in ocular microrganisms (ARMOR) 2009 surveillance study. Am J Ophthalmol. 2011;152:567–574.
- Asbell PA, Sanfilippo CM, Pillar CM, et al. Antibiotic resistance among ocular pathogens in the United States: five-year results from the antibiotic resistance monitoring in ocular microorganisms (ARMOR) surveillance study. JAMA Ophthalmol. 2015;133:1445–1454.
- Tarabishy AB, Jeng BH. Bacterial conjunctivitis: a review for internists. Cleve Clin J Med. 2008;75:507–512.
- Alvarez-Lorenzo C, Yanez F, Barreiro-Iglesias R, et al. Imprinted soft contact lenses as norfloxacin delivery systems. J Control Rel. 2006;113:236–244.
- De Campos AM, Sanchez A, Alonso MJ. Chitosan nanoparticles: a new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to cyclosporin A. Int J Pharm. 2001;224:159–168.
- Lin HH, Ko SM, Hsu LR, et al. The preparation of norfloxacin-loaded liposomes and their in-vitro evaluation in pig’s eye. J Pharm Pharmacol. 1996;48:801–805.
- Gratieri T, Gelfuso GM, de Freitas OD, et al. Enhancing and sustaining the topical ocular delivery of fluconazole using chitosan solution and poloxamer/chitosan in situ forming Gel. Eur J Pharm Biopharm. 2011;79:320–327.
- Thimmasetty MK, Mandal S, Prabhushankar GL, et al. Formulation and evaluation of an in situ gel-forming ophthalmic formulation of moxifloxacin hydrochloride. Int J Pharm Investig. 2012;2:78–82.
- Vijaya C, Goud KS. Ion-activated in situ gelling ophthalmic delivery systems of azithromycin. Indian J Pharm Sci. 2011;73:615–620.
- Majithiya RJ, Ghosh PK, Umrethia ML, et al. Thermoreversible-mucoadhesive gel for nasal delivery of sumatriptan. AAPS PharmSciTech. 2006;7:67.
- Agrawal AK, Das M, Jain S. In situ gel systems as' smart' carriers for sustained ocular drug delivery. Expert Opin Drug Deliv. 2012;9:383–402.
- Reed K, Li A, Wilson B, et al. Enhancement of ocular in situ gelling properties of low acyl gellan gum by use of ion exchange. J Ocul Pharmacol Ther. 2016;32:574–582.
- Cao Y, Zhang C, Shen W, et al. Poly(N-isopropylacrylamide)-chitosan as thermosensitive in situ gel-forming system for ocular drug delivery. J Control Release. 2007;120:186–194.
- Tang YF, Du YM, Hu XW, et al. Rheological characterisation of a novel thermosensitive chitosan/poly (vinyl alcohol) blend hydrogel. Carbohydrate Polym. 2007;67:491–499.
- Agrawal AK, Gupta PN, Khanna A, et al. Development and characterization of in situ gel system for nasal insulin delivery. Pharmazie. 2010;65:188–193.
- Ameeduz Z, Ali J, Bhatnagar A, et al. Chitosan nanoparticles amplify the ocular hypotensive effect of carteolol in rabbits. Int J Biol Macromol. 2014;65:479–491.
- Ameeduz Z, Ali J, Khan N, et al. Carteolol loaded corboxymethyl tamarind kernel polysaccharide nanoparticles for ophthalmic delivery: box-behnken design, in vitro, ex vivo assessment. Sci Adv Mater. 2014;6:1–13.
- Kortejarvi H, Yliperttula M, Dressman JB. Bio waiver monographs for immediate release solid oral dosage forms: ranitidine hydrochloride. J Pharm Sci. 2005;94:1617–1625.
- Khan N, Ameeduzzafar Aqil M, et al. Development and evaluation of a novel in situ gel of sparfloxacin for sustained ocular drug delivery: in vitro and ex vivo characterization . Pharm Dev Technol. 2015;20:662–669.
- Costa MC, Barden AT, Andrade JM, et al. Quantitative evaluation of besifloxacin ophthalmic suspension by HPLC, application to bioassay method and cytotoxicity studies. Talanta 2014;119:367–374.
- INVITOX. Frame, data bank of in vitro techniques in toxicology: hen’s eg test, INVITOX Protocol 15; Ergatt/Frame; 1990.
- Vinardell MP, Maciancomparative M. Comparative study of the HET-CAM test and the Draize eye test for assessment of irritancy potential. Toxcol In Vitro. 1994;8:467.
- ICH Q2R1. Topic Q2 (R1): Validation of analytical procedures: text and methodology International Conference on Harmonization, Geneva, Switzerland; 2005.
- Jiang Y, Meng X, Wu Z, et al. Modified chitosan thermosensitive hydrogel enables sustained and efficient anti-tumor therapy via intratumoral injection. Carbohydr Polym. 2016;144:245–253.
- Chenite A, Chaput C, Wang D, et al. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials. 2000;21:2155–2161.
- Maruoka S, Matsuura T, Kawasaki K, et al. Biocompatibility of polyvinylalcohol gel as a vitreous substitute. Curr Eye Res. 2006;31:599–606.
- Duan Y, Cai X, Du H, et al. Novel in situ gel systems based on P123/TPGS mixed micelles and gellan gum for ophthalmic delivery of curcumin. Colloids Surf B Biointerfaces. 2015;1(128):322–330.
- Gupta H, Velpandian T, Jain S. Ion- and pH-activated novel in-situ gel system for sustained ocular drug delivery. J Drug Target. 2010;18:499–505.
- Geethalakshmi A, Karki R, Jha SK, et al. Sustained ocular delivery of brimonidine tartrate using ion activated in situ gelling system. Curr Drug Deliv. 2012;9:197–204.
- Khafagy ES, Morishita M, Onuki Y, et al. Current challenges in non-invasive insulin delivery systems: a comparative review. Adv Drug Del Review. 2007;59:1521–1546.