Abstract
Soybean lipoxygenase, recombinant rice allene oxide synthase-1 and rice allene oxide cyclase were covalently immobilized on nanoporous rice husk silica using two types of linkers: glutardialdehyde and polyethylene glycol. The immobilization efficiency achieved using glutardialdehyde-linked rice husk silica was higher than that achieved using polyethylene glycol-linked rice husk silica (50–92% and 25–50%, respectively). Immobilization on both types of matrices significantly decreased the specific activities of the immobilized enzymes. Solid-phase reaction yields of the enzymes were determined relative to the yields observed for the solution-phase reactions. Yields of the solid-phase reactions catalyzed by immobilized soybean lipoxygenase, rice allene oxide synthase-1, and rice allene oxide cyclase ranged from 50% to 230% and were dependent on both the enzymes and linkers used. Production of cis(+)-12-oxophytodienoic acid from α-linolenic acid by consecutive reactions using all three enzymes in a co-immobilization system resulted in 83.6% and 65.1% yields on glutardialdehyde-linked and epichlorohydrin-polyethylene glycol-linked rice husk silica, respectively. Our results suggest that immobilization of biosynthetic enzymes of the octadecanoid pathway on rice husk silica may be an efficient method for the in vitro production of oxylipins. Additionally, enzyme immobilizations on rice husk silica matrices may be more broadly applicable for producing physiologically important compounds in other biosynthetic pathways.
Introduction
Oxylipins are a group of lipid-derived signalling compounds found in both plants and animals that play important roles in defence and development. Oxylipin synthesis in plants is a multistep process initiated by the conversion of polyunsaturated fatty acids (PUFAs) into their corresponding hydroperoxides, catalyzed by lipoxygenase (LOX). One such PUFA, α-linolenic acid (LnA), is converted by LOX into 13-hydroperoxy-9,11,15-octadecatrienoic acid (HPOTE). This product is further metabolized by consecutive reactions catalyzed by allene oxide synthase (AOS) and allene oxide cyclase (AOC) to produce cis(+)-12-oxophytodienoic acid (cis(+)-12-OPDA) as part of the jasmonic acid biosynthetic pathway (). The compound, cis(+)-12-OPDA, is a plant hormone that regulates plant growth and developmental processes [Citation1–3]. In mammalian cells, this hormone has been shown to display cytoprotective effects [Citation4]. Despite the importance of cis(+)-12-OPDA in both plants and animals, the in vivo regulation of oxylipin biosynthesis is difficult, due to the complicated network of signalling molecules that modulate this pathway, the unstable intermediates, and the different cellular locations of the individual enzymes required [Citation3]. Therefore, a well-controlled in vitro synthesis of cis(+)-12-OPDA may overcome these in vivo obstacles and may benefit the agriculture and biomedicine industries.
Figure 1. The allene oxide synthase pathway resulting in the production of cis(+)-12-oxophytodienoic acid from α-linolenic acid (18:3). The pathway requires the cooperative action of 13-LOX, AOS and AOC. In the absence of AOC, the unstable 12,13-EOT intermediate is non-enzymatically converted to α-ketol, γ-ketol and the racemic mixture of 12-OPDA as indicated by the dotted pathway.
Solution-phase enzymatic synthesis has several drawbacks, which limit its use in in vitro production of compounds, such as low operational stability, high cost and difficulty in enzyme recovery and reuse. The instability of allene oxide produced by AOS in the oxylipin pathway is another hurdle in the production of cis(+)-12-OPDA in solution-phase conditions () [Citation5]. Immobilization of enzymes on support matrices may overcome these drawbacks by improving the stability of the immobilized enzymes and would solve the problem of enzyme recovery and reuse, thereby improving both cost-effectiveness and efficiency of the synthesis. Many studies have already reported on immobilization of enzymes related to the oxylipin pathway, such as the immobilization of LOX, using the adsorption method, on glutenin and gliadin as well as on glass and glass wool [Citation6,Citation7]. Several methods were developed to covalently immobilize LOX with cyanogen bromide (CNBr), glutardialdehyde (GDA) and oxirane acrylic beads; however, these methods failed to stabilize the enzymes [Citation8,Citation9]. Rice husk silica is known to be an efficient matrix for protein immobilization, owing to its nanoporous structure (pore size approximately 4–5 nm) and the availability of functional groups on the silica surface [Citation10–12]. In addition, silica from rice husk – an abundantly available agricultural residue in rice-producing countries – serves as a safe, cheap and environmentally friendly source [Citation12–14]. Studies have described covalent immobilization of enzymes on GDA-activated silica and silica nanoparticles involving modification of the support surface with organosilane species to generate covalently bound organic moieties [Citation15,Citation16]. In this study, two methods were employed for chemical modification of the RHS surfaces: the first method used 3-aminopropyltriethoxysilane (APTES) and GDA, while the other method used APTES with epichlorohydrin (ECH)-activated polyethylene glycol (PEG). These methods allowed for the formation of covalent linkages between the enzymes and the modified matrix. LOX, AOS and AOC – enzymes essential for the production of cis(+)-12-OPDA – were immobilized on rice husk silica (RHS) matrices independently and then all together to develop the co-immobilized system. These RHS matrix systems were then used to synthesize cis(+)-12-OPDA in vitro. This study provides an efficient method for the in vitro production of cis(+)-12-OPDA. Further optimization may broaden this method’s application for large-scale production of this jasmonate compound.
Materials and methods
Materials
Soybean lipoxygenase (E.C. 1.13.11.12) (soyLOX) was purchased from Sigma-Aldrich (USA). Recombinant rice allene oxide synthase (OsAOS1) and rice allene oxide cyclase (OsAOC) were purified as previously described [Citation17]. The chemicals used in RHS modification (3-aminopropyltriethoxysilane (APTES), glutardialdehyde (GDA), epichlorohydrin (ECH) and polyethylene glycol (PEG) 8000), bovine serum albumin (BSA) and the chemicals used in the enzymatic assays (bicinchoninic acid (BCA), xylenol orange disodium salt) were purchased from Sigma-Aldrich (USA). All other chemicals and reagents used were of the highest purity.
Preparation of nanoporous RHS
Nanoporous RHS was prepared as previously reported [Citation10,Citation18]. Briefly, clean rice husk samples were treated with HCl for 6–10 h at 80 °C. The sample was then filtered, washed with distilled water, and heated to 380 °C. Further heating to 580 °C in ambient air produced white, nanoporous silica powder that was 99.8% pure with a surface area of 250 m2·g−1 and a pore diameter of 4 nm. The RHS was then pre-treated by heating to 700 °C for 4 h and cooled to room temperature in ambient air before undergoing modification.
Covalent immobilization of proteins on modified RHS
Covalent immobilization of proteins on GDA-linked RHS (RHS-APTES-GDA-proteins)
RHS was activated by silanization, using APTES as a coupling agent [Citation19]. Briefly, 10 mg of RHS was mixed with a solution of 2% w/v APTES and 80% ethanol followed by incubation at 40 °C for 2 h with gentle shaking. The mixture was centrifuged to separate the activated RHS from the solution. The RHS was washed several times with 80% ethanol and 50 mM sodium phosphate buffer (pH 7.2). A solution of 1% GDA in 50 mM sodium phosphate buffer (pH 7.2) was added to 10 mg activated RHS and the mixture was gently shaken at 25 °C for 4 h to produce GDA-linked RHS. The proteins were covalently immobilized on GDA-linked RHS by incubating them together at 25 °C for 24 h with gentle shaking, following by centrifugation at 3000 × g to recover the immobilized proteins from the solution. The proteins that were non-specifically adsorbed were washed out of the matrix with 1 M NaCl and 1% Tween 20, followed by 50 mM sodium phosphate buffer (pH 8.0). The concentration of proteins before and after immobilization was measured by the BCA assay method. The masses of the immobilized proteins were calculated by subtracting the mass of the non-immobilized proteins from the total mass. The immobilization efficiency was calculated as a percentage of immobilized protein out of the total protein.
Covalent immobilization of proteins on ECH-PEG-linked RHS (RHS-APTES-ECH-PEG-proteins)
RHS was activated with APTES as described above. Polyethylene glycol (PEG) (20 mg) was activated by incubating with 4 ml 50% v/v epichlorohydrin (ECH) for 12 h at 25 °C followed by bubbling with N2 for 6 h, which resulted in the introduction of highly reactive oxirane groups at both ends of the PEG molecules [Citation19]. The ECH-PEG complexes (≈ 20 mg) were coupled to 10 mg activated RHS by gentle shaking at 25 °C for 12 h. Proteins were immobilized onto the ECH-PEG-linked RHS following the same protocol described above for immobilizing enzymes onto the GDA-linked RHS.
Determination of LOX and AOS activity using the ferrous oxidation-xylenol orange (FOX) method
The FOX reagent was prepared by mixing 25 mM ferrous ammonium sulfate in 2.5 M sulphuric acid with 125 µM xylenol orange salt in 0.1 M sorbitol at a 1:100 ratio. The assay was performed by adding 1 ml FOX reagent to 100 µl sample and the reaction was incubated at RT for 15 min. A UV-Vis spectrophotometer (Shimadzu UV-2550) was used to measure the concentration of Fe3+-xylenol as an indirect indicator of the HPOTE concentration (ε = 267,000 M−1·cm−1 at 560 nm) [Citation20]. The specific activity of soyLOX was determined by monitoring the formation of HPOTE from the breakdown of its substrate, LnA. The specific activity of AOS was determined by monitoring the consumption of HPOTE in a soyLOX-coupled assay, as previously reported [Citation21].
Determination of LOX activity by monitoring the absorbance at 234 nm
Free and immobilized LOX activities were examined using a UV–Vis spectrophotometer (UV-2550, Shimadzu) by measuring the concentration of HPOTE that was formed from LnA oxidation (ε = 25,000 M−1·cm−1 at 234 nm) [Citation22]. LnA was used at a concentration of 0.5 mM for both reaction conditions. Reactions using free soyLOX were performed directly in a quartz cuvette and HPOTE formation was continuously measured. Reactions using immobilized soyLOX were performed in a falcon tube, quenched at specific time points by centrifugation to separate the immobilized enzyme from the reaction mixture, and its absorbance was measured to determine the HPOTE concentration.
Determination of LOX, AOS and AOC activities by reverse-phase high-performance liquid chromatography (RP-HPLC) analysis
All reactions were performed in 50 mM sodium phosphate buffer (pH 8.0) at 25 °C. LnA in 50 mM sodium phosphate buffer (pH 8.0) containing 0.02% Emulphogene was used as the substrate for soyLOX. The product of the above reaction, HPOTE, served as the substrate for the OsAOS1 reaction, while the product of the OsAOS1 reaction, 12,13-epoxyoctadeca-9,11,15-trienoic acid (EOT), was used as the substrate for OsAOC. In the assays measuring OsAOS1 activity, α-ketol – the non-enzymatically transformed product of EOT – was measured instead of the unstable EOT. Product yields of the reactions using immobilized soyLOX, OsAOS1 and OsAOC were determined based on the concentrations of HPOTE, α-ketol and cis-OPDA respectively. Yields were expressed relative to the concentrations observed for reactions using free-enzymes. Products were separated from the immobilized enzymes by centrifugation (3000 × g, 5 min, 25 °C). Products from both the free and immobilized enzyme systems were analyzed by RP-HPLC using 80% methanol and 0.01% acetic acid in double-distilled water (ddH2O) as solvent A and 100% methanol as solvent B [Citation23].
Recycling of immobilized systems
Enzyme reactions were performed at 25 °C. After each reaction, immobilized enzymes were recovered by centrifugation at 3000 × g for 5 min at RT and washed with 0.05 M sodium phosphate buffer (pH 8.0) before being used to initiate a new cycle. The amounts of product after each cycle – HPOTE, α-ketol and cis-OPDA were produced using soyLOX, OsAOS1 and OsAOC, respectively – were determined using RP-HPLC. The activities of recycled enzymes that were individually immobilized onto RHS matrices were assessed over the course of 10 cycles. Recycling of the co-immobilized LOX-AOS-AOC system was evaluated by measuring the amount of final product (cis-OPDA) after five cycles. All recycling percentages of immobilized enzymes were expressed relative to the product amounts measured in their respective first cycles.
Results and discussion
Covalent immobilization efficiencies of enzymes on modified RHS
Fourier transform infrared spectroscopy (FTIR) was used to analyze the chemical characteristics of the modified RHS [Citation24,Citation25] and the spectral data evidenced the covalent immobilization of BSA protein on GDA-linked RHS (Supplementary Figure S1). The immobilization efficiencies of the enzymes are shown in . GDA-linked RHS exhibited approximately 2-fold higher immobilization efficiencies for all enzymes compared to ECH-PEG-linked RHS. The differences in the immobilization efficiencies between GDA and ECH-PEG-linked RHS might be due to differences in the lengths of the spacer arms and enzyme immobilization mechanisms. The short linker of GDA provided more opportunity for the proteins to attach to the matrix while the long arm of ECH-PEG limited the attachment of proteins to RHS. Among the three oxylipin biosynthetic enzymes used for covalent immobilization, OsAOS1 displayed the lowest immobilization efficiency, while OsAOC exhibited the highest. Differences in the immobilization efficiencies between these enzymes were attributed to their distinct physical and chemical characteristics. SoyLOX is the largest of the three enzymes with an approximate molecular weight of 108 kDa. The molecular weight of OsAOS1 is approximately 54 kDa, while OsAOC is approximately 23 kDa. Due to the high immobilization efficiency of OsAOC, these data suggest that molecule size may be inversely correlated with immobilization efficiency. However, this suggestion is confounded by the low immobilization efficiency of OsAOS1. OsAOS1 required detergent to remain soluble due to its high hydrophobicity [Citation26], which may have impeded its immobilization onto the matrices. These enzyme characteristics may have affected the co-immobilization efficiencies as well. Pre-incubation of the three enzymes together would have provided time for protein-protein interactions to form before being immobilized onto the matrices [Citation17]. It is possible that this step would have increased the immobilization efficiency by increasing protein stability.
Table 1. Immobilization efficiency of the covalent immobilization of proteins on modified RHS.
Specific activities of free and covalently immobilized enzymes on modified RHS
Specific activities of immobilized soyLOX and OsAOS1 on GDA- and ECH-PEG-linked RHS were measured by xylenol orange assay and compared to the activities observed for the free-enzyme conditions. Both immobilization methods resulted in significant reductions of specific activities compared to the free-enzyme conditions. In particular, the enzymes immobilized on GDA-linked RHS exhibited very low relative specific activities (). This phenotype might be due to the limited mobility of the immobilized enzymes on the matrices. This effect might be more pronounced in the GDA-linked system where the enzymes are closer to the matrix surface due to the short length of the spacer arms. In contrast, the ECH-PEG-linked system has a longer spacer arm, which would provide more conformational freedom for the immobilized enzyme and easier access to the substrate [Citation19]. Hence, although the immobilization efficiency of enzymes on the ECH-PEG-linked RHS was lower, the specific activities of the enzymes were higher than on GDA-linked RHS. Moreover, the adsorption of the reaction products on RHS appears to affect their quantification.
Table 2. Comparison of the specific activities of soyLOX and OsAOS1 in free and covalently immobilized systems on GDA- and ECH-PEG-linked RHS.
Comparison of the kinetic profiles of soyLOX in the free and immobilized enzyme reaction systems
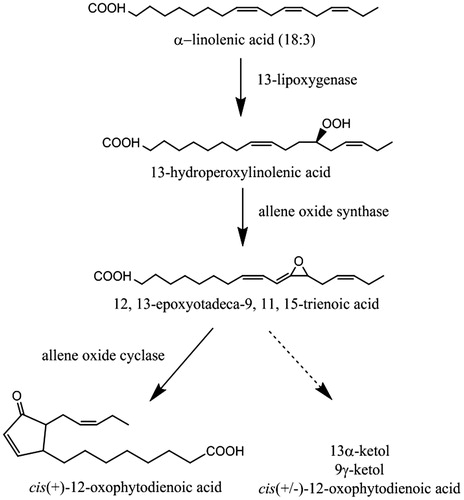
The specific activity of soyLOX was reduced to 2.5% and 23% in the GDA-linked and ECH-PEG-linked systems respectively, compared to the activity observed in the free-enzyme system (). Interestingly, the difference in the kinetic courses of the enzyme was clear (). In the free-enzyme system, the final concentration of LOX product (HPOTE) was highly dependent on the concentration of soyLOX (). These data indicate that free soyLOX was inactivated during catalytic turnover as previously suggested by the enzyme-initiated catalytic mechanism of LOX [Citation27]. This result was also observed for soyLOX immobilized on ECH-PEG-linked RHS (). In contrast, the final concentration of HPOTE was independent from soyLOX concentration in the GDA-linked RHS immobilization system (). Immobilization of LOX on GDA-linked RHS may therefore solve the issue of LOX inactivation during catalytic turnover. Consistent with this result, soyLOX-recycling experiments indicated that immobilized soyLOX was more stable on GDA-linked RHS than ECH-PEG-linked RHS ().
Figure 2. The concentration-dependent comparison of reaction kinetics of free and immobilized soyLOX. LnA (0.1 mM) was used as the substrate. HPOTE was continuously measured in the free soyLOX system (A) and discontinuously measured in the immobilized soyLOX systems on GDA- (B) and ECH-PEG-linked RHS (C). Data from the discontinuous enzyme assays are represented as the mean ± SD (n = 3).
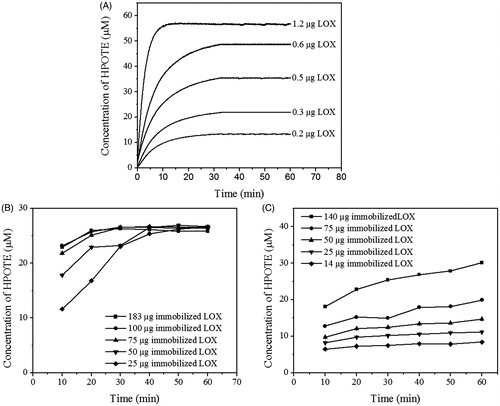
Dependence of cis-OPDA production on the molar ratio of OsAOC to OsAOS1 in the GDA-linked RHS co-immobilization system
Mixtures of OsAOS1 and OsAOC at different molar ratios were covalently co-immobilized on modified RHS. The substrate, HPOTE (0.5 mM), was used to determine the optimum ratio of OsAOC to OsAOS1 in the production of cis-OPDA, as measured by RP-HPLC. Cis-OPDA production was saturated at a 40:1 molar ratio of OsAOC to OsAOS1 (). This result indirectly suggests that AOS may interact with AOC, with the ability to shuttle the unstable allene oxide intermediate (EOT in ) between the active sites of the two enzymes [Citation28].
Figure 3. The production of cis-OPDA as a function of the molar ratio of OsAOC to OsAOS1 in the co-immobilized system of OsAOS1 and OsAOC on GDA-linked RHS. HPOTE (0.5 mM) was used as the substrate for the co-immobilized AOS-AOC on GDA-linked RHS. The reaction was carried out in 0.05 mM sodium phosphate buffer (pH 8.0) at RT for 1 h. Data are represented as the mean ± SD (n = 3).
Relative product yields of free and RHS-linked immobilized enzymes
Relative product yields from reactions catalyzed by soyLOX, OsAOS1 and OsAOC were analyzed by RP-HPLC [Citation17,Citation26]. Although enzyme immobilization using either linker resulted in significant reductions in the specific activities of immobilized enzymes (), the product yields relative to their respective free-enzyme conditions were high (). Specifically, product yield using immobilized soyLOX was greater than 50% when the reaction reached steady state after 1 h. In the reactions using immobilized OsAOS1, the production of α-ketol after 30 min was much higher than the levels produced in the presence of free-enzyme, an approximate two-fold increase for GDA-linked OsAOS1 and a 1.5-fold increase for the ECH-PEG-linked conditions. These results suggest that immobilization of OsAOS1 improved catalytic activity, and that immobilized OsAOS1 can be used to generate large amounts of stable product. The amount of cis-OPDA produced by OsAOC immobilized on GDA- and ECH-PEG-linked RHS was nearly the same as that produced by free OsAOC after 1 h, suggesting immobilized OsAOC was stable and catalytically active. In the covalent co-immobilization experiments, soyLOX, OsAOS1 and OsAOC were mixed together at a 40:1:40 molar ratio, which was previously determined to be the optimum condition for product formation [Citation17]. Catalytic activity of the covalently co-immobilized LOX-AOS-AOC on GDA- and ECH-PEG-linked RHS was evaluated based on the production of the final product, cis-OPDA, from LnA. The product yields in both of the co-immobilization systems were high enough to produce cis-OPDA ().
Table 3. Relative product yields of immobilized enzymes on modified RHS.
Recycling of covalently immobilized enzymes on modified RHS
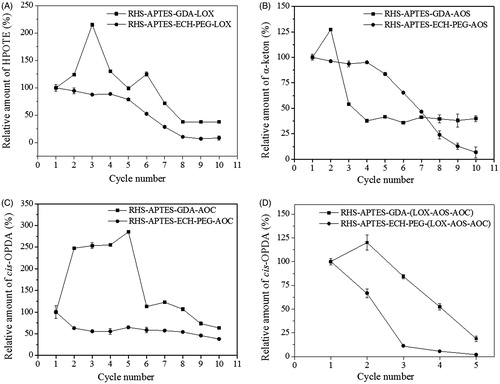
Immobilized enzyme stabilities were evaluated based on their activity after multiple cycles of recycling. We observed that enzymes immobilized on GDA-linked RHS demonstrated better recyclability than those immobilized on ECH-PEG-linked RHS (). The immobilized soyLOX, OsAOS1 and OsAOC on GDA-linked RHS could be reused at least 10 times. On ECH-PEG-linked RHS, immobilized soyLOX and OsAOC also showed high activity after ten cycles of reuse; however, immobilized OsAOS1 exhibited a dramatic decrease in catalytic activity after 10 cycles of reuse (). The co-immobilized systems using both GDA- and ECH-PEG-linked RHS were not as stable as the immobilization of individual enzymes (). In the co-immobilized system, cis-OPDA production decreased steadily with each cycle of reuse. In order to maintain the catalytic activity at 50% or greater, the co-immobilized system on GDA-linked RHS could be recycled up to three times, whereas on ECH-PEG-linked RHS, enzymes could only be recycled up to two times. It was observed that some cycles reusing immobilized and co-immobilized enzymes on GDA-linked RHS exhibited increased product formation (). This might be due to adsorption of products to the matrix, as the nanoporous structure of RHS is efficient for the adsorption of small molecules. It was also possible that the substrate accumulated inside the matrix, which may have become exposed to the enzymes after several uses and washes. This phenomenon was not observed for enzymes immobilized on ECH-PEG-linked RHS. This might be due to the long spacer arm of ECH-PEG [Citation19], which positioned the enzymes far from the surface of the matrix and would have decreased the likelihood of contact between substrates adsorbed to RHS and immobilized enzymes.
Figure 4. Reusability of immobilized soyLOX, OsAOS1 and OsAOC on GDA- and ECH-PEG-linked RHS. (A) Immobilized soyLOX. (B) Immobilized OsAOS1. (C) Immobilized OsAOC. (D) Co-immobilized LOX-AOS-AOC. Product amounts were calculated relative to the amount of product generated in the first cycle of enzyme use. Data are represented as the mean ± SD (n = 3).
Conclusions
RHS bears a nanoporous structure and acts as an efficient matrix for enzyme immobilization. In this study, we showed that RHS could be modified for covalent immobilization of enzymes. The three enzymes related to the oxylipin pathway – soyLOX, OsAOS1 and OsAOC– were covalently immobilized on RHS modified with either GDA or ECH-PEG. In general, enzymes immobilized on GDA-linked RHS exhibited better results than ECH-PEG-linked RHS, both for the single-enzyme immobilization conditions, as well when all three enzymes were co-immobilized on the matrix. Single-enzyme immobilization displayed higher immobilization efficiency and recyclability than co-immobilization. GDA-linked RHS was therefore considered a good matrix for covalent immobilization of soyLOX, OsAOS1, and OsAOC. The co-immobilized enzymes on RHS modified with APTES and GDA resulted in high yields of cis(+)-12-OPDA and the ability of the enzyme to be reused for many cycles. Further optimization of LOX-AOS-AOC co-immobilization is necessary to improve the activity, product formation efficiency and stability of these enzymes for maximum in vitro production of cis(+)-12-OPDA.
Thu_Bao_Le_et_a-_supplemental_content.docx
Download MS Word (335.3 KB)Acknowledgements
This article is based on a thesis submitted for the Master of Science degree program in the Department of Molecular Biotechnology, College of Agriculture and Life Sciences, Chonnam National University, Gwangju, South Korea.
Disclosure statement
All authors report no scientific and/or financial conflicts of interest in regard to this work.
Funding
This research was financially supported by NRF-2015R1D1A1A01058366.
References
- Stintzi A, Weber H, Reymond P, et al. Plant defense in the absence of jasmonic acid: the role of cyclopentenones. Proc Natl Acad Sci USA. 2001;98:12837–12842.
- Taki N, Sasaki-Sekimoto Y, Obayashi T, et al. 12-Oxo-phytodienoic acid triggers expression of a distinct set of genes and plays a role in wound-induced gene expression in Arabidopsis. Plant Physiol. 2005;139:1268–1283.
- Dave A, Graham IA. Oxylipin signaling: a distinct role for the jasmonic acid precursor cis-(+)-12-oxo-phytodienoic acid (cis-OPDA). Front Plant Sci. 2012;3:42.
- Taki-Nakano N, Ohzeki H, Kotera J, et al. Cytoprotective effects of 12-oxo phytodienoic acid, a plant-derived oxylipin jasmonate, on oxidative stress-induced toxicity in human neuroblastoma SH-SY5Y cells. Biochim Biophys Acta. 2014;1840:3413–3422.
- Hamberg M, Fahlstadius P. Allene oxide cyclase: a new enzyme in plant lipid metabolism. Arch Biochem Biophys. 1990;276:518–526.
- Allen JC. Soybean Lipoxygenase. 1. Purification, and the effect of organic solvents upon kinetics of the reaction. Eur J Biochem. 1968;4:201–208.
- Graveland A. Modification of the course of the reaction between wheat flour lipoxygenase and linoleic acid due to adsorption of lipoxygenase on glutenin. Biochem Biophys Res Commun. 1970;41:427–434.
- Yamane T. Air-oxidation of linoleic acid by lipoxygenase-containing particles suspended in water-insoluble organic solvent. In: Chibata I, Fukui S, Wingard LB, editors. Enzyme engineering. Boston (MA): Springer; 1982. p. 141–142.
- Maguire NM, Mahon MF, Molloy KC, et al. Chemoenzymatic synthesis of some macrocyclic C13-lactones. J Chem Soc Perkin Trans 1. 1991;8:2054–2056.
- Chon H, Chon MJ, Han CS, et al. Method for preparing porous silica, porous silica molding material, and nanosized silica particle derived from rice husk. Korean Patent 396457; 2003.
- Byun SC, Jung IO, Kim MY, et al. Morphology of the cross section of silica layer in rice husk. J Nanosci Nanotechnol. 2011;11:1305–1309.
- Adam F, Appaturi JN, Iqbal A. The utilization of rice husk silica as a catalyst: review and recent progress. Catal Today. 2012;190:2–14.
- Chandrasekhar S, Satyanarayana K, Pramada P, et al. Review processing, properties and applications of reactive silica from rice husk – an overview. J Mater Sci. 2003;38:3159–3168.
- Prasad R, Pandey M. Rice husk ash as a renewable source for the production of value added silica gel and its application: an overview. Bull Chem React Eng Catal. 2012;7:1–25.
- Daglioglu C, Zihnioglu F. Covalent immobilization of trypsin on glutaraldehyde-activated silica for protein fragmentation. Artif Cells Nanomed Biotechnol. 2012;40:378–384.
- Tamturk H, Yüksekdag H. Acetylcholinesterase immobilized onto PEI-coated silica nanoparticles. Artif Cells Nanomed Biotechnol. 2016;44:443–447.
- Yoeun S. Mechanistic studies on the formation of allene oxide and cyclopentadiene derivatives in oxylipin pathway [PhD]. Gwangju (Korea): Chonnam National University; 2014.
- Lee SY, Han CS. Nano filter from sintered rice husk silica membrane. J Nanosci Nanotechnol. 2006;6:3384–3387.
- Buthe A, Wu S, Wang P. Nanoporous silica glass for the immobilization of interactive enzyme systems. In: Minteer SD, editor. Enzyme stabilization and immobilization: methods and protocols. New York (NY): Humana Press; 2011. p. 37–48.
- Jiang ZY, Woollard AC, Wolff SP. Hydrogen peroxide production during experimental protein glycation. FEBS Lett. 1990;268:69–71.
- Ha SB, Lee BC, Lee DE, et al. Molecular characterization of the gene encoding rice allene oxide synthase and its expression. Biosci Biotechnol Biochem. 2002;66:2719–2722.
- Vick BA. A spectrophotometric assay for hydroperoxide lyase. Lipids. 1991;26:315–320.
- Stenzel I, Hause B, Miersch O, et al. Jasmonate biosynthesis and the allene oxide cyclase family of Arabidopsis thaliana. Plant Mol Biol. 2003;51:895–911.
- Gunda NSK, Singh M, Norman L, et al. Optimization and characterization of biomolecule immobilization onsilicon substrates using (3-aminopropyl)triethoxysilane (APTES) and glutaraldehyde linker. App Surf Sci. 2014;305:522–530.
- Kayhan S, Sari N, Nartop D. Nanoplatforms attached Schiff bases by condensation method; Investigation of glucose oxidase enzyme as biocatalysts. Artif Cells Nanomed Biotechnol. 2015;43:224–229.
- Yoeun S, Kim J, Han O. Cellular localization and detergent dependent oligomerization of rice allene oxide synthase-1. J Plant Res. 2015;128:201–209.
- Jang S, Huon T, Kim K, et al. Regiochemical and stereochemical evidence for enzyme-initiated catalysis in dual positional specific maize lipoxygenase-1. Org Lett. 2007;9:3113–3116.
- Agrawal GK, Tamogami S, Han O, et al. Rice octadecanoid pathway. Biochem Biophys Res Commun. 2004;317:1–15.
