?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Poly (lactic-co-glycolic acid) (PLGA) is a widely used biodegradable polymer for preparation of polymeric biodegradable carriers that shows attractive features and offers possibilities to tune the physicochemical characteristics, drug release properties and biological behaviour of the PLGA-based nanospheres. Double emulsion solvent evaporation methods fail when it comes to encapsulation of highly hydrophilic drugs such as doxorubicin (Dox). The reason for this defect is due to the rapid drug diffusion into the external aqueous phase before solidification of the PLGA nanospheres. In this study, we present a comprehensive comparison between the four different fabrication methods of PLGA nanospheres with a focus on double emulsion solvent evaporation-based methodologies to achieve the effective method for co-encapsulation of superparamagnetic iron oxide nanoparticles (SPIONs) and Dox into the PLGA polymer in terms of the size of nanoparticles, particle size distribution and drug loading. Therefore, the optimized ratio of the different phases and other process parameters of these methods is discussed. In conclusion, the prepared SPIO/Dox-PLGA nanospheres (NPs) via a modified double emulsion solvent evaporation method were found to be a promising delivery system in terms of particle size distribution, drug loading, release profile as well as magnetic properties for tumour therapeutic and diagnostic purposes.
Introduction
Small drug molecules are extensively used for the treatment of various types of diseases. The clinical application of these molecules has been restricted due to their fast clearance or unfavourable biodistribution, low bioavailability and poor stability necessitating frequent dosing [Citation1,Citation2]. Nowadays, the special focus of scientific advanced technologies is on tackling the restrictions associated with traditional drug delivery through evolution of innovative pharmaceutical products. The aforementioned obstacles can be prevented by encapsulating of these drugs into delivery systems. For example, encapsulation technologies allow controlled release of drug, which make a more patient-friendly dosing regimen. Colloidal carriers possess several attractive applications in biomedicine and biotechnology. Polymeric biodegradable nanoparticles, polymeric micelles, dendrimers, liposomes, nanotubes and nanocords are some kinds of colloidal carriers employed in medicine that serve as drug reservoirs which not only create local therapeutic effect but also deliver a variety of drugs, ranging from small molecules to proteins, genes and vaccines to target organs through site specific delivery [Citation3,Citation4]. Poly (lactic-co-glycolic acid) (PLGA) is a widely used biodegradable polymer for preparation of polymeric biodegradable carriers [Citation5,Citation6]. PLGA is available in different copolymer compositions, molecular weights and various capping groups, that shows attractive features and offers possibilities to tune (a) physicochemical characteristics (e.g. hydrophobicity, zeta potential), (b) drug release properties (e.g. delayed, prolonged, triggered) and (c) biological behaviour (e.g. bioadhesion, improved cellular uptake) of the PLGA nanospheres [Citation7]. Drug loaded PLGA carriers can be given easily via subcutaneous, intramuscular or intravascular injection. The therapeutic value of such PLGA carriers can be improved by delivering safe doses of drugs and overcoming issues like rapid systemic clearance, cytotoxic effects or poor local distribution. Double emulsification (DE) and single emulsion (SE) solvent evaporation methods can be utilized in encapsulation of different kinds of active agents in polymers. Oil–water (O/W) method, a SE process, is generally employed for the encapsulation of insoluble or poorly water soluble drugs. For example, 5-fluorouracil [Citation8] and cisplatin [Citation9] are two types of drugs that have been encapsulated via this method. However, this method fails when it comes to the encapsulation of highly hydrophilic drugs where the drug either diffuses into the continuous phase or remains undissolved in the organic solvent.
Therefore, multiple emulsions play an important role in such cases. Double emulsion method is the most common type of multiple emulsion methods. In this process, both hydrophobic and hydrophilic kinds of drugs can be encapsulated. Double emulsion, as a complex system, is also termed as “emulsion of emulsion” [Citation10]. Although the first published paper on double emulsion method dates back to 89 years ago [Citation11], detailed investigation on this method had been started in the late 1970s. Water–oil–water (W/O/W) and oil–water–oil (O/W/O) are two common types of double emulsions applied for the encapsulation of different agents. For W/O/W preparation, the water soluble drug solubilized in the inner aqueous phase (W1) is dispersed in an oil phase containing lipophilic emulsifier at first. This primary emulsion then is dispersed into a secondary aqueous phase (W2) containing hydrophilic emulsifier [Citation10,Citation12]. A large number of hydrophilic drugs has been encapsulated via this method [Citation13,Citation14]. Double emulsion systems have shown beneficial features in cancer therapy as it leads to the targeted delivery and prolonged release of drugs. The encapsulation efficiency (EE) and drug release properties of such systems could be greatly improved by alteration in the type and concentration of different oils and emulsifiers utilized in the system. Florence and Whitehill [Citation15] showed that three types of double emulsion droplets could be prepared in double emulsion process in which usually one of them is predominant. Type A, as the simplest system, consists of relatively small droplets in an internal aqueous phase. Type B, with larger droplets size, composed of several small droplets (less than 50 in number) in an internal aqueous phase. In type C, numerous droplets of internal aqueous phase (W1) are encapsulated which produce the largest size of droplets as compared to those of types A and B. It has been shown that the type C would be the most promising for the preparation of polymeric carriers in drug delivery systems in that it allows the slow controlled release of entrapped agent from the internal aqueous phase or the polymeric matrix. In order to encapsulate the active molecule such as chemotherapeutic drugs into PLGA polymer, several encapsulation techniques have been applied for the manufacturing of PLGA microspheres. Microencapsulation by solvent evaporation is the common technology utilized in pharmaceutical industries for the fabrication of drug-loaded PLGA microspheres, e.g. O/W, water–oil–water (W1/O/W2), water–oil–oil (W/O1/O2) or solid–oil–water (S/O/W) [Citation16]. Apart from these conventional methods, novel emulsification technologies such as microfluidics and membrane emulsification have also been adopted to the manufacture PLGA microspheres [Citation17–22]. The selection of a method giving adequate drug encapsulation usually depends on the hydrophilicity or hydrophobicity of the drugs [Citation23]. Shortly, in double emulsion method, there are two main steps; in the first step, the primary emulsion (W1/O) is formed by mixing the proper ratio of the inner aqueous solution (e.g. Dox solution) into an organic solution (e.g. PLGA in dichloromethane (DCM) or chloroform) using either mechanical stirring or sonication. Then, the primary emulsion (W1/O) is emulsified with the outer aqueous phase containing appropriate stabilizer (e.g. polyvinyl alcohol (PVA) solution) resulting in the formation of W1/O/W2 emulsion with monodisperse droplets [Citation24,Citation25]. Finally, the organic solvent (O) is evaporated from the dispersed phase via the rotary evaporator or simple stirring at ambient temperature to the point of the polymer hardening resulted in the encapsulation of the drug. In this work, we aim to present a comprehensive comparison between the four different fabrication methods for PLGA nanospheres preparation (O/W, W1/O/W2, W1/O1, 2/W2 and O1/W1/O2/W2) with a focus on the double emulsion solvent evaporation-based methodologies to achieve the effective method for the co-encapsulation of superparamagnetic iron oxide nanoparticles (SPIONs) and Dox into the PLGA polymer in terms of the nanoparticle size distribution and drug loading. These parameters are vital in determining the in vivo fate of the nanospheres as they affect the uptake rates of the nanospheres by reticuloendothelial system (RES) and the maximum passive tumour targeting by enhanced permeability and retention effect (EPR) which finally leads to an added therapeutic value. Nanocarriers with size ranging from 100 to 200 nm are known to have favourable EPR effect as they could pass tumour vasculature [Citation26,Citation27]. Successful encapsulation of drugs like paclitaxel [Citation27], cisplatin [Citation28], temozolomide [Citation29], docetaxel [Citation30] has been reported in PLGA polymer nanoparticles. Dox is derived by chemical semisynthesis from a bacterial species (wild type strains of Streptomyces) and widely used as chemotherapeutic agent [Citation31]. As a DNA intercalating agent, Dox could effectively treat various types of cancers, including haematological malignancies (like leukaemia, blood cancers and lymphoma), and several types of soft tissue sarcomas and carcinoma (solid tumours) [Citation32–35]. Dox is a highly hydrophilic drug (approximately 10 mg per 1 ml of water) that prevents it from being highly encapsulated into hydrophobic polymers such as PLGA which is a challenging job [Citation36]. SPIONs are currently used for their potential applications as magnetic resonance imaging (MRI) contrast agents in clinical diagnosis, targeted therapy, delivery vectors and hyperthermia [Citation37,Citation38]. In this study, we used SPIONs and Dox to synthesize the SPIO/Dox-PLGA nanospheres (hereafter NPs) for dual therapeutic and diagnostic applications. NPs were synthesized using an O/W SE method and a current W1/O/W2 double emulsion and then these two techniques were compared with two different modified multiple emulsion solvent evaporation methods, including O1/W1/O2/W2 and W1/O1,2/W2 double emulsion methods. In W1/O/W2 method, an aqueous solution of Dox hydrochloride in deionized water (dH2O) was used, while in the O1/W1/O2/W2 and W1/O1,2/W2 emulsion methods, a chloroform solution of Dox was prepared by extraction of the drug into the organic phase using triethylamine (TEA). We only discussed the effects of these four different methods on the particle size and drug loading content (LC) of prepared NPs. Consequently, some other process parameters of emulsions such as the amount of PLGA polymer, Dox and SPIONs were not changed between these methods.
Materials and methods
Materials
Iron (III) chloride hexahydrate (FeCl3.6H2O, 99%), iron (II) chloride tetrahydrate (FeCl2.4H2O, 99%) and ammonium hydroxide (5 M) were purchased from Sigma-Aldrich (St. Louis, MO). Doxorubicin hydrochloride (Dox-HCl) was purchased from Sigma-Aldrich (St. Louis, MO). Carboxy-terminated poly (lactide-co-glycolide) (PLGA-COOH, Resomer RG503H, lactic/glycolic acid ratio (LG) 50:50, MW: 35 kDa) and poly (lactide-co-glycolide)-b-poly (ethylene glycol) (Resomer PLGA-PEG, PEG 9%, LG 50:50, 0.72 dl/g, MW: 40:5 kDa) were obtained from Boehringer Ingelheim (Ingelheim am Rhein, Germany). All the materials were of analytical grade.
Synthesis of SPIONs
SPIONs were synthesized in our laboratory as described in the previously reported method [Citation39]. Briefly, 4 ml of a NH4OH solution (5 M) was added to a deoxygenated solution of Fe (III) chloride (0.486 mg, 30 ml) and Fe (II) chloride (0.19 mg, 15 ml) over 2 min under nitrogen (N2) atmosphere while stirring on a magnetic stir plate for 20 min. Next, 250 mg oleic acid (OA) was added dropwise into the suspension and heated to 80 °C while being stirred to evaporate the ammonia for 1 h, and was then cooled to room temperature. Finally, the black precipitated magnetite gel was separated by applying an external magnetic field, washed twice with deionized water and acetone. The lyophilized SPIONs were stored at –20 °C until use.
Preparation of NPs
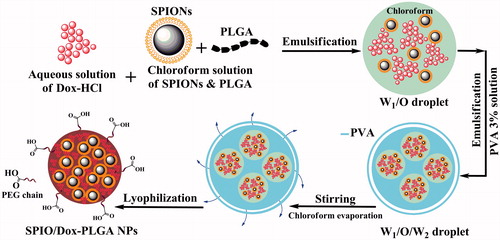
Simple W1/O/W2 emulsion method
Briefly (), an aqueous solution of Dox (1 mg Dox in 500 µl of deionized water) (W1) was emulsified in 3 ml of a stirring chloroform solution of PLGA and SPIONs (16 mg of PLGA 503H, 4 mg PLGA-PEG 9% and 5 mg SPIONs) (O) followed by sonication for 30 s (15 W). Next, the obtained W1/O emulsion was added immediately to 8 ml of 3% (w/v) PVA aqueous solution (W2) followed by ultrasonication for 30 s (50 W) in an ice bath (MSE; Soniprep 150 probe sonicator, London, UK). PVA is one of the most commonly used polymeric surfactants in order to stabilize the dispersed phase. Finally, the obtained W1/O/W2 emulsion was added dropwise to 10 ml of 1% (w/v) PVA aqueous solution and allowed for chloroform to evaporate for 3 h. The obtained NPs were washed three times with distilled water followed by centrifugation at 21,000×g for 20 min at 4 °C to remove the PVA and the non-encapsulated Dox from the outer aqueous phase. Finally, the nanoparticle suspensions were freeze dried and stored at 4 °C until use.
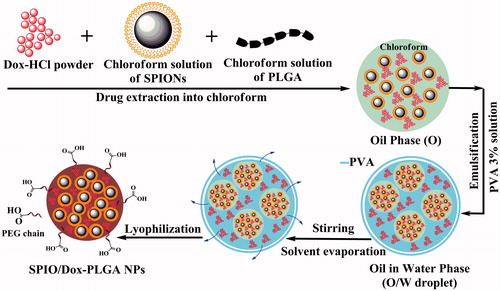
Simple O/W emulsion method
In this method (), 0.5 ml of a chloroform solution of SPIONs (5 mg per 0.5 ml) and 1 mg of Dox-HCl powder was added to 3 ml of a stirred chloroform solution of PLGA (O) (16 mg of PLGA 503H, 4 mg PLGA-PEG 9%) and then was stirred for 1 h at room temperature to extract Dox moiety into chloroform, the organic phase. Then, the obtained O phase was added immediately to 8 ml of 3% (w/v) PVA aqueous solution (W) followed by ultrasonication for 30 s (50 W) in an ice bath to obtain an O/W emulsion. To evaporate the organic solvent, the resulting O/W emulsion was added dropwise to 10 ml of 1% (w/v) PVA aqueous solution under mechanical stirring for a period of 3 h at room temperature. The obtained NPs were washed, lyophilized and stored at 4 °C similar to that used for W1/O/W2 emulsion method.
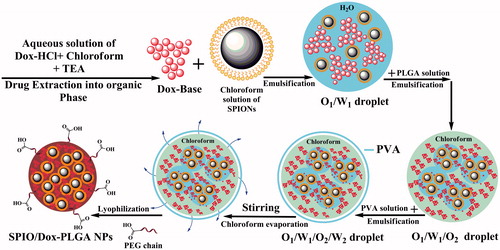
Modified multiple O1/W1/O2/W2 emulsion method
In this method (), we used a chloroform solution of Dox prepared by extracting the Dox-HCl into the organic phase as described in our previous study [Citation40]. Briefly, 5 mg SPIONs was dispersed in 0.5 ml chloroform to prepare a primary organic phase (O1). The inner aqueous solution (W1) was prepared as follows: 1 mg Dox-HCl as a hydrophilic drug was dissolved in 100 µl double-distilled water and neutralized with 3.0 equivalent of TEA and was added immediately to the primary organic phase (O1) to prevent Dox instability issues under stirring condition for 1 h. After one hour, 900 µl deionized water was added to this solution and stirred for 10 min followed by ultrasonication for 30 s (15 W) in an ice bath to obtain an O1/W1 emulsion. To obtain an O1/W1/O2 double emulsion, the primary emulsion (O1/W1) was emulsified in 3 ml of a stirred chloroform solution of PLGA (O2) (16 mg of PLGA 503H and 4 mg PLGA-PEG 9%) followed by ultrasonication for 30 s (15 W) in an ice bath. The resulting O1/W1/O2 was added immediately to 8 ml of 3% (w/v) PVA aqueous solution (W2) and was emulsified again for 30 s by ultrasonication (50 W) in an ice bath. To evaporate the organic solvent, the resulting O1/W1/O2/W2 emulsion was added dropwise to 10 ml of 1% (w/v) PVA aqueous solution under mechanical stirring for 3 h at room temperature. The obtained NPs were washed three times with distilled water by centrifugation at 21,000×g for 20 min at 4 °C. Finally, the NPs were washed, lyophilized and stored at 4 °C until use.
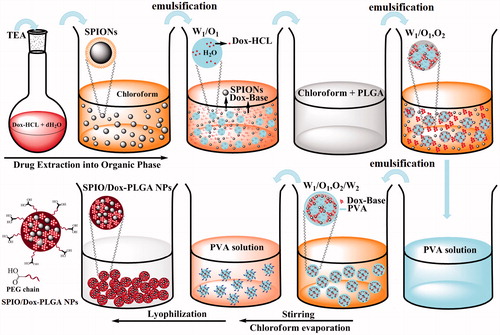
Modified multiple W1/O1,2/W2 emulsion method
According to our previous study, preparation of the NPs was performed using a modified multiple emulsion solvent evaporation method () [Citation41]. First, 1 ml chloroform solution of SPIONs (5 mg/ml) was prepared as a primary organic phase (O1). Then, 1 mg Dox-HCl was dissolved in 100 µl double-distilled water and neutralized with 3.0 equivalent of TEA to prepare an inner aqueous solution (W1). Next, the primary organic phase (O1) was immediately poured into the inner aqueous solution (W1). The resulting solution was stirred for 1 h at 25 °C for maximum extraction of Dox into chloroform. This obtained W1/O1 emulsion was emulsified in an organic solution of PLGA (O2) (16 mg of PLGA 503H and 4 mg PLGA-PEG 9% in 1 ml chloroform) by ultrasonication for 30 s (15 W) in an ice bath to obtain a W1/O1,2 emulsion. The prepared emulsion was added immediately to 8 ml of 3% (w/v) PVA aqueous solution (W2) and was emulsified by ultrasonication for 30 s (50 W). Finally, the resulting W1/O1,2/W2 emulsion was added dropwise to 10 ml of 1% (w/v) PVA aqueous solution and was stirred for 3 h to evaporate the chloroform. The obtained NPs were washed, lyophilized and stored at 4 °C similar to that used for W1/O/W2 emulsion method.
Characterizations of NPs
Nanoparticle size distribution and morphology
The nanoparticle size distribution was determined by dynamic light scattering (DLS) using a Malvern Zeta Sizer (NANO-ZS, Malvern, UK). Briefly, the particle suspensions were diluted 10 times in deionized water and then analysed with a Zetasizer at a scattering angle of 90°. All of the measurements were performed at 25 °C. The morphological examination of NPs was carried out with an atomic force microscopy (AFM, JPK NanoWizard II, JPK Instrument, Berlin, Germany). Briefly, the suspension of NPs (0.5 mg/ml) dispensed onto the glass cover slips and dried at room temperature for 24 h. The measurements were performed in the tapping mode and dehydrated state in air using ACT cantilevers.
Encapsulation efficiency and loading content of Dox
To determine the EE and LC of NPs, 1 mg of lyophilized NPs was dissolved in 1 ml of acetone for complete degradation of the PLGA matrix for 24 h. As the loaded SPIONs in our PLGA formulation were completely insoluble in acetone, an external magnetic field was utilized to remove the insoluble magnetite particles from the solution. The supernatant was collected. Also, Dox at this concentration of used NPs (1 mg/ml), is not at its limit of solubility in acetone. Acetone is a volatile organic solvent and the determination of the Dox solution absorbance in acetone is difficult via spectrofluorometer. Therefore, to determine the Dox concentration, the acetone was evaporated from the solution using a vacuum centrifuge and then the obtained red pellet containing Dox was re-dissolved in 1 ml dimethyl sulphoxide (DMSO) followed by measuring the Dox concentration (Ex: 470 nm, Em: 590 nm) using a Jasco FP-6200 spectrofluorometer (Tokyo, Japan). All the experiments were carried out in triplicate. The EE an LC of Dox was evaluated using the following equations, respectively [Citation42].
(1)
(1)
(2)
(2)
In vitro release study
To measure the in vitro Dox release from NPs, phosphate buffered saline (PBS, pH 7.4) and acetate buffer solution (pH 5.5) were used as the release media. A suspension of NPs, at the final concentration of 0.2 mg Dox/2.5 ml buffer, was added into a centrifuge tube. The centrifuge tube was placed in an incubator shaker and vibrated horizontally at 100 rpm at 37 °C. At selected time intervals (0.5, 2, 4, 8, 12, 24, 36, 48, 60 and 72 h followed by 4, 6, 8, 10, 12, 14, 18, 20, 26, 32 and 36 days), the tubes were centrifuged at 14,000×g for 20 min and the supernatants were analysed spectrofluorimetrically (Ex: 470 nm, Em: 590 nm), which was replaced by the same amount of buffer each time [Citation43].
Results and discussion
Preparation of NPs
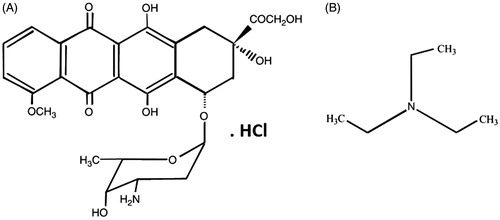
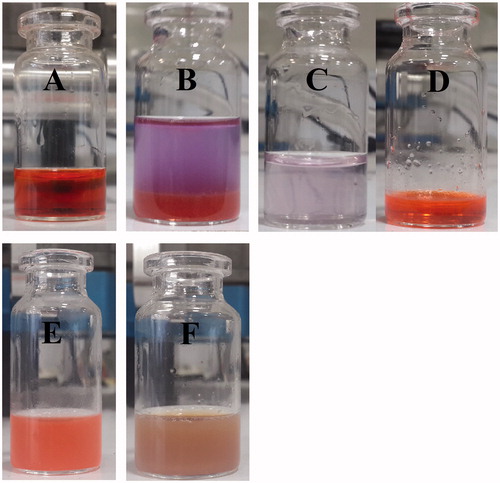
Doxorubicin hydrochloride (C27H30ClNO11, MW: 579.99 g/mole), a weak amphipathic base (pKa: 8.3), is a highly hydrophilic drug (approximately 10 mg per 1 ml of water) that makes its encapsulation into PLGA nanospheres a challenging job. Tewes et al. [Citation44] showed that Dox is more hydrophobic at pH around 8.6–9, meaning that drug affinity to the organic phases increases when the pH of the aqueous phase increases from 4 to 9, within the Dox pKa value ranges (8.2 and 9.6). This affects Dox EE. They showed that there is an amino sugar at the structure of Dox () that is protonated at low pH (4–6) resulting in cationic Dox. Above pH 6, the fraction of positively charged amine groups decreases in favour of neutral molecular form of Dox (Neutral Dox). At above pH (7.5), the phenol group of Dox is deprotonated and then the fraction of neutral Dox is again reduced (anionic Dox). They used an initial aqueous phase pH 8.6 (in borate buffer) to favour Dox lipophilicity to extract the Dox into DCM. The increase in the Dox affinity to the organic phase, which is a major concern to prevent Dox instability issues, has also been investigated by several groups [Citation44–47]. In the pH range investigated, the results confirmed that the optimum pH for extraction of Dox to the organic phase is the one that favours the neutral (uncharged) form of Dox molecules. It would be better not to perform the experiments above pH 9, because of the high Dox degradation rate at pH exceeding this value [Citation48]. Therefore, the conditions favourable for the drug lipophilicity can be adopted to formulate Dox-loaded PLGA nanospheres. In our present study, the chemical conversion strategy of Dox-HCl into its free base form (deprotonated hydrophobic Dox) through a chemical reaction with TEA (N (CH2CH3)3, MW: 101.19 g/mole) was utilized to improve Dox loading into PLGA nanospheres [Citation47]. According to the molar mass and chemical formula of Dox and TEA that have been mentioned above, a TEA-Dox molar ratio of ∼3 (∼3.0 equivalent of TEA) should be used to neutralize the Dox-HCl to favour Dox lipophilicity for Dox extraction into organic solvent without further favouring Dox instability. In this study, the effects of different kinds of organic solvents such as DCM and chloroform in terms of maximum dispersion and colloidal stability of SPIONs were also examined. The SPIONs dispersed in chloroform had a colloidal stability for up to one year under 4 °C without any sign of aggregation or increasing size of nanoparticles, especially during the preparation of NPs compared with DCM. An increase in the Dox affinity to the organic phase (chloroform) as opposed to aqueous phase has also been reported between pH 5 and 7 [Citation46]. Therefore, we conducted further formulation experiments with chloroform as a suitable organic solvent in our studies. Aqueous solutions of Dox present a red colour () at pH < pKa: 8.3 (maximum adsorption of Dox at 485 nm), yet solutions at pH above 10.5 show a purple colour () (maximum adsorption of Dox shifts to 550 and 592 nm) [Citation49]. Dox in the free base form shows an orange colour (). In the O/W method, a certain amount of pure Dox hydrochloride powder was added directly to organic phase, but in the W1/O/W2 method, an aqueous solution of the Dox hydrochloride in dH2O was used. In contrast to the O/W and W1/O/W2 methods, in the O1/W1/O2/W2 and W1/O1,2/W2 methods, a chloroform solution of Dox was prepared by extraction of the drug into the organic phase using TEA to favour Dox lipophilicity to improve the EE and LC of Dox. These four different methods were applied to find the best way of the NPs preparation in terms of nanoparticle size, polydispersity index (PDI) and drug LC. Size and PDI of NPs have a vital role in accumulation and penetration of NPs within the tumour site through EPR effect as well as in the blood clearance rate of administered NPs after IV injection through the RES in cancer therapy [Citation50]. Drug LC is also very important for in vivo efficacy studies because it not only shows how much Dox is finally administered into each animal, but also used to administer the least amount of drug-loaded NPs with maximum anti-proliferation effects on cancer cells.
Figure 6. Aqueous solutions of Dox-HCl present a red colour at pH < pKa: 8.3 (A). A chemical conservation strategy was utilized to extract Dox-HCl into its free base form (protonated Dox which is hydrophobic) through a chemical reaction with TEA to improve Dox loading into the PLGA nanospheres (B). Whereas aqueous solution of Dox-HCl at pH above 10.5 shows a purple colour (C), Dox in the free base form shows an orange colour (D). Dox-loaded PLGA nanospheres (E) and SPIO/Dox loaded PLGA nanospheres (NPs) (F) with an excellent colloidal stability obtained by W1/O1,2/W2 methods.
EE, LC, particle size and polydispersity index
To attain the maximum drug loading of Dox in nanospheres with narrow size distribution, some different preparation methods were compared to find the best way for preparation of NPs. shows a comparison between the EE and LC results of four different preparation methods used in this study as well as the final particle size of obtained NPs. It is quite obvious when Dox-HCl was neutralized with TEA in O1/W1/O2/W2 and W1/O1, 2/W2 double emulsion method, Dox in the organic phase was hardly diffused into the aqueous phase for the EE was about 70.2 ± 2.3 and 87.6 ± 2.8 which is about 1.73 and 2.15 folds higher than the EE obtained by the W1/O/W2 methods, respectively (). The low amount of EE in O/W SE method is mainly controlled by Dox–HCl diffusion and limited solubility of the drug in chloroform. The comparison between the results of W1/O/W2, O1/W1/O2/W2 and W1/O1, 2/W2 double emulsion methods clearly demonstrated that the W1/O1, 2/W2 method for co-encapsulation of SPIONs and Dox gained better results in terms of the EE, LC and particle size distribution, thus W1/O1, 2/W2 used for further studies. Regarding the DLS results (), only one peak (peak 1) was observed in NPs, indicating narrow particle size distribution and mono-modal population of the NPs.
Figure 7. Particle size distribution of NPs prepared by W1/O1,2/W2 method. (A, B) The particle size distribution of NPs using DLS and AFM analysis, respectively.
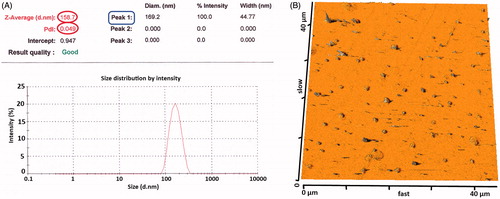
Table 1. Ingredients for preparation of NPs as well as particle size, PDI, LC and EE.
In vitro release study
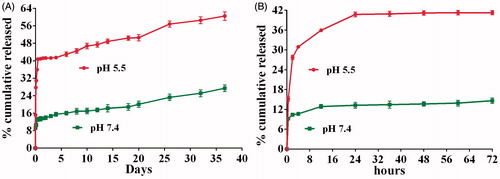
Faster Dox release of NPs at low pHs would be beneficial to increase the anti-proliferation effect of Dox inside the cellular organelles such as lysosomes (pH is about 5.5), while limiting its release in blood circulation. shows the In vitro Dox release from NPs in pH 7.4 PBS and pH 5.5 Acetate buffers at 37 °C. An almost 2.5-fold increase in the cumulative Dox release at pH 5.5 over pH 7.4 was observed. For both acetate and PBS media, there was an initial burst release during the first 2 h post-incubation followed by slow release within 12 h (13 and 36% for pH 7.4 and pH 5.5, respectively) (). After that, a continuous release in both buffers within 20 days was exhibited (20.2 ± 0.5% in pH 7.4 and 50.1 ± 0.5% in pH 5.5). Over 36 days, the Dox release profile of NPs showed a relatively fast drug cumulative release phase reaching 27.4 ± 0.3% and 60.1 ± 0.6% in pH 7.4 and pH 5.5, respectively. Totally, a nearly threefold higher cumulative Dox release was observed in pH 5.5 than in pH 7.4, which could be resulted from the improved solubility of protonated Dox at lower pH [Citation44]. In conclusion, the NPs appear to be suitable to reach as low as possible release in blood, and as higher as release in lysosome that depends on the pH of media, drug diffusion and matrix-erosion mechanisms [Citation51].
Diagnostic applications
In this study, we introduced an effective method for co-encapsulation of SPIONs and Dox into the PLGA nanospheres via W1/O1, 2/W2 method. Based on our previous results, SPIONs showed a mean size of ∼20 nm with narrow particle size distribution [Citation39]. In another study, we prepared NPs via W1/O1, 2/W2 method similar to this study except that we used PLGA-PEG-COOH instead of PLGA-PEG to conjugate the aptamer-NH2 molecules, as a targeting ligand, on the surface of NPs via EDC/NHS technique [Citation41]. The mean size of non-conjugated NPs was 165 nm with a narrow particle size distribution, with the SPIONs loading of 16% and saturation magnetization value of 6.5 emu/g. These results indicated that the most of initial SPIONs had been encapsulated into the PLGA polymer matrix. According to the saturation magnetization values reported in previous studies, the values of 7.3 emu/g [Citation52] and 4.5 emu/g [Citation53] were appropriately sensitive to be considered as a highly efficient contrast agent in the tumour site at MRI technique and could be promising NPs for simultaneous diagnostic and therapeutic applications.
Conclusions
Since Dox has a high solubility in water, the low affinity of Dox to the PLGA polymer could result in low entrapment efficiency. To improve the entrapment efficiency and LC of Dox, the chloroform solution was utilized to extract Dox into this organic phase using TEA to neutralize Dox in favour of Dox lipophilicity. The results of the current study clearly demonstrated that the W1/O1, 2/W2 double emulsion method produced better results in terms of particle size, PDI, drug loading, release profile and magnetic properties for co-encapsulation of SPIONs and Dox in PLGA nanospheres. Thus, the method could be applied as an appropriate method for the preparation of SPIONs/Dox PLGA NPs suitable for therapeutic and diagnostic applications.
Acknowledgements
The data presented in this report were a part of J. Mosafer PhD thesis (Grant number: 920565) supported by Nanotechnology Research Center, Mashhad University of Medical Sciences (MUMS).
Disclosure statement
The authors declare that they have no conflicts of interest.
Additional information
Funding
References
- Chen Z. Small-molecule delivery by nanoparticles for anticancer therapy. Trends Mol Med. 2010;16:594–602.
- Vrignaud S, Benoit J-P, Saulnier P. Strategies for the nanoencapsulation of hydrophilic molecules in polymer-based nanoparticles. Biomaterials. 2011;32:8593–8604.
- Zafar N, Fessi H, Elaissari A. Cyclodextrin containing biodegradable particles: from preparation to drug delivery applications. Int J Pharm. 2014;461:351–366.
- Jahanshahi M, Babaei Z. Protein nanoparticle: a unique system as drug delivery vehicles. Afr J Biotechnol. 2008;7:4926–4934.
- Sah H, Thoma LA, Desu HR, et al. Concepts and practices used to develop functional PLGA-based nanoparticulate systems. Int J Nanomed. 2013;8:747.
- Danhier F, Ansorena E, Silva JM, et al. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161:505–522.
- Iqbal M, Zafar N, Fessi H, et al. Double emulsion solvent evaporation techniques used for drug encapsulation. Int J Pharm. 2015;496:173–190.
- Boisdron-Celle M, Menei P, Benoit J. Preparation and characterization of 5‐fluorouracil‐loaded microparticles as biodegradable anticancer drug carriers. J Pharm Pharmacol. 1995;47:108–114.
- Verrijk R, Smolders IJ, Bosnie N, et al. Reduction of systemic exposure and toxicity of cisplatin by encapsulation in poly-lactide-co-glycolide. Cancer Res. 1992;52:6653–6656.
- Garti N, Bisperink C. Double emulsions: progress and applications. Curr Opin Colloid Interface Sci. 1998;3:657–667.
- Siegfried A. Angleterre d’aujourd’hui; 1924.
- Schuch A, Deiters P, Henne J, et al. Production of W/O/W (water-in-oil-in-water) multiple emulsions: droplet breakup and release of water. J Colloid Interface Sci. 2013;402:157–164.
- Okochi H, Nakano M. Preparation and evaluation of w/o/w type emulsions containing vancomycin. Adv Drug Deliv Rev. 2000;45:5–26.
- Sinha V, Trehan A. Biodegradable microspheres for protein delivery. J Control Release. 2003;90:261–280.
- Florence A, Whitehill D. Some features of breakdown in water-in-oil-in-water multiple emulsions. J Colloid Interface Sci. 1981;79:243–256.
- Ramazani F, Chen W, van Nostrum CF, et al. Strategies for encapsulation of small hydrophilic and amphiphilic drugs in PLGA microspheres: state-of-the-art and challenges. Int J Pharm. 2016;499:358–367.
- Falke LL, van Vuuren SH, Kazazi-Hyseni F, et al. Local therapeutic efficacy with reduced systemic side effects by rapamycin-loaded subcapsular microspheres. Biomaterials. 2015;42:151–160.
- Kazazi-Hyseni F, Landin M, Lathuile A, et al. Computer modeling assisted design of monodisperse PLGA microspheres with controlled porosity affords zero order release of an encapsulated macromolecule for 3 months. Pharm Res. 2014;31:2844–2856.
- Ramazani F, Chen W, Van Nostrum CF, et al. Formulation and characterization of microspheres loaded with imatinib for sustained delivery. Int J Pharm. 2015;482:123–130.
- Xu Q, Hashimoto M, Dang TT, et al. Preparation of monodisperse biodegradable polymer microparticles using a microfluidic flow‐focusing device for controlled drug delivery. Small. 2009;5:1575–1581.
- Ye M, Kim S, Park K. Issues in long-term protein delivery using biodegradable microparticles. J Control Release. 2010;146:241–260.
- Zhang Z, Bi X, Li H, et al. Enhanced targeting efficiency of PLGA microspheres loaded with Lornoxicam for intra-articular administration. Drug Deliv. 2011;18:536–544.
- Li M, Rouaud O, Poncelet D. Microencapsulation by solvent evaporation: state of the art for process engineering approaches. Int J Pharm. 2008;363:26–39.
- Qi F, Wu J, Hao D, et al. Comparative studies on the influences of primary emulsion preparation on properties of uniform-sized exenatide-loaded PLGA microspheres. Pharm Res. 2014;31:1566–1574.
- Vladisavljević GT, Schubert H. Influence of process parameters on droplet size distribution in SPG membrane emulsification and stability of prepared emulsion droplets. J Membr Sci. 2003;225:15–23.
- Liu D, Mori A, Huang L. Role of liposome size and RES blockade in controlling biodistribution and tumor uptake of GM 1-containing liposomes. Biochim Biophys Acta (BBA)-Biomembr. 1992;1104:95–101.
- Guo J, Gao X, Su L, et al. Aptamer-functionalized PEG–PLGA nanoparticles for enhanced anti-glioma drug delivery. Biomaterials. 2011;32:8010–8020.
- Dhar S, Gu FX, Langer R, et al. Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt (IV) prodrug-PLGA–PEG nanoparticles. Proc Natl Acad Sci. 2008;105:17356–17361.
- Ling Y, Wei K, Zou F, et al. Temozolomide loaded PLGA-based superparamagnetic nanoparticles for magnetic resonance imaging and treatment of malignant glioma. Int J Pharm. 2012;430:266–275.
- Ling Y, Wei K, Luo Y, et al. Dual docetaxel/superparamagnetic iron oxide loaded nanoparticles for both targeting magnetic resonance imaging and cancer therapy. Biomaterials. 2011;32:7139–7150.
- Carvalho C, Santos RX, Cardoso S, et al. Doxorubicin: the good, the bad and the ugly effect. CMC. 2009;16:3267–3285.
- Xu W-H, Han M, Dong Q, et al. Doxorubicin-mediated radiosensitivity in multicellular spheroids from a lung cancer cell line is enhanced by composite micelle encapsulation. Int J Nanomed. 2012;7:2661.
- Blay J-Y, Leahy MG, Nguyen BB, et al. Randomised phase III trial of trabectedin versus doxorubicin-based chemotherapy as first-line therapy in translocation-related sarcomas. Eur J Cancer. 2014;50:1137–1147.
- Xu Q, Leong J, Chua QY, et al. Combined modality doxorubicin-based chemotherapy and chitosan-mediated p53 gene therapy using double-walled microspheres for treatment of human hepatocellular carcinoma. Biomaterials. 2013;34:5149–5162.
- Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106:3725–3732.
- Mao JN, Li AJ, Zhao LP, et al. PEG-PLGA nanoparticles entrapping doxorubicin reduced doxorubicin-induced cardiotoxicity in rats. Adv Mater Res. 2014;912:263–268.
- Wu W, Wu Z, Yu T, et al. Recent progress on magnetic iron oxide nanoparticles: synthesis, surface functional strategies and biomedical applications. Sci Technol Adv Mater. 2016;16:023501.
- Yigit MV, Moore A, Medarova Z. Magnetic nanoparticles for cancer diagnosis and therapy. Pharm Res. 2012;29:1180–1188.
- Mosafer J, Abnousb K, Tafaghodi M, et al. Preparation and characterization of uniform-sized PLGA nanospheres encapsulated with oleic acid-coated magnetic-Fe 3 O 4 nanoparticles for simultaneous diagnostic and therapeutic applications. Colloids Surf A: Physicochem Eng Aspects. 2017;514:146–154.
- Mosafer J, Teymouri M, Abnous K, et al. Study and evaluation of nucleolin-targeted delivery of magnetic PLGA-PEG nanospheres loaded with doxorubicin to C6 glioma cells compared with low nucleolin-expressing L929 cells. Mater Sci Eng C. 2017;72:123–133.
- Mosafer J, Abnous K, Tafaghodi M, et al. In vitro and in vivo evaluation of anti-nucleolin-targeted magnetic PLGA nanoparticles loaded with doxorubicin as a theranostic agent for enhanced targeted cancer imaging and therapy. Eur J Pharm Biopharm. 2017;113:60–74.
- Wohlfart S, Khalansky AS, Gelperina S, et al. Efficient chemotherapy of rat glioblastoma using doxorubicin-loaded PLGA nanoparticles with different stabilizers. PLoS One. 2011;6:e19121.
- Alibolandi M, Ramezani M, Abnous K, et al. In vitro and in vivo evaluation of therapy targeting epithelial-cell adhesion-molecule aptamers for non-small cell lung cancer. J Control Release. 2015;209:88–100.
- Tewes F, Munnier E, Antoon B, et al. Comparative study of doxorubicin-loaded poly (lactide-co-glycolide) nanoparticles prepared by single and double emulsion methods. Eur J Pharm Biopharm. 2007;66:488–492.
- Sanson C, Schatz C, Le Meins JF, et al. A simple method to achieve high doxorubicin loading in biodegradable polymersomes. J Control Release. 2010;147:428–435.
- Kataoka K, Matsumoto T, Yokoyama M, et al. Doxorubicin-loaded poly (ethylene glycol)–poly (β-benzyl-l-aspartate) copolymer micelles: their pharmaceutical characteristics and biological significance. J Control Release. 2000;64:143–153.
- Khemani M, Sharon M, Sharon M. pH dependent encapsulation of doxorubicin in PLGA. Ann Biol Res. 2012;3:4414–4419.
- Raghunand N, Mahoney BP, Gillies RJ. Tumor acidity, ion trapping and chemotherapeutics: II. pH-dependent partition coefficients predict importance of ion trapping on pharmacokinetics of weakly basic chemotherapeutic agents. Biochem Pharmacol. 2003;66:1219–1229.
- Cheung BC, Sun TH, Leenhouts JM, et al. Loading of doxorubicin into liposomes by forming Mn 2+-drug complexes. Biochim Biophys Acta (BBA)-Biomembr. 1998;1414:205–216.
- Acharya S, Sahoo SK. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv Drug Deliv Rev. 2011;63:170–183.
- Magenheim B, Levy M, Benita S. A new in vitro technique for the evaluation of drug release profile from colloidal carriers-ultrafiltration technique at low pressure. Int J Pharm. 1993;94:115–123.
- Hu F, Neoh K, Kang E. Synthesis and in vitro anti-cancer evaluation of tamoxifen-loaded magnetite/PLLA composite nanoparticles. Biomaterials. 2006;27:5725–5733.
- Cui Y, Xu Q, Chow PK, et al. Transferrin-conjugated magnetic silica PLGA nanoparticles loaded with doxorubicin and paclitaxel for brain glioma treatment. Biomaterials. 2013;34:8511–8520.