Abstract
The aim of this study is to develop thermo-sensitive in situ gelling formulation of KCL based on poly(N-isopropylacrylamide)/hyaluronic acid. The prepared preparations were characterized for in vitro gelation, drug release and antifungal activity. In this study, drug content of prepared gels was found to be in the range of 91–96%. The pH value was in the range of 6.0–7.5. By measuring the gelation temperature of the prepared PN-HA thermogelling solutions, it was 33 °C. In vitro release showed that the release of KCL from in situ gels was moderate without burst effects. The KCL PN-HA in situ gels were well-tolerated by the rabbits, and no macroscopic signs of irritation, redness, or other toxic effects were observed. From in vivo antimicrobial study, KCL PN-HA in situ gels may be concluded to have a better cure percent in clinical profile and negative growth of Candida albicans in microbiological test. Therefore, this new formulation could prove to be a novel ocular dosage form able to prolong the residence time and to control the release of drug when administered into the eye.
Introduction
Ophthalmic delivery is one of the most challenging tasks for pharmaceutical practitioners [Citation1]. The unique structure of the eye restricts the entry of drug molecules into the desired site. More than 90% of the marketed ophthalmic preparations are produced in the form of eye drops, which are washed away from the eye by various mechanisms, such as lachrymation, tear dilution, and turnover, resulting in low ocular bioavailability of drugs [Citation2]. In addition, the human cornea, including the epithelium, substantia propria, and endothelium, limits the entry of drug molecules [Citation3]. As a result of these factors, <5% of the administered drug enters the eye.
To overcome these problems, a variety of ophthalmic vehicles, such as viscous solutions, ointments, gels or polymeric inserts have been trying to extend the ocular residence time of medications for topical application to the eye [Citation4]. The corneal contact time has been increased to varying degrees by these vehicles. However, they do not receive consistent approval because of blurred vision (e.g. ointments) or lack of patient adherence (e.g. inserts). Therefore, good ocular bioavailability of a drug by following topical delivery remains a challenge and need to be addressed [Citation5,Citation6].
Generally biodegradable nanoparticles of natural polysaccharides have attracted much attention because of their good biocompatibility, biodegradability and protective actions [Citation7,Citation8]. Another interesting approach to ocular drug delivery is the use of hydrogel systems. Hydrogels are hydrophilic polymers that can be swollen in water or water solvents. However, preformed hydrogels do not allow precise or reproducible administration of drugs, and after administration they often generated in blurred vision, scabby eyelids, and tears. Therefore, the most promising method is to use in situ formed hydrogels, which can be instilled as eye drops and immediately gelled in contact with the eye [Citation9].
In situ gel can be divided into three types according to their phase transition properties: temperature sensitive, pH sensitive, and ionic strength sensitive. Temperature-sensitive material mainly include block copolymer and poloxamer [Citation10]. pH-sensitive material include cellulose acetate phthalate and acrylic acid polymers, which, by changing the pH value of the environment, can promote the transition [Citation11]. Among ion-sensitive material, sodium alginate and deacetylase gellan gum are the two most commonly used [Citation12,Citation13].
Temperature-sensitive polymers such as poly(N-isopropylacrylamide) (PN) have been widely used in drug delivery systems to release drugs in response to the changes in surrounding temperature [Citation14]. Copolymerization of PN with hyaluronic acid (HA) has shown success in increasing the lower critical solution temperature (LCST) of PN from 32 °C to the temperatures above physiological temperature, which is more suitable for ophthalmic application. HA is a liner glycosaminoglycan composed of repeating disaccharide units of N-acetyl-d-glucosamine and d-glucuronic acid. HA widely exists in human body and is the major component of the extracellular matrix. Due to its good biocompatibility and biodegradability, HA shows excellent potential for application in drug delivery, wound healing and tissue engineering but the rapid degradation of HA compromise its effectiveness for tissue engineering [Citation15,Citation16].
Ketoconazole (KCL) is a synthetic imidazole drug used primarily to treat different types of body fungal infections. The drug works by inhibiting the enzyme cytochrome P450 14α-demethylase, which contributes to the synthesis of the fungal ergosterol [Citation17]. When KCL is administered for treatment of fungal eye infections, it is characterized by a very short duration of action since its elimination half-life in the aqueous humor and cornea is ∼19 and 43 min, respectively [Citation18]. Although KCL is characterized by its high lipid solubility (log p = 4.74), which may support permeation through biological membranes, especially through the corneal epithelium, the drug high molecular weight (MW; 531.44 Da) hinders its transport. Moreover, due to its high hydrophobicity and poor aqueous solubility, KCL cannot be formulated into the common aqueous formulations, leading to a low absorption and bioavailability.
Thus, the aim of this study is to develop an in situ gelling formulation of KCL based on PN-HA with respect to their concentrations in the simulated tear fluid (STF, pH 7.4). The prepared preparations were characterized for in vitro gelation, drug release, antifungal activity and permeation through epithelial cell lines. This new formulation could prove to be a novel ocular dosage form able to prolong the residence time and to control the release of drug when administered into the eye.
Material and methods
Material
KCL was given by Yinxing Medical Tech Ltd. (Hubei, China; patch number: 20160223). PN-HA compound was synthetized from Weida Biopharmaceutical Co., Ltd (Wuhan, China), (HA; MW =1000 Da). Purified water from Milli-Q system (Millipore, Bedford, MA) was used throughout the experiment. All other reagents were of commercially analytical grade.
Preparation of KCL PN-HA in situ gel
Thermo-sensitive ophthalmic in situ gel was prepared by dissolving a certain amount of PN-HA in 10 ml double-distilled water, followed by keeping under magnetic stirring at 4 °C for 30 min. Then, KCL (0.5%, w/v), chlorhexidine acetate (0.01%, w/v), and glycerol (2%, w/v) were slowly added to the system and mixed well for at least 30 min. Once completely dissolved, the solution was cooled to a temperature below 10 °C. The pH of the formulation was between 6.0 and 8.0. In this formulation, PN-HA served as a gel base, and chlorhexidine acetate, a preservative.
Drug content, osmotic pressure and pH measurements
Gel equivalent to 10 mg of KCL was dissolved in 100 ml distilled water and determined by HPLC method. Osmotic pressure and pH measurements of KCL PN-HA in situ gel were performed by OSMOMAT 030-D (Gonotec GmbH, Berlin, Germany), S400 (Mettler Toledo, Zurich, Switzerland), respectively. Experiments were performed in triplicate.
Sol-gel transition
The test tube inverting method was used to determine the sol-gel transition temperatures of the copolymer sol in water [Citation19]. Each sample at a given concentration was prepared by dissolving the copolymer in distilled water in a vial and stored at 4 °C for 24 h. The vials containing 20 ml copolymer sol were immersed in an oil bath at different setting temperatures and allowed to reach equilibrium. The sample was regarded as a “gel” when flow was no longer visually observed within 30 s by inverting the vial with a temperature increment of 2 °C per step. Then, the viscosity of the formulation, either in solution or in gel, was determined with a rotational viscometer using a proper sample. Measurements were performed using suitable spindle number at different rotation rates. The viscosity was read directly from the viscometer display. All measurements were made in triplicate.
Stability study
The proposal of stability study was modified mainly based on the guiding principles of the Chinese Pharmacopoeia. KCL in situ gel was placed in three stable boxes kept at 4 °C, room temperature, and 40 °C, and saturated sodium chloride solution was added and maintained at a relative humidity of 75 ± 5%. Then, at 0 day, 30 days, 60 days and 90 days of the test, they were observed to find if the gel content, appearance, gelation, and viscosity were changed or not.
In vitro release
The in vitro release of KCL from the gels was measured through a cellulose acetate dialysis membrane employing classic paddle method. Briefly, 0.1 ml KCL gels were immersed into 250 ml distilled water as a dissolution medium at 33 ± 0.5 °C. It was stirred at the paddle speed of 50 rpm for 48 h. At predetermined time intervals, 2 ml of the medium was withdrawn, diluted with the dissolution medium and filtered using a 0.45 μm syringe filter. The concentration of the drug was determined at 350 nm by using HPLC method [Citation20]. Meanwhile, the release of MCL raw material (100 mg) was monitored under control.
In vivo eye irritation test
The in vivo eye irritation test of the KCL PN-HA in situ gels and the commercial KCL eye drops were performed in two groups of 12 New Zealand White rabbits. The first group received 25 μL of the KCL PN-HA in situ gels, which was instilled into the lower conjunctival sac of the rabbit’s right eye, while the left eye was kept as a control without manipulation. The second group received the same volume of commercial KCL eye drops, administered as for the first group. The test eye was observed at 0, 5, 10, and 30 min and 1, 6, 12, 24, 48, and 72 h for changes to the cornea, iris, conjunctiva, and chemosis compared to the control. The degree of eye irritation was scored following the classical Draize test.
In vivo antimicrobial study
Twenty-four New Zealand White rabbits were used for in vivo antimicrobial studies. The 24 rabbits were divided into two groups with group A assigned for KCL PN-HA in situ gels while group B was assigned for commercial KCL eye drops. Both eyes of each rabbit were inoculated with fungi (Candida albicans). In each group, the left eye of all rabbits received the placebo as a control. The right eye for one group received the KCL PN-HA in situ gels while the other group received commercial KCL eye drops. The rabbits were sedated by ketamine. The rabbits’ corneas were marked with trephine 7 mm, and removal of surface epitheliumin the form of scraping was done. Washing the eyes with topical tobramycin was made. Exactly 100 μL (500,000 spores) of the spore suspension of fungal strain was injected into the corneal stroma. The rabbits’ eyes were examined every day. Both formulations was applied three times per day (every 8 h) for 10 days. These formulae were started 48 h following inoculation.
Follow-up of size and depth of corneal ulcer, and signs of inflammation in the form of severe iritis or hypopyon was done for each rabbit and recorded according to their enumeration. After antimicrobial studies, the rabbits were scarified after 12 days from inoculation. The corneas were excised at limbal margin and each cornea was used for microbiological and pathological evaluation.
Statistical analysis
Data were statistically described in terms of mean ± standard deviation (±SD). Student’s t-test was used to evaluate associations between two groups’ data. All p < .05 were considered statistically significant. All statistical calculations were done using computer programs SPSS (Statistical Package for the Social Science; SPSS Inc., Chicago, IL) version 21 for Microsoft Windows.
Results and discussion
Characterization of gel
On the basis of our previous study, aqueous solutions of PN-HA were chosen as precursors of in situ-forming hydrogels. In this study, drug content of prepared gels was found to be in the range of 91–96%. The pH value of the optimized formulation was in the range of 6.0–7.5. The state of in situ-forming hydrogels before and after gelation were shown in . In order to investigate the effect of the temperature during the gelation time, the fluidity of PN-HA solutions was measured using the viscosity method [Citation21].
An acceptable ophthalmic thermogelling solution must have a gelation temperature in the range of 32–36 °C so as to be in liquid form at room temperature and to form a gel phase instantly in orbital cavity. The results of viscosity measurement showed that the preparations behaved like a fluid, but formed a rigid gel when exposed to increasing temperature. By measuring the gelation temperature of the prepared PN-HA thermogelling solutions, it was 33 °C ().
Figure 2. Mean viscosity–temperature profiles of KCL PN-HA in situ gels. KCL (0.5%, w/v), PN-HA (1%, w/v), chlorhexidine acetate (0.01%, w/v), and glycerol (2%, w/v), (n = 3).
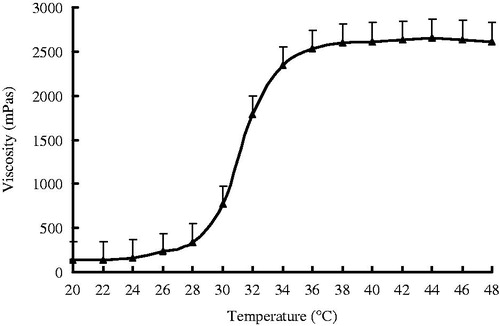
These results could be due to the addition of PN-HA, leading to the formation of thermogelling solution due to the following: (i) with the increase of temperature, the arrangement of the whole system is broken, from homogeneous one to heterogeneous one. (ii) PN has hydrophilic amino group and hydrophobic isopropyl at the same time, which makes the PN linear aqueous solution and the PN crosslinked hydrogel also show temperature sensitive characteristics.
There were negligible alterations in the initial values of viscosity of the formulations over a storage period of 90 days. The samples were also analyzed for drug content by the HPLC method. Again, the drug degraded to a negligible extent, and the percentage of drug degradation is <5%. Many factors affected the stability of a pharmaceutical product, including the stability of the active ingredient(s), and the potential interaction between active and inactive ingredients. To calculate the shelf life of the formulation, extensive stability data are collected according to the International Conference on Harmonization guidelines ().
Table 1. The stability studies of the KCL in situ gel during the 3 months.
In vitro release
In vitro drug release behavior of KCL in situ gels was studied using a dialysis membrane method. The release profiles of free drug and in situ gels were shown in . A very fast release behavior of free KCL was observed, whereas the cumulative release rate of KCL gels was much slower followed by a sustained release. In free KCL group, 95% of KCL were released in the first 2 h. In contrast, only 30% KCL were released from gels in the first 2 h (p < .01). The results showed that the release of KCL from in situ gels was moderate without burst effects. It was most likely that PN-HA underwent a rapid sol-gel transition when exposed to 33 °C dissolution medium as confirmed by the viscosity experiment. During the hydrogel formation, a portion of KCL might be loaded into the hydrogel phase, and thus the drug release became slow. The in vitro release was kinetically analyzed according to zero-order, first-order, and the diffusion-controlled release mechanism. The relative high correlation coefficient values obtained from the analysis of the amount of the drug released versus the square root of time indicated the release followed the Higuchi (Higuchi, 1962) kinetic model, as shown in .
Figure 3. In vitro release profiles of KCL in situ gels from three batches. Release experiments were carried out in distilled water as a dissolution medium at 33 ± 0.5 °C. Each point represents the mean value of three different mean ± SD. △: free drug; ▲: KCL in situ gels.
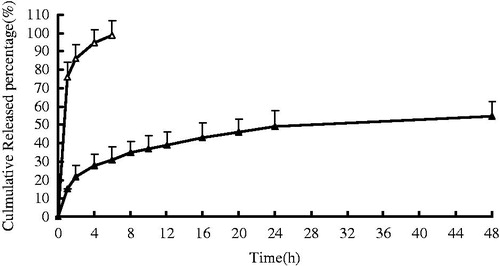
Table 2. Correlation coefficients for kinetic analysis of release data for KCL in situ gels.
Eye irritation test
The results of the eye irritation test of the KCL PN-HA in situ gels and the commercial KCL eye drops studied in New Zealand White rabbits are shown in . KCL PN-HA in situ gels did not irritate rabbits’ eyes, as seen in the total score of an eye irritation assessment, equaling to zero. Compared to the commercial KCL eye drops group, the eyes of the rabbits in the group with KCL PN-HA in situ gels looked normal. The KCL PN-HA in situ gels were well-tolerated by the rabbits, and no macroscopic signs of irritation, redness, or other toxic effects were observed. Therefore, KCL PN-HA in situ gels can be used as a safe formulation for ophthalmic use.
Table 3. Score obtained from eye irritation assessment of KCL PN-HA in situ gels and commercial KCL eye drops in New Zealand white rabbits.
In vivo antimicrobial study
All rabbits after 48 h from inoculation started receiving treatment. The rabbits’ corneas before treatment suffered from deep stromal infiltration and a surrounding stromal edema and inflammation. shows the percentage of cured animal treated with both formulations (p < .05). The cure was judged by improvement in size and depth of ulcer and hypopyon with improvement of stromal edema and obvious healing epithelium. The histological changes observed in the sections of the excised corneal tissues are shown in . In our study, the highest cure rate was obvious in the eyes which received KCL PN-HA in situ gels (91.7%), followed by the eyes which received commercial KCL eye drops (66.7%), and then the eyes which received placebo control (0%), with a significant difference between three groups (p < .05). From all of these results, KCL PN-HA in situ gels may be concluded to have a better cure percent in clinical profile and negative growth of C. albicans in microbiological test. In addition, it showed an absence of mild inflammation on histopathology. This makes this formulation a good candidate for further clinical trials on human corneas.
Figure 4. Pathological observation of the corneal tissues in the established rabbits’ models. (×5000). A: normal; B: KCL PN-HA in situ gels; C: commercial KCL eye drops; D: negtive control.
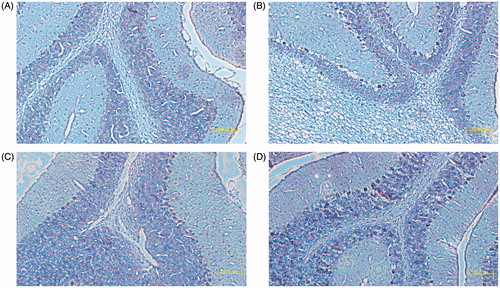
Table 4. Effect of different KCL antifungal formulations on rabbits’ corneas (n = 12).
Acknowledgment
We wish to express our thanks to Dr. Yang Wang (Department of Pharmacy, Fudan University, Shanghai, China) for her help in the design of animal experiments.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Alami-Milani M, Zakeri-Milani P, Valizadeh H, et al. Novel pentablock copolymers as thermosensitive self-assembling micelles for ocular drug delivery. Adv Pharm Bull. 2017;7:11–20.
- Kotreka UK, Davis VL, Adeyeye MC. Development of topical ophthalmic in situ gel-forming estradiol delivery system intended for the prevention of age-related cataracts. PLoS One. 2017;12:e0172306.
- Le Bourlais C, Acar L, Zia H, et al. Ophthalmic drug delivery systems–recent advances. Prog Retin Eye Res. 1998;17:35–58.
- Dominguez-Godinez C, Carracedo G, Pintor J. Diquafosol delivery from silicone hydrogel contact lenses: improved effect on tear secretion. J Ocul Pharmacol Ther. 2017; DOI: 10.1089/jop.2016.0193.
- Shen HH, Chan EC, Lee JH, et al. Nanocarriers for treatment of ocular neovascularization in the back of the eye: new vehicles for ophthalmic drug delivery. Nanomedicine (Lond). 2015;10:2093–2107.
- Hartnett TE, O’Connor AJ, Ladewig K. Cubosomes and other potential ocular drug delivery vehicles for macromolecular therapeutics. Expert Opin Drug Deliv. 2015;12:1513–1526.
- Yadav M, Ahuja M. Preparation and evaluation of nanoparticles of gum cordia, an anionic polysaccharide for ophthalmic delivery. Carbohydr Polym. 2010;81:871–877.
- Kaur H, Ahuja M, Kumar S, et al. Carboxymethyl tamarind kernel polysaccharide nanoparticles for ophthalmic drug delivery. Biol Macromolecules. 2012;50:833–839.
- Agrawal AK, Das M, Jain S. In situ gel systems as ‘smart’ carriers for sustained ocular drug delivery. Expert Opin Drug Deliv. 2012;9:383–402.
- Matanović MR, Kristl J, Grabnar PA. Thermoresponsive polymers: insights into decisive hydrogel characteristics, mechanisms of gelation, and promising biomedical applications. Int J Pharm. 2014;472:262–275.
- Singh NK, Lee DS. In situ gelling pH- and temperature-sensitive biodegradable block copolymer hydrogels for drug delivery. J Control Release. 2014;193:214–227.
- Sharma A, Sharma J, Kaur R, et al. Development and characterization of in situ oral gel of spiramycin. Biomed Res Int. 2014;2014:876182.
- Mahdi MH, Conway BR, Smith AM. Evaluation of gellan gum fluid gels as modified release oral liquids. Int J Pharm. 2014;475:335–343.
- Sundaresan V, Menon JU, Rahimi M, et al. Dual-responsive polymer-coated iron oxide nanoparticles for drug delivery and imaging applications. Int J Pharm. 2014;466:1–7.
- Zhang Y, Heher P, Hilborn J, et al. Hyaluronic acid-fibrin interpenetrating double network hydrogelprepared in situ by orthogonal disulfide cross-linking reaction for biomedical applications. Acta Biom Ater. 2016;38:23–32.
- Talaat WM, Haider M, Kawas SA, et al. Chitosan-based thermosensitive hydrogel for controlled drug delivery to the temporomandibular joint. J Craniofac Surg. 2016;27:735–740.
- Loose DS, Kan PB, Hirst MA, et al. Ketoconazole blocks adrenal steroidogenesis by inhibiting cytochrome P450-dependent enzymes. J Clin Invest. 1983;71:1495–1499.
- Zhang J, Wang L, Gao C, et al. Ocular pharmacokinetics of topically-applied ketoconazole solution containing hydroxypropyl beta-cyclodextrin to rabbits. J Ocul Pharmacol Ther. 2008;24:501–506.
- Yu L, Zhang Z, Zhang H, et al. Biodegradability and biocompatibility of thermoreversible hydrogels formed from mixing a sol and a precipitate of block copolymers in water. Biomacromolecules. 2010;11:2169–2178.
- Venishetty VK, Parikh N, Sistla R, et al. Application of validated RP-HPLC method for simultaneous determination of docetaxel and ketoconazole in solid lipid nanoparticles. J Chromatogr Sci. 2011;49:136–141.
- Lv Z, Chang L, Long X, et al. Thermosensitive in situ hydrogel based on the hybrid of hyaluronic acid and modified PCL/PEG triblock copolymer. Carbohydr Polym. 2014;108:26–33.

