?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The anti-infection ability and skin regeneration are important aspects on the progress of wound healing, which needs an ideal wound dressing that not only resists bacteria but also promotes skin regeneration. In this study, zinc oxide/silver/polyvinylpyrrolidone/polycaprolactone (ZnO/Ag/PVP/PCL) nanofibres were prepared through electrospinning. Firstly, zinc oxide nanoparticles (ZnONPs) and silver nanoparticle (AgNPs) were synthesized respectively. Secondly, the two nanoparticles were mixed with polyvinylpyrrolidone (PVP) and polycaprolactone (PCL) to obtain the nanofibres. The results of scanning electron microscopy (SEM) showed that ZnONPs and AgNPs were 40.07 ± 9.70 nm and 37.46 ± 12.02 nm, respectively. After electrospinning, the nanofibres were 368.22 ± 123.96 nm in diameter. Infrared spectroscopy revealed that ZnONPs/AgNPs bimetallic nanomaterials were physically embedded in the nanofibres. The antibacterial effects against Staphylococcus aureus and Escherichia coli of ZnO/Ag/PVP/PCL nanofibres were significantly better than these of the single metal material-loaded nanofibres. More importantly, the combination of ZnO and Ag reduced the cytotoxicity of ZnO/Ag/PVP/PCL bimetallic nanofibres toward fibroblasts. These findings demonstrated that ZnO/Ag/PVP/PCL bimetallic nanofibres should be of greater interest than the single metal nanomaterial-loaded nanofibres in inhibiting growth of bacteria.
Introduction
More recently, metal nanomaterials had attracted the scientists’ attention in the field of anti-infection because of their ability to overcome existing drug resistance at suitable doses, which can increase cellular uptake and reduce efflux of drugs from the microbial cell [Citation1]. Zinc oxide nanoparticles (ZnONPs), which possess both antibacterial and anti-inflammatory properties and accelerate the healing of both acute and chronic wounds, are an ideal material for using in inhibiting growth of bacteria [Citation2–6]. In addition, zinc is necessary for the human body as a trace element [Citation7]. However, a lower concentration of zinc oxide inhibits but does not kill microbes and is likely to allow some microbial cells to live and therefore develop resistance when exposed to drug [Citation1,Citation8,Citation9]. It is a contradiction that high concentrations of zinc oxide nanoparticles used would induce intolerance in the body. Therefore, a reliable drug delivery technology that can maintain the superiority of ZnONPs for anti-infection and reduce the dose of administration for drug while not reducing its efficacy on killing bacteria is highly desired.
Silver nanoparticles (AgNPs), another metal materials investigated for a long time, are an alternative antibacterial agent to inhibit/kill bacteria and show a significantly better antibacterial capacity than many antibiotics [Citation10–13]. Similar to ZnO, high amount of Ag used would result in resistance and serious side effects. More importantly, high amount of Ag nanoparticles used trend to aggregate resulting in decreased stability, which in turn reduce the antibacterial efficacy. Although attempts on the development of ZnO or Ag formulations have been made, the optimal antibacterial efficacy and the reduced toxicity still remain challenges [Citation14–16]. Alternatively, Ag materials, especially Ag ions, can enhance the antimicrobial activity of ZnO [Citation17]. In combination, therapy-based ZnO and Ag, both amount of ZnO and Ag could be reduced without reducing the therapeutic efficacy. Furthermore, the ZnO/Ag bimetallic nanomaterials would centralize all advantages of both drugs and provide a general means to maximize therapeutic efficacy, overcome treatment resistance and reduce adverse effects [Citation14]. Consequently, this combination therapy based on ZnO/Ag bimetallic nanomaterials will be a better method to overcome the limitations of metal materials encountered in antimicrobial therapy.
In practical use, polyvinylpyrrolidone (PVP)/polycaprolactone (PCL)-based electrospun nanofibres were performed subsequently to encapsulate ZnO/Ag bimetallic nanomaterials (ZnO/Ag/PVP/PCL). Therefore, in the study, we aim to fabricate ZnO/Ag/PVP/PCL nanofibres with superior antibacterial activity and good biocompatibility by electrospinning technology. After acquisition of ZnONPs and AgNPs, respectively, ZnO/Ag/PVP/PCL nanofibres were prepared by electrospinning. The products were characterized by scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FT-IR) and differential scanning calorimetry (DSC). Cytotoxicity to human skin fibroblasts (HSFs) and antimicrobial activity against Staphylococcus aureus and Escherichia coli of the prepared nanofibres were studied.
Materials and methods
Materials
Silver nitrate was supplied by the Guanghua Sci-Tech Co, Ltd. (Guangzhou, China), and zinc acetate and trifluoroethanol were supplied by Aladdin (Shanghai, China). Oxalate and polyvinylpyrrolidone K30 were purchased from the Sinopharm Chemical Reagent Co, Ltd. (Shanghai, China). Polyvinylpyrrolidone K90 was purchased from Tokyo chemical industry Co, Ltd. (Tokyo, Japan). Polycaprolactoneand dimethyl sulphoxide was obtained from Sigma-Aldrich (St. Louis., MO). 3–(4,5-Methylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was procured from Solon (OH). Mueller–Hinton agar and Mueller–Hinton broth were supplied by the Solarbio (Bejijing, China).
Synthesis and characterization of ZnONPs and AgNPs
Synthesis of ZnONPs
ZnO nanoparticles were synthesized via an oxalate decomposition method [Citation18]. In brief, 1.36 g of anhydrous zinc acetate was added to a round-bottomed flask with 25 ml of ethanol. The reaction system was stirred for 1 h until it formed white solution. The reaction mixture was elevated to 50 °C and stirred for another 30 min. Afterwards, 1.0 g of oxalic acid dihydrate was rapidly added to the reaction vessel under reflux for 3 h. The precipitate was separated from the mixture followed by drying at 75 °C for a whole night. After cooled down, the dried product was ground and calcined at 550 °C for 2 h. The reaction equations are as follows:
Synthesis of AgNPs
In the study, 1.0 g of polyvinylpyrrolidone (PVP K30) was added to a 50 ml round-bottomed flask with 10 ml of ethanol followed by stirred and heated to 50 °C. 10 ml of AgNO3 solution containing ethanol was quickly added into the round-bottomed flask. The concentration of AgNO3 in the final solution was 1.2%. The mixture was kept stirring at 50 °C for another 4 h until the colour of solution did not change. In order to obtain a higher yield of AgNPs, the reaction solution was subjected to centrifugation at 14,000 rpm for 30 min. The lower layer liquid containing the precipitate was separated and then steamed at 40 °C under vacuum.
Characterization of ZnONPs and AgNPs
The morphology of ZnONPs and AgNPs was examined by an S-3000 N SEM equipment (Hitachi, Japan). Briefly, specimens of ZnONPs were uniformly blown on the conductive adhesive followed by placing under SEM to observe their morphology. Similarly, 3 ∼ 5 drops of synthesized AgNPs solution were placed in the conductive adhesive followed by drying. The average particle sizes of ZnONPs and AgNPs were calculated by randomly selecting 50 nanoparticles from the image, respectively.
3 ∼ 5 mg of ZnONPs and AgNPs were, respectively, dissolved in 10 ml of double-distilled water followed by ultrasonic. Then, the samples were characterized by an ultraviolent spectrometer (TU-1810, Beijing Purkinje General Instrument Co., Ltd. China).
The quantity of ZnO and Ag present in the products was determined as follows: 25 mg of the prepared ZnONPs and AgNPs were added to different volumetric flasks, respectively. The samples were dissolved with the dilute nitric acid aqueous solution, which were measured by an atomic absorption spectrophotometer (Z5000, Hitachi).
Preparation of ZnO/ag/PVP/PCL nanofibres
A total mixture of 12% of PVP K90 and PCL was dissolved in 2,2,2-trifluoroethanol in various proportions (PVP, PVP/PCL = 3/1, PVP/PCL = 1, PVP/PCL = 1/3, PCL) to prepare PVP/PCL nanofibres. The polymer solutions were stirred for at least 24 h at room temperature and filled into a syringe with a needle (inter diameter 0.8 mm). Electrospinning was performed in a high-voltage power source. The prepared nanofibres were collected on a 15 × 15 cm aluminium foil, which was electrically grounded. The applied voltages, working distance and velocity were optimized.
After acquisition of the optimal PVP/PCL nanofibres (PVP/PCL = 1/3), the drugs including ZnONPs and AgNPs were loaded into the PVP/PCL nanofibres at different concentrations (1% of ZnONPs (w/v), 1% of AgNPs, 0.25% of ZnONPs + 0.75% of AgNPs, 0.5% of ZnONPs + 0.5% of AgNPs). All the parameters were the same as mentioned earlier. As a result, ZnO/PVP/PCL nanofibres, Ag/PVP/PCL nanofibres, ZnO/Ag/PVP/PCL nanofibres (ZnO/Ag = 1) and ZnO/Ag/PVP/PCL nanofibres (ZnO/Ag = 1/3) were obtained followed by drying under vacuum at 40 °C for 2 h, respectively.
Characterization of the prepared nanofibres
Fabrication and physicochemical characteristics of the nanofibres
The morphology and diameter of the prepared nanofibres were determined via SEM. The diameter of nanofibres was recorded and calculated by randomly selecting 50 nanofibres from the image through Image J software (National Institutes of Health, Bethesda, MD). Fourier transformation infrared spectroscopy (Frontier, PerkinElmer, Waltham, MA) was used to study the structure and chemical bond of the polymer and nanoparticles. The infrared spectra of PVP/PCL (1/3) nanofibres, Ag/PVP/PCL nanofibres, ZnO/PVP/PCL nanofibres and ZnO/Ag/PVP/PCL nanofibres were measured from 4000 cm−1 to 650 cm−1. Thermodynamic properties of the prepared nanofibres were also studied using a differential scanning calorimeter instrument (1100LF, METTLER, Switzerland). The prepared nanofibres were placed in pans and heated from 20 to 450 °C. The heating rate of the instrument was 10 °C per minute.
Water vapour transmission rate
The water vapour transmission rate of the prepared nanofibres was determined by an ASTEM E 96 desiccant method [Citation19]. In brief, the samples were divided into nine groups: PVP nanofibres, PCL nanofibres, PVP/PCL nanofibres (1/3), PVP/PCL nanofibres (1/1), PVP/PCL nanofibres (3/1), Ag/PVP/PCL nanofibres, ZnO/PVP/PCL nanofibres, ZnO/Ag/PVP/PCL nanofibres and non-woven fabrics (used as the control group) [Citation20]. Some allochroic silica gel was put into small cups accompanied with the nanofibres and non-woven fabrics followed by sealing. These cups were placed in a test chamber at 37 °C with a humidity of 75%. The weight change was recorded at a predetermined time and the water vapour transmission rate was calculated by the following formulas.
(1)
(1)
(2)
(2)
RWVP represents water vapour transmission rate (gh−1· cm−2·mmHg−1); W represents the quantity of water permeating the nanofibres (gh−1); A represents the area of the small cup mouth (cm2); ΔP represents the pressure difference on both sides of nanofibres (mmHg); d represents the thickness of nanofibres (cm); and P means the permeability of nanofibres.
Swelling degree and weight loss
The prepared nanofibres and non-woven fabrics were immersed in phosphate buffer solution (PBS, pH = 7.4) at 37 °C for 24 h. The swelling degree and weight loss of the nanofibres were calculated based on the weight change by the following formulas.
(3)
(3)
(4)
(4)
Among the two equations, Wt means wet weight of the nanofibres after immersed in PBS, while Wd means dry weight of the nanofibres after immersed in PBS. Wo represents the initial weight of the nanofibres.
Antibacterial study
Antibacterial activity of the nanofibre formulations was studied using a disc diffusion method and an antibacterial kinetic test with bacterial strains of Staphylococcus aureus and Escherichia coli. The bacterial suspensions in Mueller–Hinton broth with a concentration of 0.5 McFarland were prepared (1.5 × 108 cfu/mL). Then, the suspension was diluted several times to achieve a bacteria concentration at 105 cfu/mL.
Firstly, the nanofibre formulations were cut into circular sheet mat (15 mg; 14.00 mm in diameter) by a circular punch. 100 μL of the diluted bacterial suspension (105 cfu/mL) were coated on the surface of Mueller–Hinton agar (MHA) medium and spread evenly with a triangular glass rod. The disc-shaped nanofibres were adhered to the surface of the medium and the culture dishes were incubated at 37 °C for 24 h.
Bactericidal kinetic study was tested by measuring the OD value of cultured bacterial suspension. Thirty milligrams of cropped nanofibre formulations were added to the sterilized culture tubes containing 10 mL of bacterial suspension (105 cfu/mL), which was cultured at 37 °C for 20 h. At desired time intervals, 150 μL of bacterial suspension were collected for absorbance measurement at 600 nm (Molecular Devices, Sunnyvale, CA).
Cytotoxicity measurement of ZnONPs/AgNPs and the nanofibre formulations
In this study, the potential cytotoxicity of the nanoparticles and nanofibres was evaluated against the human skin fibroblasts (HSFs). Four types of drug-containing medium, including ZnONPs, AgNPs, ZnO/Ag nanoparticles (1/1) and ZnO/Ag nanoparticles (1/3), were prepared at a concentration of 125 ng/mL (ZnO/Ag equivalent), respectively. Then, the original medium in the 96-well plates was replaced by the drug-containing medium followed by incubation at 37 °C for 2 days. Simultaneously, the sterilized nanofibre formulations were added to another 96-well plate before 100 μL of fresh medium was added. At 2- and 4-d post-treatment, the cytotoxicity of ZnONPs/AgNPs and the nanofibres was evaluated via a MTT test. The adherent fibroblasts were purged twice with PBS after the culture medium was sucked away. Another 180 μL of fresh medium was added into the wells before 20 μL of MTT regent (5 mg/mL) was added. The fibroblasts were incubated for another 4 h at 37 °C. Then, 200 μL of dimethyl sulphoxide (DMSO) was added to the wells after the medium was sucked away. The absorbance was measured by a Spectra max plus microplate reader (Molecular Devices) at 490 nm.
Statistical analysis
The data are expressed as the mean plus or minus the standard deviation (mean ± SD). Statistical significance of differences among groups was evaluated using one-way analysis of variance (ANOVA). Differences were considered statistically significant at p < .05.
Results and discussion
Characterization of ZnONPs and AgNPs
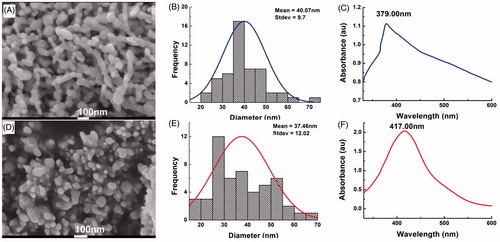
As shown in , it can be obviously observed that ZnONPs and AgNPs were successfully prepared. The morphology of both ZnONPs and AgNPs was spherical in shape and the average diameters of ZnONPs and AgNPs were 40.07 ± 9.70 and 37.46 ± 12.02 nm, respectively. The ultraviolent-visible spectroscopy of both two types of nanoparticles was also investigated and the results were showed in . It can be concluded that the maximum absorption band of ZnONPs and AgNPs is 379 and 417 nm, respectively. Simultaneously, the mass concentration of ZnO and Ag included in the nanopartilces was 84% and 89%, respectively.
Optimization of nanofibre formulations
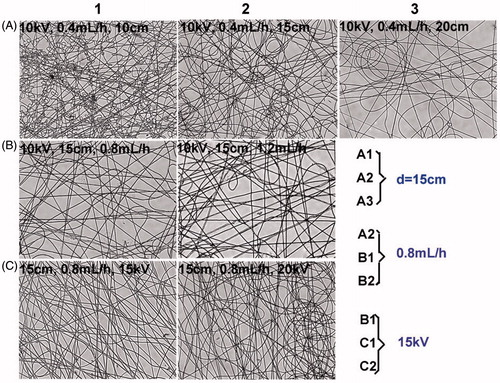
The effects of voltage (10, 15 and 20 kV), flow rate (0.4, 0.8 and 1.2 mL/h) and distance from the nozzle to the receiving plate (10, 15 and 20 cm) on the morphologies of electrospun nanofibres were investigated. Obviously, the nanofibres exhibited the best morphology at the voltage of 15 kV, the flow rate of 0.8 mL/h and the distance from the nozzle to the receiving plate of 15 cm ().
Figure 2. Optimization of nanofibre formulations on the voltage, flow rate and collected distance. The prepared nanofibres were observed by a microscope (200×).
Due to the high hydrophobicity of PCL, hydrophilic PVP was involved into the nanofibres to improve the swelling degree and change other properties of the formulations. To optimize the PVP/PCL proportion, the morphology, water vapour permeability and swelling degree were performed. shows the micrographs of PVP/PCL nanofibres collected on the surface of a glass slide. When high concentration of PVP was used, many fibres collected were crooked. More orderly fibres were observed and the beaded fibres were disappeared gradually as the concentration of PVP decreased. And the satisfactory results were obtained in PVP/PCL (1/3) and PCL groups.
Figure 3. Influence of PVP/PCL proportion on nanofibre formulations. (A) Micrographs of PVP/PCL nanofibres at different mass ratios. (B) Influence of various proportion of PVP/PCL on the water vapour permeability. (C) Influence of various proportion of PVP/PCL on the swelling degree. (D) Influence of various proportion of PVP/PCL on the weight loss. *p < .05; **p < .01; ***p < .001, compared with the control group (n = 3).
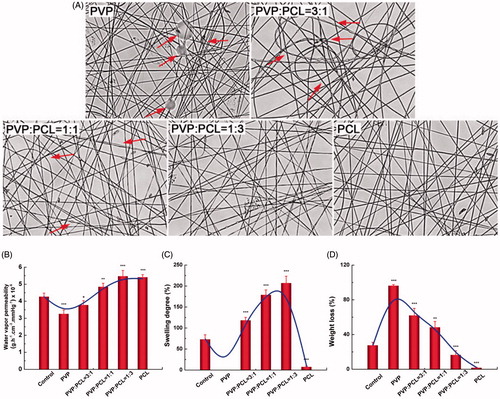
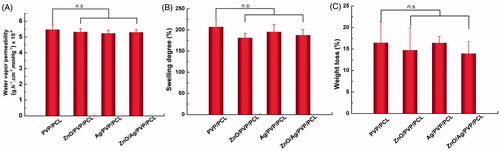
The permeability of moisture and gases (water vapour permeability) is desired in wound dressings to keep the wound comfortable. As a result, water vapour permeability of the nanofibre formulations was determined by an ASTEM E 96 desiccant method. In the study, non-woven fabrics were set as the control group [Citation20]. It can be seen from that the water vapour permeability of pure PVP nanofibres and PVP/PCL nanofibres (3/1) was weaker than non-woven fabrics (p < .05), while the other groups including PVP/PCL (PVP/PCL = 1), PVP/PCL (PVP/PCL = 1/3) and PCL nanofibres were superior to the non-woven fabrics (p< .05). The highest water vapour permeability was obtained in the PVP/PCL nanofibres (1/3) group.
Swelling degree and weight loss are two important parameters to evaluate the preparation of the nanofibres. The swelling degree of PVP nanofibres could not be detected because PVP was almost completely soluble in water. The addition of PVP improved the swelling degree of the nanofibres (). When the PVP/PCL proportion was 1: 3, the swelling degree reached to 200% approximately. The weight loss of the nanofibres had positive correlation with the amount of PVP (). Compared with non-woven fabrics, PVP/PCL (1/3) and PCL group showed the obviously lower weight loss (p< .05). Following with these findings, PVP/PCL (1/3) was performed during the electrospinning process to prepare drug-loaded nanofibres.
Fabrication and characterization of drug-loaded nanofibres
Electrospinning is a feasible, efficient and economy technique that was employed to obtain nanofibres for using in many fields. We selected a 1:3 mass ratio of PVP and PCL to prepare drug-loaded nanofibres, including Ag/PVP/PCL, ZnO/PVP/PCL and ZnO/Ag/PVP/PCL nanofibres (ZnO/Ag = 1/3).
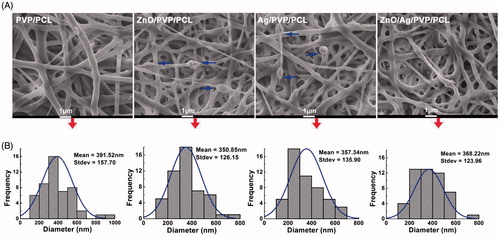
The morphology of nanofibre formulations was investigated by SEM and TEM. SEM images revealed that all the nanofibres were smooth in shape, but the irregular clumps exposed outside of the fibres in ZnO/PVP/PCL and Ag/PVP/PCL groups (), which were considered as the inhomogeneous nanofibres. In the ZnO/Ag/PVP/PCL group, the nanoparticle masses disappeared and ZnO/Ag bimetallic nanoparticles were evenly distributed in the nanofibres (). The average diameters of the nanofibres in the PVP/PCL, Ag/PVP/PCL, ZnO/PVP/PCL and ZnO/Ag/PVP/PCL groups were 391.52 ± 157.70 nm, 357.34 ± 135.90 nm, 350.85 ± 126.15 nm and 368.22 ± 123.96 nm, respectively (). In addition, the ZnO/Ag bimetallic nanoparticles were encapsulated inside the nanofibres. These findings suggested that the single metal nanoparticles clustered easily when electrospinning, which could be overcome by the combination of ZnONPs and AgNPs.
Figure 4. Morphology and in vitro characteristics of ZnO/Ag/PVP/PCL composite nanofibres. (A) Drug-loaded nanofibre formulations visualized by scanning electron microscope (SEM), Scale bar: 1 μm; arrows represent nanoparticle mass. (B) The fibre diameter distribution of drug-loaded nanofibres.
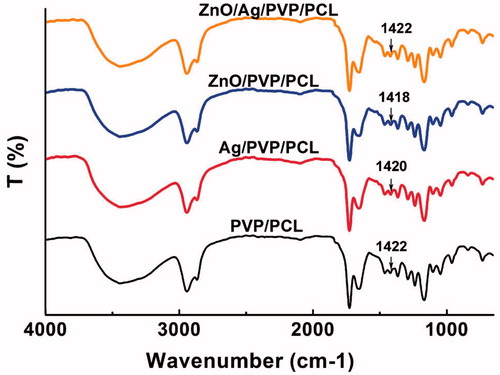
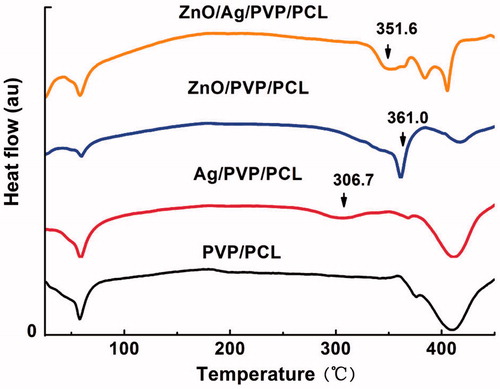
FT-IR is a reliable method for analysing molecular-specific interactions in polymer blends because it is especially sensitive to polymer microstructure. As shown in , the absorption bands of PVP/PCL nanofibres were 2944 cm−1 (C-H asymmetric stretching), 2868 cm−1 (C-H symmetric stretching), 1652 cm−1 (C = O stretching in primary amide), 1464 cm−1 and 1422 cm−1 (C-H deformation), 1366 cm−1 (C-O stretching symmetric) and 1238 cm−1 (C-O-C stretching asymmetric), respectively. After drug entrapment, Ag/PVP/PCL, ZnO/PVP/PCL and ZnO/Ag/PVP/PCL did not change the absorption bands, suggesting that ZnONPs and AgNPs were physically entrapped in the nanofibres through van der Waals forces [Citation21,Citation22]. In addition, differential scanning calorimetry (DSC) was also used to investigate the thermal and crystalline properties of the nanofibres formulations. It can be seen from that the tested materials show the low glass transition temperature (60 °C, higher than the physiological human body temperature). The exothermic peaks of ZnO/PVP/PCL nanofibres and Ag/PVP/PCL nanofibres were 361.0 and 306.7 °C (), respectively, while the exothermic peak of ZnO/Ag/PVP/PCL nanofibres was 351.6 °C. This would be attributed to the formation of bimetallic nanofibres based on ZnONPs and AgNPs. The results showed that the ZnO/Ag bimetallic nanopartilces were dispersed in the nanofibres without aggregation (). Otherwise, the aggregation leaded to reduced antibacterial efficacy [Citation23].
After loaded into the PVP/PCL nanofibres, ZnONPs or/and AgNPs did not influence the water vapour permeability of relative drug-loaded formulations compared with PVP/PCL nanofibres (p> .05) (). In addition, except for a high permeability and hygroscopicity, an ideal wound dressing requires a high degree of swelling and little weight loss. Similarly, in swelling degree and weight loss tests, no significant differences between the drug-loaded nanofibres and PVP/PCL nanofibres were observed (p> .05) (). These findings suggested that the encapsulation of ZnONPs, AgNPs or ZnO/Ag bimetallic nanoparticles does not change the physicochemical properties of PVP/PCL nanofibres.
Antibacterial activity
Staphylococcus aureus (Gram positive) and Escherichia coli (Gram negative) were used to evaluate the antibacterial activity of the nanofibre formulations. Nanofibres with better antibacterial effect can promote wound healing more prominently. In order to assess the antibacterial effect, different mass ratios of ZnO/Ag in the nanofibres were performed to inhibit Staphylococcus aureus and Escherichia coli, which were used as the test organism. The results revealed that different antibacterial activities against both bacteria were obtained after treatment of ZnO- or/and Ag-loaded nanofibres (). At the same time, Ag could improve the antibacterial efficiency of ZnO based on the nanofibres. Compared with ZnO/Ag/PVP/PCL (ZnO/Ag = 1/1), ZnO/Ag/PVP/PCL (ZnO/Ag = 1/3) nanofibres improved the antibacterial efficiency because of the addition of Ag. Among them, ZnO/Ag/PVP/PCL (ZnO/Ag = 1/3) group showed the best antibacterial activity for Escherichia coli. Simultaneously, ZnO/Ag/PVP/PCL (ZnO/Ag = 1/3) and Ag/PVP/PCL groups were the best two groups for Staphylococcus aureus. No antibacterial activity was observed in PVP/PCL group.
Figure 8. The antibacterial effect of the nanofibre formulations against Staphylococcus aureus and Escherichia coli. (A) Inhibition zone of the various contents of drug-loaded nanofibres. (B) Bactericidal kinetic study of Staphylococcus aureus and (C) Bactericidal kinetic study of Escherichia coli. *p< .05; #p<.05, compared with the Ag/PVP/PCL, ZnO/Ag/PVP/PCL (ZnO/Ag =1) and ZnO/PVP/PCL, respectively (n = 3).
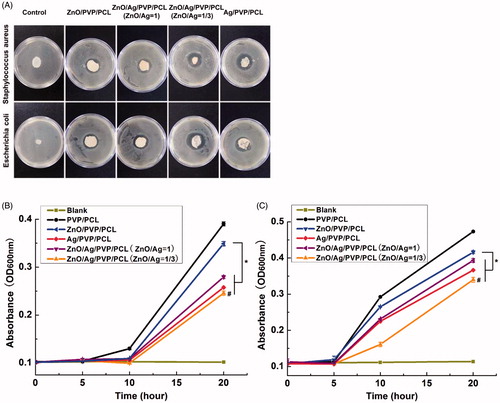
The results of bactericidal kinetic study showed in reflected the growth of Staphylococcus aureus and Escherichia coli in the Mueller–Hinton broth medium. The absorbance value (OD value) increased as the bacteria grew. From the results, we could find that the growth of Staphylococcus aureus and Escherichia coli was inhibited by ZnO/Ag/PVP/PCL nanofibres (ZnO/Ag = 1/3) and Ag/PVP/PCL nanofibres. ZnO/Ag/PVP/PCL nanofibres (ZnO/Ag = 1/3) were superior to the single metal nanopaticles (ZnONPs or AgNPs)-loaded nanofibres and ZnO/Ag/PVP/PCL nanofibres (ZnO/Ag = 1) in inhibiting bacteria. Especially for Escherichia coli, ZnO/Ag/PVP/PCL nanofibres (ZnO/Ag = 1/3) showed the highest antibacterial activity.
As reported, AgNPs can induce bacteria death by destroying cell wall or membrane, disturbing protein synthesis and processing, preventing DNA replication and disturbing the antioxidative system [Citation11,Citation12]. Ag + ions released from AgNPs can attach directly onto cell surfaces and change the membrane permeability, resulting in induction and accumulation of intracellular ROS. On the other hand, ZnONPs are also a well-known antibacterial material that produces ROS to attack the bacterial cells. In our study, hybrid ZnO/Ag nanosystems were shown to be more efficient than single components (ZnONPs or AgNPs) due to synergistic effects of bimetallic combination [Citation24]. Moreover, Ag ions can enhance the antimicrobial activity of ZnO by ROS production [Citation11,Citation17]. Therefore, the enhancement of antibacterial activity induced by the incorporated metal-loaded nanofibres may be caused by the increased oxidative stress followed by cell damage.
Cytotoxicity of ZnO/Ag/PVP/PCL nanofibres
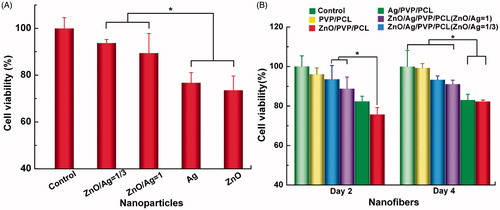
In the study, the MTT test was used to evaluate potential cytotoxicity of the prepared nanofibres, including PVP/PCL, ZnO/PVP/PCL, Ag/PVP/PCL, ZnO/Ag/PVP/PCL (ZnO/Ag = 1) and ZnO/Ag/PVP/PCL nanofibres (ZnO/Ag = 1/3). The ZnONPs alone showed slight cytotoxicity towards HSF cells at a concentration of 125 ng/mL (), while reducing the amount of ZnO (the mixed nanoparticles) could increase the cell viability (p<.05). It can conclude that the combined use of ZnONPs and AgNPs could reduce the cytotoxicity but not reduce the antibacterial effect. Similarly, a slight cytotoxicity was observed in ZnO/PVP/PCL nanofibre-treated group after a long-time administration, while ZnO/Ag bimetallic nanofibres showed no cytotoxicity (). The property of cell adhesion was improved by ZnO/Ag/PVP/PCL nanofibres compared with ZnO/PVP/PCL and Ag/PVP/PCL (data not shown). It represents that ZnO/Ag/PVP/PCL nanofibres, especially ZnO/Ag/PVP/PCL nanofibres (ZnO/Ag = 1/3), had lower cytotoxicity and better biocompatibility than Ag/PVP/PCL and ZnO/PVP/PCL nanofibres at a lower concentration. The results suggested that the cytotoxicity of ZnO could be reduced by the ZnO/Ag bimetallic nanoparticles. Therefore, we selected a 1:3 mass ratio of ZnO/Ag to prepare the electrospun nanofibres.
Figure 9. Cytotoxicity of ZnO and Ag against the human skin fibroblasts (HSFs). (A) Cell viability of ZnONPs and AgNPs towards HSFs. (B) Cell viability of the prepared nanofibres towards HSFs at day 2 and 4. *p<.05 (n = 3).
Due to the advantages of antibacterial activity, anti-inflammatory and antiwrinkle properties, promotion of tissue regeneration and so on, ZnONPs had become an ideal nanomaterial for bacteriostasis. And zinc is essential for life as a trace element. However, a lower concentration of ZnONPs shows the ability to inhibit but does not kill microbes and therefore may develop resistance when exposed to drug frequently. On the other hand, both single metal nanoparticles including ZnONPs and AgNPs clustered readily when used (), which resulted in decreased stability and reduced antibacterial efficacy [Citation23,Citation25]. In addition, nanoparticles with smaller particle size possess stronger antibacterial activity but slightly higher toxicity than the larger ones [Citation10,Citation26]. In our study, the average diameter of ZnONPs was 40.07 ± 9.70 nm, which were prepared by a solution-gel method [Citation18] and the average diameter of AgNPs was 37.46 ± 12.02 nm, which were prepared by a PVP-capped method [Citation27,Citation28]. These were just the reasons that would induce the cytotoxicity of ZnONPs and AgNPs. To overcome these problems, the major method was to decrease the amount of used drugs, which always reduce the therapeutic effect. In the light of this, the combination of two drugs would keep or even rise the therapeutic effect with lower toxicity. In order to improve the antibacterial effect and reduce the side effect and the resistance; therefore, an appropriate amount of AgNPs, which also possesses the strong bactericidal ability and anti-inflammatory effect were synergistically used with the nano-zinc oxide. As a consequence, electrospun nanofibres for ZnO/Ag bimetallic nanoparticles can contribute to the improved antibacterial activity with low toxicity. ZnO/Ag bimetallic nanofibres not only increased the antimicrobial effect, but also reduced the cytotoxicity of drugs towards fibroblasts. Therefore, the combination of ZnO and Ag in the form of the composite nanofibres has clearly not only improved the antibacterial efficacy but also reduced the cytoxicity of the two materials.
Conclusions
In conclusion, we have developed a new wound dressing that combines ZnONPs and AgNPs to produce an improved antimicrobial activity against both Gram-negative and Gram-positive bacteria. The prepared ZnO/Ag/PVP/PCL nanofibres showed the excellent gaseous exchange ability, a strong haemostatic ability and a low weight loss rate. Simultaneously, the bimetallic nanofibres could synergistically improve the antibacterial effect. These preliminary findings provide a new candidate in treating the increasing antibacterial resistance and infection.
Disclosure statement
The authors declare that they have no competing interests.
References
- Pelgrift RY, Friedman AJ. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv Drug Deliv Rev. 2013;65:1803.
- Raguvaran R, Manuja BK, Chopra M, et al. Sodium alginate and gum acacia hydrogels of ZnO nanoparticles show wound healing effect on fibroblast cells. Int J Biol Macromol. 2017;185–191.
- Lu Z, Gao J, He Q, et al. Enhanced antibacterial and wound healing activities of microporous chitosan-Ag/ZnO composite dressing. Carbohydr Polym. 2016;156:460–469.
- Díez-Pascual AM, Díez-Vicente AL. Wound healing bionanocomposites based on castor oil polymeric films reinforced with chitosan-modified ZnO nanoparticles. Biomacromolecules. 2015;16:2631.
- Rakhshaei R, Namazi H. A potential bioactive wound dressing based on carboxymethyl cellulose/ZnO impregnated MCM-41 nanocomposite hydrogel. Mater Sci Eng C. 2017;73:456–464.
- Vasile BS, Oprea O, Voicu G, et al. Synthesis and characterization of a novel controlled release zinc oxide/gentamicin-chitosan composite with potential applications in wounds care. Int J Pharm. 2014;463:161–169.
- Pedersen L, Andersen-Ranberg K, Hollergaard M, Nybo M. Quantification of multiple elements in dried blood spot samples. Clin Biochem. 2017;50:703–709.
- Umair M, Sumera S, Rafay A, et al. Antibacterial, structural and optical characterization of mechano-chemically prepared ZnO nanoparticles. PLoS One. 2016;11:e0154704.
- Rădulescu M, Andronescu E, Cirja A, et al. Antimicrobial coatings based on zinc oxide and orange oil for improved bioactive wound dressings and other applications. Rom J Morphol Embryol. 2016;57:107.
- Zhang XF, Liu ZG, Shen W, et al. Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. IJMS. 2016;17:1534.
- Zhang Y, Gao X, Zhi L, et al. The synergetic antibacterial activity of Ag islands on ZnO (Ag/ZnO) heterostructure nanoparticles and its mode of action. J Inorg Biochem. 2014;130:74–83.
- Singh H. DJaYT. Biosynthesis of silver nanoparticles using Aeromonas sp. THG-FG1.2 and its antibacterial activity against pathogenic microbes. Artif Cells Nanomed Biotechnol. 2017;45:584–590.
- Francis S, Joseph S, Koshy EP, et al. Microwave assisted green synthesis of silver nanoparticles using leaf extract of elephantopus scaber and its environmental and biological applications. Artif Cells Nanomed Biotechnol. DOI:10.1080/21691401.2017.1345921
- Yamani NE, Collins AR, Rundénpran E, et al. In vitro genotoxicity testing of four reference metal nanomaterials, titanium dioxide, zinc oxide, cerium oxide and silver: towards reliable hazard assessment. Mutagenesis. 2017;32:117–126.
- Zhang T, Wang L, Chen Q, et al. Cytotoxic potential of silver nanoparticles. Yonsei Med J. 2014;55:283–291.
- Wiesenthal A. Nanoparticles: small and mighty. Int J Dermatol. 2011;50:247–254.
- Liu Y, Kim HI. Characterization and antibacterial properties of genipin-crosslinked chitosan/poly(ethylene glycol)/ZnO/Ag nanocomposites. Carbohydr Polym. 2012;89:111.
- Jafari A, Ghane M, Arastoo S. Synergistic antibacterial effects of nano zinc oxide combined with silver nanocrystales. Afr J Microbiol Res. 2011;5:5465–5473.
- Vargas EAT, Baracho NCDV, Brito JD, et al. Hyperbranched polyglycerol electrospun nanofibers for wound dressing applications. Acta Biomater. 2010;6:1069–1078.
- Montaser AS, Abdel-Mohsen AM, Ramadan MA, et al. Preparation and characterization of alginate/silver/nicotinamide nanocomposites for treating diabetic wounds. Int J Biol Macromol. 2016;92:739–747.
- Li C, Fu R, Yu C, et al. Silver nanoparticle/chitosan oligosaccharide/poly(vinyl alcohol) nanofibers as wound dressings: a preclinical study. Int J Nanomed. 2013;8:4131–4145.
- Madhumathi K, Kumar PTS, Abhilash S, et al. Development of novel chitin/nanosilver composite scaffolds for wound dressing applications. J Mater Sci: Mater Med. 2010;21:807–813.
- Siddiq AM, Parandhaman T, Begam AF, et al. Effect of gemini surfactant (16-6-16) on the synthesis of silver nanoparticles: a facile approach for antibacterial application. Enzyme Microb Technol. 2016;95:118–127.
- Mallevre F, Fernandes TF, Aspray TJ. Silver, zinc oxide and titanium dioxide nanoparticle ecotoxicity to bioluminescent Pseudomonas putida in laboratory medium and artificial wastewater. Environ Pollut. 2014;195:218–225.
- Shrivastava S, Bera T, Roy A, et al. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology. 2007;18:225103.
- Adamcakova-Dodd A, Stebounova LV, Kim JS, et al. Toxicity assessment of zinc oxide nanoparticles using sub-acute and sub-chronic murine inhalation models. Part Fibre Toxicol. 2014;11:15.
- Pereira RF, Bártolo PJ. Traditional therapies for skin wound healing. Adv Wound Care (New Rochelle). 2016;5:208.
- Tian L, Wang P, Zhao Z, et al. Antimicrobial activity of electrospun poly(butylenes succinate) fiber mats containing PVP-capped silver nanoparticles. Appl Biochem Biotechnol. 2013;171:1890–1899.