Abstract
Purpose: As a kind of difficult to cure tumour, malignant gliomas have attracted widespread attention. The proliferation and immune escape of tumour cells were closely related to the development of malignant gliomas. The aim of this study was to investigate the role of endothelin B receptor (NTBR) in gliomas.
Methods: RT-PCR was used to detect the expression of NTBR mRNA in glioma tissue and glioma cell lines. The expression of NTBR in glioma tissues was detected by immunohistochemistry. MTT assay was used to detect the viability of U87 cells after adding NTBR. Cell cloning assay was used to detect the cell proliferation ability. Western blot was used to detect the expression of TGF-β and the expression of Treg after adding NTBR to U87.
Result: The expression of NTBR in glioma tissues and cells was significantly higher than that in the control group by RT-PCR. After adding NTBR, cell proliferation of U87 was significantly enhanced and TGF-β and Treg were significantly expressed. It was suggested that NTBR could contribute to tumour immune escape in glioma, and it was found that there was a positive correlation between NTBR expression and different stages in malignant gliomas.
Conclusion: Endothelin B receptor can increase the proliferation of glioma cells and tumour immune escape. The expression of endothelin B is closely related to the clinical stage of glioma.
Introduction
Malignant gliomas include undeveloped astrocytoma and glioblastoma, both of which were highly invasive brain tumours and the most common intracranial primary tumours in clinical neurosurgery. The incidence in brain neurological tumours accounted for 50%. In recent years, the incidence of glioma showed a trend in younger [Citation1,Citation2]. The development of glioblastoma was a multistep process, during which many regulatory factors were affected. At present, the treatment of brain tumours was surgical treatment, radiotherapy, and chemotherapy. However, these treatments are not ideal and the prognosis is still low. Studies have shown that the median survival rate after treatment was only 12–14 months and there was no significant improvement in malignant glioma [Citation3–6].
Endothelin family is composed of three isomer members, ET-1, ET-2, and ET-3, and their individual amino acid residues are different. ET-1 exists in the cardiovascular. Endothelin is composed of 21 amino acids with molecular weight of 2400 D, with the N-terminal structure determining its affinity with the receptor and C-terminal structure determining its binding position with the receptor. The important function of ET-1 is related to angiogenesis. Endothelin will undergo biological effects in a paracrine or autocrine way, for example vascular strain steady-state balance, neural crest development, ovarian cycle, cell proliferation, anti-angiogenesis, and inflammatory infiltration [Citation7–9]. In order to regulate these physiological effects, endothelin activity is divided into two categories: one is the endothelin A receptor, and the other is the endothelin B receptor. Endothelin B receptors can bind to three endothelin families, but endothelin A is more affinity with ET-1 and ET-2. Endothelin and its receptors have been shown to exhibit different forms in many diseases. Recent studies have shown that endothelin B receptors are involved in two important human diseases, namely cardiovascular disease and tumours. The high expression of endothelin B receptor or excessive stimulation can lead to atherosclerotic lesions, tissue fibrosis, and atherosclerotic plaque of the heart [Citation10–12]. In the case of tumours, the pathological role of endothelin receptor B plays an important role in the peripheral blood vessels surrounding the tumour: (a) increasing angiogenesis and promoting tumour cell proliferation; (b) promoting macrophage release of matrix metalloproteinases and promoting tumour cell infiltration and metastasis; and (c) reducing the toxicity of T cells to tumour cells and reducing immune surveillance and causing tumour escape, and in this way, many kinds of tumour cells are presented. Recent studies have shown that overexpression of melanoma cells in endothelin receptor B contributes to tumour development and infiltration [Citation13,14], but has not been reported in gliomas.
Tumour cells could escape the surveillance and killing of immune system through a variety of mechanisms. One of the important mechanisms is the production of immunosuppressive cytokines TGF-β. TGF-β is a pleiotropic cytokine that plays an important role in tumour and immunoregulation. T cell-derived TGF-β has been shown to play an important role in the inhibition of anti-tumour immunity, but the role of tumour-derived TGF-β in this process has yet been clear. Our study focuses on the role of endothelin B receptors in gliomas and the effects on immune escape.
Materials and methods
Collection of samples
Glioma tissue specimens in 41 cases and 20 cases of adjacent normal tissue specimens were collected from July 2014 to December 2014 in our hospital surgical resection, of which surgical resection of glioma tissue lymph node metastasis was observed in 25 cases and no lymphatic metastasis in 16 cases. Clinical staging of cases is in accordance with the 2009 International Anti-Cancer Association TNM staging standards: 3 cases in Stage 0, 6 cases in Stage I, 4 cases in Stage II, 15 cases in Stage III, and 13 cases in Stage IV. No chemotherapy and radiotherapy were performed before surgery. All cases were diagnosed by pathology. Tumour tissue acceptance criteria were as follows: (a) patients and their families agree and sign; (b) detailed case reports; and (c) pathological detailed diagnostic results (the tumour consists of mature astrocytes).
Reagents and apparatus
U251, U87, C6 glioma cell lines, and normal astrocytes (RA) were purchased from Shanghai Institute of Chinese Academy of Sciences (Shanghai, China); NTBR monoclonal antibody was purchased from Epitomics (CA, USA); foetal bovine serum, RPMI-1640 supplemented from Hyclone (UT, USA), and the like were purchased from Gibco (CA, USA); ChemiDoc™ XRS gel imaging system is from Bio-Rad (CA, USA); and MTT is from Sangon Biotech (Shanghai, China).
MTT assay
The tumour cells were inoculated into 96-well plates. When the cell density reached 50%, the recombinant protein NTBR was added to the experimental group and DMSO was added to the other group. After 12 h, 20 μL of 5 mg/mL MTT was added and cultured for 4 h. The culture medium was discarded and 150 μL of DMSO was added to each well. The crystals were sufficiently dissolved by shaking, and the OD value was measured at 560 nm. The relative proliferation rate was calculated using the OD value at 630 nm as a reference.
Colony formation assay
The tumour cells were inoculated into 6-well plates. When the cell density reached 50%, the recombinant protein NTBR was added to the experimental group and DMSO was added to the other group. After 48 h, the cells were fixed with 10% formaldehyde and stained with 0.1% crystal violet for 30 min at room temperature. The dye solution was shaken gently and washed with distilled water. The culture plate was immersed in paper and dried to take a photograph.
Real-time fluorescent quantitative PCR
Reverse transcription kit was purchased from TaKaRa (Beijing, China). Fresh tissue was placed in a mortar and the liquid nitrogen and rapidly ground to a powder. Total RNA extraction from glioma tissue and glioma cell lines (U251, U87, and C6) was performed using the Trizol method. After RNA was slightly dried, 20 μL of DEPC was added to dissolve and the RNA concentration was measured by ultramicro spectrophotometer. The RNA was converted into a complementary DNA (cDNA) using a reverse transcriptase or stored at −80 °C. The primers sequences of NTBR and GADPH were shown in .
Table 1. Primers of RT-PCR.
Detection of NTBR protein in tissue
At the end of the treatment, the tissues were fixed by transcranial perfusion with 100 ml 4% (w/v) paraformaldehyde (pH 7.4). The slices were prepared according to the above-described method. After clearing the paraffin, the samples were washed with PBS for 5 min three times. The samples were placed in citric acid buffer solution for high pressure boil for 5 min and then washed with PBS for 5 min three times. The samples were soaked with 0.3% hydrogen peroxide at room temperature for 30–60 min and then washed with PBS for 5 min three times. The sample was placed in foetal bovine serum 10% for half an hour, then incubated with primary antibody overnight, and then washed with PBS for 5 min three times. After washing, the samples were incubated in the secondary antibody (1:100) at 37° C for 1 h. The tissue was placed in DAB agent for coloration. After termination of the reaction, a drop of permount over the tissue was placed on each slide and a coverslip was added. The slides were viewed using a microscope.
Flow cytometry
The erythrocyte lysate was centrifuged and the supernatant was discarded. The precipitate was washed 3 times with 0.5% BSA for 5 min. Antibodies were added at a ratio of 1:100 and incubated for 30 min in the dark. After centrifugation of 350 g for 5 min, the supernatant was discarded. The precipitate was washed with 0.5% BSA 3 times and the supernatant was discarded. The final precipitation was resuspended with 200 μl 0.5% BSA for detection. (If not timely detection, the cell should resuspend and fixed with 400 μl 1% paraformaldehyde.)
Western blotting
Tissue proteins were extracted using general protein kits (Beyotime, China). All protein samples were adjusted to equal concentrations, followed by addition of bromophenol blue. The bubbles were removed under the board. Equal amounts of proteins were loaded on SDS/PAGE. 6 μL of protein marker was added at the same time. The protein samples were separated according to a predetermined voltage. Then, the protein was transferred to nitrocellulose membranes and blotted with primary antibodies at a dilution of 1:1000, followed by secondary antibodies. Detection was performed using the LI-COR Odyssey Scanning Infrared Fluorescence Imaging System (LI-COR, Lincoln, NE).
Statistical analysis
RT-PCR, Q-PCR, and Western blot results were analyzed by ImageJ software (Bethesda, MD, USA). All experimental dates were analyzed using SPSS 17.0 (Armonk, NY, USA). Each experiment is repeated more than three times. The paired t-test was used to compare the two groups. p < .05 represents a significant difference.
Results
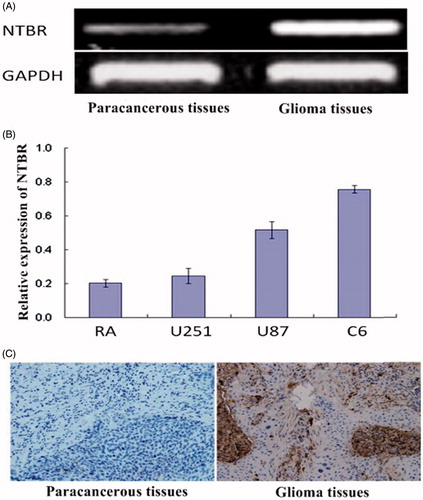
Expression of NTBR in glioma tissue and glioma cells
RT-PCR results showed that the expression of NTBR in glioma tissues was significantly higher than that in adjacent normal tissues (0.68 ± 0.05 vs. 0.23 ± 0.00), and the difference was statistically significant (p < .05) (). As shown in , Q-PCR results showed that the relative expression of NTBR in glioma cell lines U251, U87, and C6 was significantly higher than that in normal astrocytes (RA) (0.24 ± 0.04, 0.51 ± 0.05, 0.52 ± 0.02) vs. (0.20 ± 0.02), respectively. U87 was used for the next experiment because of its moderate expression. As shown in , immunohistochemistry showed that the positive expression of NTBR in glioma tissues was higher than that in adjacent normal gliomas (5.49 ± 0.48) vs. (0.89 ± 0.02). The difference was statistically significant (p < .01).
Figure 1. NTBR expression in glioma tissue and glioma cells. (A) RT-PCR was used to detect the expression of NTBR in paracancerous tissues and glioma tissues; (B) NTBR expression in glioma cell lines; (C) NTBR immunohistochemistry was used to detect the expression of NTBR in paracancerous tissues and glial tumours changes in the tumour.
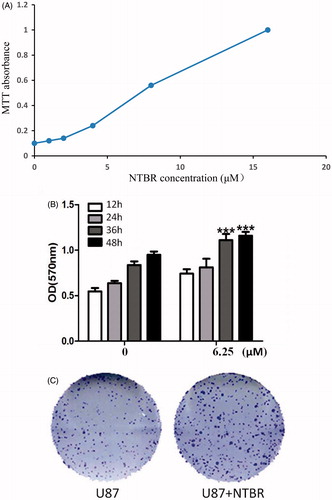
NTBR promotes the proliferation of malignant glioma cells
Based on the above study, we knew that the relative expression of NTBR in glioma cell lines was significantly high, and U87 was moderate in expression, so U87 was used for the next experiment. The recombinant endothelin B receptor was added to the glioma cells, and the IC50 showed an effective dose of 6.25 μM (). Cell viability was measured by MTT assay after adding DMSO (control group) and endothelin B receptor 6.25 μM (experimental group) in U87. The results showed that the cells were rapidly proliferated after incubation with endothelin B receptor for 36 h and 48 h, and there were statistically significant differences (). Cell cloning experiments showed that the ability of single-cell proliferation was significantly enhanced by the effect of 6.25 μM endothelin B receptor ().
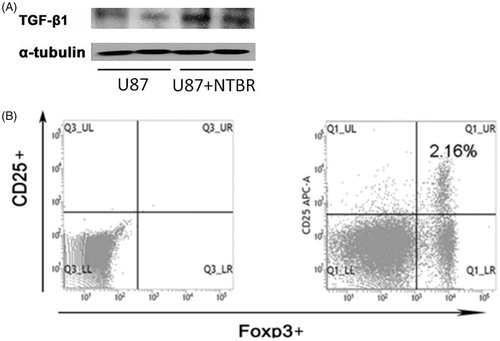
NTBR promotes malignant glioma cell immune escape
In order to further validate the effect of NTBR on tumour immune escape, the expression of TGF-β was detected by Western blot in glioma cell line U87 after treated with 6.25 μM endothelin B receptor, and DMSO-treated cell as control. The results showed that the expression of TGF-β significantly increased when endothelin B receptor was added (). The expression of Treg was favourable for the growth of tumour cells, so we examined the expression of Treg after U87 addition of endothelin B receptor. As shown in , the level of Treg was significantly elevated under the stimulation of endothelin B receptor.
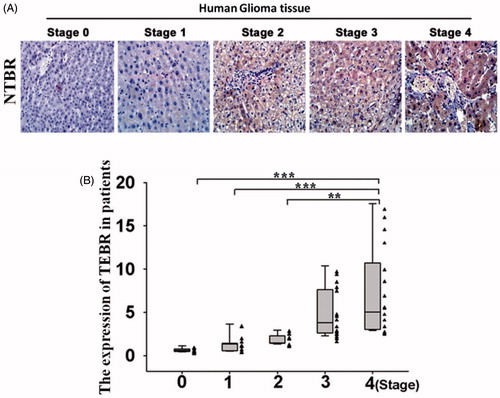
NTBR regulates tumour staging in glioma cells
Glioma patients have a higher level of NTRB, whether there was a relationship between high level of NTRB and tumour staging. The results of immunohistochemistry showed that the expression of NTRB was low in patients with early glioma, and the expression of NTRB was the highest in the Stage IV patients (). As shown in , clinical statistical analysis showed that there was a significant difference in this expression. In all, NTRB plays a regulatory role in the development of glioma.
Discussion
The occurrence and development of gliomas is a multifactorial, multistep process that ultimately leads to the activation of oncogenes and the inactivation of tumour suppressor genes. There is a considerable evidence that endothelin plays an important role in tumour suppressor or cancer progression, regulating the tumour cell proliferation, apoptosis, signal transduction, and the pathogenesis of the tumour in different tumours [Citation15]. As an oncogene, the expression of endothelin is upregulated in many types of cancer cells. Recent studies have shown that expression of NTBR in lung cancer cells is higher than that in normal lung cells and NTBR is closely related to the development of NSCLC [Citation16]. Our study showed that the expression of NTBR in glioma tissue was higher than that in adjacent tissues, as well as the expression in glioma cell lines U251, U87, and C6 was higher than that in normal astrocytes (RA). Although this study did not explain why the expression of NTBR in the cell line was different, we speculated that it may be due to the differences in the invasive cell lines. As NTBR level was moderate in the U87, we can rule out the high and low NTBR expression on the experimental results of the impact, so U87 was selected as experimental cell lines.
The infinite multiplication of tumour makes tumour cells to continue to split and proliferation, making the synthesis of protein much higher than the decomposition in tumour cells, even leading to the body in cachexia and further deterioration of the disease. It has been reported that endothelin can regulate the biological behaviour of tumour cells by targeting a kind of molecules [Citation17,Citation18]. Therefore, the cell proliferation of U87 cells was detected by MTT after adding NTBR. The results showed that proliferation of U87 cells was significantly increased and significant statistical difference after 36-h incubation, which indicated that NTBR could promote the growth of malignant gliomas.
It is the so-called tumour immune escape that tumour cells can escape the identification and attack of immune system through a variety of mechanisms. When malignant cells appear in the body, the immune system can recognize and remove tumour cells through the immune mechanism specifically, thereby inhibiting the development of the tumour. However, malignant cells in some cases can escape the surveillance of immune system and rapidly proliferate to form a tumour. On the one hand, the body can resist the occurrence of tumours through natural and acquired immunity; on the other hand, tumour cells can escape the immune recognition and attack through various mechanisms [Citation19,Citation20]. TGF-β has a direct relationship with tumour immune escape [Citation21,Citation22]. Our study showed that the expression of TGF-β in the cells increased with the addition of NTBR recombinant protein in U87; meanwhile, the expression of Treg was also increased, which indicates that NTBR could contribute to immune escape in malignant gliomas. Further studies have shown that there is a difference in the level of NTBR in different clinical stage malignant gliomas, and the higher the clinical stage, the higher the expression of NTBR. It is of statistical significance.
In summary, endothelin B receptors increased the proliferation of glioma cells and tumour immune escape. In addition, the expression of endothelin B glioma was significantly related to clinical stage of malignant gliomas.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Disclosure statement
The authors declare that they have no conflict of interest.
References
- Xue K, Yang J, Hu J, et al. MicorRNA-133b expression associates with clinicopathological features and prognosis in glioma. Artif Cells Nanomed Biotechnol. 2017;6:1–4.
- Xu Y, Xu W, Lu T, et al. miR-126 affects the invasion and migration of glioma cells through GATA4. Artif Cells Nanomed Biotechnol. 2016;6:1247–1253.
- Attwell D, Buchan AM, Charpak S, et al. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243.
- Baker GJ, Yadav VN, Motsch S, et al. Mechanisms of glioma formation: iterative perivascular glioma growth and invasion leads to tumor progression, VEGF-independent vascularization, and resistance to antiangiogenic therapy. Neoplasia. 2014;16:543–561.
- Basş M, Greve J, Stelter K, et al. A randomized trial of icatibant in ACE-inhibitor-induced angioedema. N Engl J Med. 2015;372:418–425.
- Bazargani N, Attwell D. Astrocyte calcium signaling: the third wave. Nat Neurosci. 2016;19:182–189.
- Thorin E, Clozel M. The cardiovascular physiology and pharmacology of endothelin-1. Adv Pharmacol. 2010;60:1–26.
- Khimji AK, Rockey DC. Endothelin-biology and disease. Cell Signal. 2010;22:1615–1625.
- Seccia TM, Calo LA. Endothelin-1-induced endothelial mesenchymal transition via endothelin type B receptor stimulation: implication for chronic kidney disease. J Hypertens. 2017;35:1329–1330.
- Koehl B, Nivoit P, El Nemer W, et al. The endothelin B receptor plays a crucial role for the adhesion of neutrophils to the endothelium in sickle cell of disease. Haematologica. 2017;102:1161–1172.
- Becker BK, Speed JS, Powell M, et al. Activation of neuronal endothelin B receptors mediates pressor response through alpha-1 adrenergic receptors. Physiol Rep. 2017;5:e13077.
- Au M, Zhu M, Chang WJ, et al. Emerging role of endothelin receptor type B in regulating chondrogenic and hypertrophic changes of human mesenchymal stem cells. Osteoarthritis Cartilage. 2017;25:S423–S424.
- Meidan R, Levy N. The ovarian endothelin network: an evolving story. Trends Endocrinol Metab. 2007;18:379–385.
- Griswold DE, Douglas SA, Martin LD, et al. Endothelin B receptor modulates inflammatory pain and cutaneous inflammation. Mol Pharmacol. 1999;56:807–812.
- Salani D, Taraboletti G, Rosanò L, et al. Endothelin-1 induces an angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Am J Pathol. 2000;157:1703–1711.
- Bouffet E, Tabori U, Huang A, et al. Possibilities of new therapeutic strategies in brain tumors. Cancer Treat Rev. 2010;36:335–341.
- Binder C, Hagemann T, Sperling S, et al. Stromal endothelin B receptor-deficiency inhibits breast cancer growth and metastasis. Mol Cancer Ther. 2009;8:2452–2460.
- Brunner F, Brás-Silva C, Cerdeira AS, et al. Cardiovascular endothelins: essential regulators of cardiovascular homeostasis. Pharmacol Ther. 2006;111:508–531.
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174.
- Mansh M. Ipilimumab and cancer immunotherapy: a new hope for advanced stage melanoma. Yale J Biol Med. 2011;84:381–389.
- Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700.
- Zhang K, Zhang J, Han L, et al. Wnt/beta-catenin signaling in glioma. J Neuroimmune Pharmacol. 2012;7:740–749.