?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This study reports the synthesis and characterization of poly(ethylene glycol) coated gold@iron oxide core–shell nanoparticles conjugated with folic acid (FA–PEG–Au@IONP). Also, targeted therapeutic properties of such a nanocomplex were studied on human nasopharyngeal carcinoma cell line KB and human breast adenocarcinoma cell line MCF-7 in vitro. The synthesized nanocomplex was characterized by transmission electron microscopy (TEM), dynamic light scattering (DLS), UV–Vis spectroscopy, vibrating sample magnetometry (VSM), and Fourier transform infrared (FTIR) spectroscopy. The photothermal effects of nanocomplex on both KB and MCF-7 cell lines were studied. Cell death and apoptosis were evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and flow cytometry using an annexin V-fluorescein isothiocyanate/propidiumiodide apoptosis detection kit. It was found that nanocomplex is spherical in shape and its size is approximately 60 nm. UV–vis spectrum showed that nanocomplex has appropriate absorption near infrared region. FTIR spectra obtained from nanocomplex before and after conjugation with FA confirmed the formation of folate conjugated nanocomplex. Significant cell lethality was observed for KB (∼62%) and MCF-7 (∼33%) cells following photothermal therapy. Also, it was found that majority of the cell deaths were related to apoptosis process. It can be concluded that, the synthesized nanocomplex is an effective and promising multifunctional nanoplatform for targeted photothermal therapy of cancer.
Introduction
In recent years, there has been a great deal of attention on the development of an efficient photothermal therapy technique that can be used in minimally invasive selective cancer therapy. In photothermal therapy, lasers and photosensitizer agents play critical roles and both must be fine-tune controlled. In practice, the usage of high laser powers is not appropriate for clinical treatments of tumours, because of low tolerance threshold of the skin. Various penetration depths of different lasers is another major concern in photothermal therapy of cancer [Citation1].
Ultra–violate and visible lasers have poor tissue penetration depths and it is mandatory to design novel agents capable of absorbing the near infra-red (NIR) lasers (in the biological window) for the treatment of deep-seated tumours [Citation1,Citation2]. Plasmonic noble metal nanostructures have recently demonstrated interesting optical properties, making them powerful agents for photothermal therapy of various cancers [Citation3,Citation4]. It is well known that large amount of heat is generated upon photoexcitation of metal nanostructures at their respective localized surface plasmon resonance (LSPR) band [Citation1,Citation5]. Such a liberated heat can be efficiently utilized in photothermal therapy. Several metal nanostructures such as, spherical gold nanoparticles (AuNPs), nanorods and nanoshells have been reported as NIR laser responsive and activatable nanomaterial mediating photothermal therapy process for the destruction of malignant tumours [Citation2,Citation4]. These AuNPs can convert absorbed photons of laser into thermal energy and trigger cell destruction as a result of electron–phonon and phonon–phonon interactions [Citation2,Citation6].
Multifunctional nanomedical platform for simultaneous targeting, imaging and therapy of cancer is another new concept which must be considered. Such a nanoplatform potentially takes advantage of the integration of various nanomaterials with different properties into a single nanoscale composite. For example, nanocomposites containing nanoparticles with both magnetic and plasmonic properties have recently attracted intensive attention due to their unique potentials in simultaneous diagnosis and therapy of cancer [Citation7]. Among the various magnetic nanoparticles reported so far, iron oxide nanoparticles (IONPs) are highly advantageous for constructing multifunctional nanocomposites because of their intrinsic magnetic features. There are many publications on the potentials of IONPs in molecular imaging. Recently, the authors of the current study reported on a new type of nanocomposite having both magnetic and plasmonic components and demonstrated that it can be used as a negative contrast agent for magnetic resonance imaging (MRI) [Citation8]. It is believed that such a magneto-plasmonic nanocomposite can accelerate the transverse relaxation of water protons and thus shorten the spin–spin proton relaxation time [Citation6,Citation8]. Dong et al. [Citation9] also reported on a simple route for constructing a Fe3O4@hybrid@Au nanocomposite, in which each nanoparticle was constructed of superparamagnetic Fe3O4 nanoparticles as the core, an organic–inorganic hybrid as the mediate layer and an outer gold nanoshell. Kim et al. [Citation10] synthesized a magnetic gold nanoshell and employed them for MRI and photothermal therapy of tumour cells. They generated such a nanoshells by first assembling IONPs and gold seeds on the surface of silica particles and then growing the gold nanoshell. Additionally, several other papers reported on the synthesis of magnetic gold nanoshells by first embedding IONPs in silica particles by the Stober method and then coating with gold [Citation11–14].
Conjugation of the Au@IONP nanoshell with an appropriate targeting ligand is necessary and offers more opportunities for development of a modern strategy for efficient cancer therapy. Targeted nanomaterials selectively bind to the cancer cells and affect them, with minor effects on the healthy cells. Folic acid (FA) is one of the most promising targeting ligands to make the nanomaterials efficiently targeted and to solve the problem of non-selectivity in cancer therapy [Citation15,Citation16]. There are some other targeting ligands such as monoclonal antibodies which may have better specificity than FA, but they have at least two drawbacks: the overall dimensions of the antibodies, which make particles to diffuse poorly through biological barriers, and their immunogenicity [Citation4,Citation17]. FA (with low molecular weight; MW= 441 Da) is a vitamin whose receptor is frequently overexpressed on the surface of certain human cancer cells (like head and neck carcinoma) [Citation3]. In addition, FA is stable, inexpensive, non-immunogenic and it moves into the cell cytoplasm, which is an advantage for more efficient intracellular delivery of nanoparticles than using a cell membrane marker that is not cell internalized [Citation17]. The applications of FA in cancer nanotechnology are presented in a review paper recently published by our research group [Citation18].
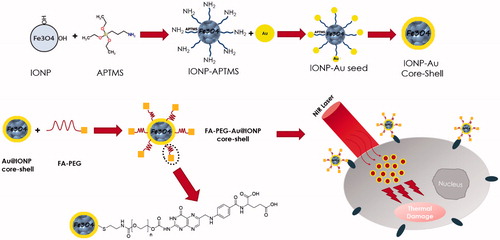
Taken together, much attention has been paid on combining magnetic components with gold nanoshells and several preparation routes have been developed. The synthesis of a targeted, biocompatible, uniform and monodisperse multifunctional nanocomplex that suitably integrates Fe3O4 nanoparticles and gold nanoshells and possesses suitable size via simple methodology is still a great challenge. In this work, the synthesis, characterization and photothermal effects of a new targeted FA–PEG–Au@IONP nanocomplex, including FA, poly (ethylene glycol) (PEG), AuNPs and IONPs were reported. To increase biocompatibility of nanocomplex, and particularly its circulating time for in vivo purposes, the surface of nanocomplex was modified with PEG as a hydrophilic, flexible and nonionic polymer [Citation19]. To solve the problem of non-selectivity, FA was used as a targeting ligand. As a good photosensitizer, gold nanoshell was selected to enhance thermal effects of laser. The resulting multifunctional nanocomplex may be useful for targeted photothermal therapy. Here, these experiments were conducted on two human cell lines expressing different levels of folate receptors; human nasopharyngeal carcinoma cell line KB and human breast adenocarcinoma cell line MCF-7. To assess the results, MTT assay and flow cytometry were performed using annexin V-PI kit. Scheme 1 illustrates the theme of the current study in brief.
Materials and methods
Materials
Iron (II) chloride tetrahydrate (>99%), iron (III) chloride hexahydrate (>99%), ammonia (32%), methanol (99.9%) and toluene (>99%) were purchased from Merck (Darmstadt, Germany). 3-Amino-propyltrimethoxysilane (97%) was purchased from Fluka (Buchs, Switzerland). Gold (III) chloride solution, cysteamine hydrochloride (>98%), poly (ethylene glycol) (PEG-10,000) and FA (>97%) were purchased from Sigma (St. Louis, MO, USA). Deionized water was used in all the experiments.
Synthesis of nanocomplex
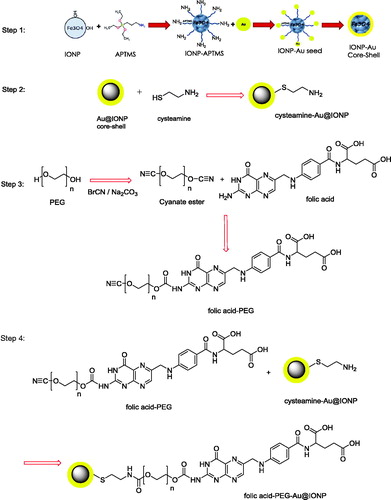
A step by step schematic illustration of synthesis of nanocomplex is presented in The details of each step of synthesis are explained below.
Preparation of Au@IONP core–shell
Au@IONP core–shell was synthesized based on the method recently published by our research group [Citation8]. Briefly, IONPs were synthesized using a modification of the co-precipitation method of Massart [Citation20]. First, 12.5 ml of NH3 (2 M) was added dropwise to a 2:1 solution of FeCl3·6H2O and FeCl2·4H2O under mechanical stirring. When the addition was completed, the sediment was separated by an external magnetic field, washed three times with methanol, dispersed in 50 ml of toluene and stored as the source of IONP colloidal solution. Then, 3-aminopropyl trimethoxysilane (APTMS) (25 µL) was added to 12.5 ml of the IONPs and sonicated for 30 min. The solution was heated for 4 h at 60 °C in an oven. The sediment was separated by a magnetic field, washed three times with methanol to remove the extra APTMS and toluene, and dispersed in methanol.
To form the gold shell on the surface of IONPs, AuNPs with a diameter of 3.5 nm were prepared as detailed in the literature [Citation21] and immobilized onto the surface of the APTMS-functionalized IONPs. For this purpose, 4 ml of APTMS-functionalized IONPs (51.5 mM Fe) in methanol was added dropwise to a 25 ml solution of undiluted AuNPs (0.198 mM Au). Next, 4 ml of methanol and 10 ml of water were added to maximize the coverage of AuNPs on the IONP surface. After 2 h of stirring at room temperature, the AuNPs were immobilized onto the surface of the IONPs. The resulting nanoparticles were separated magnetically, washed three times with DI water and dispersed in 15 ml of DI water. Finally, 0.011 g of chloroauric acid was added to the 15 ml solution of prepared Au immobilized on the IONP surface under sonication. To complete the shell formation, 0.4 ml (78.9 mM) of ascorbic acid was added. After 2 h of incubation, a continuous and complete Au shell layer was formed on the IONP surface.
Preparation of cysteamine coated Au@IONP
0.33 g cysteamine hydrochloride was dissolved in 2 ml DI water and this solution was added drop wise to the colloidal solution of Au@IONP. The mixture was stirred at room temperature for 2 h. To remove unreacted cysteamine, the mixture was placed on a permanent magnet and then washed three times with DI water.
Preparation of folic acid–PEG–Au@IONP
Terminal hydroxyl groups of PEG were activated by cyanogen bromide to react with terminal amine group of FA. PEG-10,000 (2 g), sodium carbonate (0.1 g) and cyanogen bromide (0.61 g) were dissolved in 10 ml DI water and stirred at room temperature for 10 min. Then, 1.2 g FA was added to the solution and the mixture was stirred for 2 h. This mixture was added to cysteamine coated Au@IONP and stirred at room temperature for 12 h. To remove unreacted reagents, final mixture was placed on a permanent magnet and washed with DI water three times.
Nanocomplex characterization
To determine the shell thickness and size distribution of the core–shell nanocomplex, high-resolution transmission electron microscopy (HR-TEM) was performed. Size distribution and average diameter of the synthesized nanocomplex was determined using ImageJ software (http://rsb.info.nih.gov/ij/). The UV–visible (UV–Vis) absorption spectra of the FA–PEG–Au@IONP nanocomplex dispersed in water were recorded using a Rayleigh UV-1601 instrument. The hydrodynamic size and zeta potential of the synthesized nanoparticles were measured using a Malvern Zetasizer, NANO ZS (Malvern Instruments Limited, Malvern, UK). The nanocomplex surface coating was investigated by a Fourier transform infrared (FTIR) spectrometer (Thermo scientific, Waltham, MA, USA). FTIR spectra of samples in KBr were determined at room temperature in the spectral region of 500–4000 cm−1. The magnetic properties of the nanocomplex were determined using a vibrating sample magnetometry (VSM) (MDK6, Kavir, Iran) under a magnetic field of 10 kOe. The concentrations of iron and gold in each sample were directly analysed by dissolving the core–shells in aqua regia and performing inductively coupled plasma analysis (ICP; Varian 730-ES Axial ICP-OES).
To explore the photo-absorption capability of the synthesized nanocomplex at 808 nm, the extinction coefficient was calculated according to the following equation [Citation22]:
where A is the absorbance at 808 nm, L represents the path-length (1 cm) and C represents the molar concentration of the nanocomplex. The molar concentration, C can be calculated by
where Cwt is the weight concentration, NA is Avogadro's constant, VNC and ρ respectively, represent the average volume and density of the nanocomplex.
Finally, the number of folate groups per nanoparticle was estimated using data obtained from TEM, UV–Visible spectroscopy and ICP-OES studies.
In vitro experiments
Cell culture
Human nasopharyngeal carcinoma cell line, KB, and human breast cancer cell line, MCF7, were purchased from Pasteur Institute of Iran (Tehran, Iran). These cell lines were maintained in Roswell Park Memorial Institute medium (RPMI-1640) (GIBCO, New York, NY, USA) supplemented with 10% foetal bovine serum (FBS) (GIBCO, New York, NY, USA), 300 U/ml of penicillin (Sigma-Aldrich, St. Louis, MO, USA) and 200 mg/l of streptomycin (Sigma-Aldrich, St. Louis, MO, USA). The cells were cultured as a monolayer at a density of 104 cells/cm2 in T-25 tissue culture flasks. The cultures were maintained at 37 °C in a humidified atmosphere of 5% CO2.
Study of photothermal effects
MTT assay
When the cells were 80% confluent, they were detached with trypsin-EDTA. The cells were then centrifuged at 200g for 7 min and counted using trypan blue to determine viability. Cells were seeded at a density of 5000 cells/well and incubated with nanocomplex at concentration of 50 µg/ml in flat-bottom 96-well plates for 12 h. Then, nanoparticles were removed and replaced with fresh culture medium supplemented with 10% FBS. Then, cancer cells were irradiated by near infrared laser (λ = 808 nm; 6 W/cm2; 10 min). Sufficient control groups were also considered. Plates were returned to incubator and kept in CO2 incubator overnight. Retention of regenerative potential was estimated using the MTT-tetrazolium assay, which measures the ability of metabolically active mitochondria in live cells to reduce a colourless tetrazolium compound to a blue formazan product. To perform this assay, the culture medium was removed, 100 ml of culture medium without FBS and 10 ml of MTT (5 mg/mL in PBS) were added to each well, and the plate was returned to the incubator. After 4 h, the medium was removed from the wells, and the cells were lysed with 200 ml of DMSO. After the formazan product was dissolved, the absorbance at 570 nm was measured using an ELISA reader (Stat Fax-2100 Awareness, Mountain View, CA, USA). Relative survival was represented as the absorbance of the treated sample divided by absorbance of the control group. Eight replicates were used for each condition, and the experiments were repeated at least three times.
Cell apoptosis and necrosis assays
The Annexin-V-fluorescein isothiocyanate (FITC)-propidium iodide (PI) (eBioscience, San Diego, CA, USA) apoptosis detection assay was performed to compare the apoptotic effects of nanocomplex before and after laser irradiation. As well as MTT assay, four groups for each cell line were studied: (1) control, (2) cells + nanocomplex, (3) cells + laser and (4) cells + nanocomplex + laser. After treatments, cells in each group were trypsinized and re-suspended in 500 µL 1 × binding buffer at a concentration of 1 × 106 cells/mL. Afterwards, 5 µL of Annexin V–FITC and 5 µL of PI were consecutively added into the above 1 × binding buffer and the cells were incubated for another 20 min at room temperature in dark. Finally, the quantitative analysis of cell apoptosis and necrosis were performed immediately by flow cytometry.
Statistical analysis
The results are expressed as mean values ± standard error of mean (SEM). Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Tukey’s test as the post-hoc analysis using SPSS version 12. The value of p < 0.05 was considered to be significant.
Results
Nanocomplex characterization
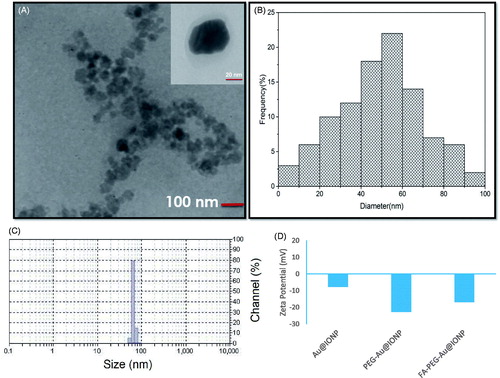
To determine nanocomplex size distribution and thicknesses of core and shell of the nanocomplex, high-resolution HR-TEM was performed. As shown in , TEM images showed that the prepared nanocomplex is spherical in shape and has a good core/shell structure. Thicknesses of core and shell were estimated to be approximately 50 and 10 nm, respectively. The size distribution of the synthesized nanocomplex was also examined using dynamic light scattering (DLS) (). It was found that hydrodynamic size of the synthesized nanocomplex is approximately 71 nm which is consistence with TEM results. Also, zeta potentials of Au@IONP, PEG–Au@IONP and FA–PEG–Au@IONP nanocomplexes were measured which were −7.82, −22.74 and −16.94 mV, respectively ().
Figure 2. (a) TEM image (inset is the high-resolution TEM image), (b) size distribution histogram of TEM image, (c) dynamic light scattering (DLS) profile of the prepared nanocomplex (PdI: 0.24). (d) zeta potentials of various nanoparticles synthesized in this study.
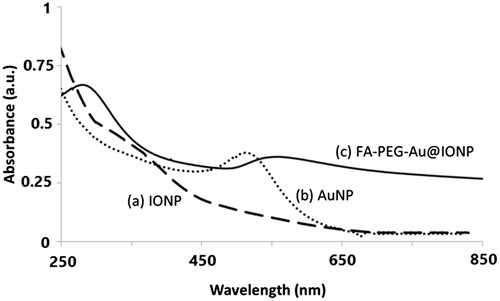
The UV–Vis absorption spectra of the nanocomplex dispersed in water is shown in . As shown this figure, FA–PEG–Au@IONP nanocomplex in comparison with AuNPs or IONPs has higher NIR absorption at 808 nm. Accordingly, it may be stated that the synthesized nanocomplex is a suitable and efficient agent for photothermal therapy. As described in previous section, the extinction coefficient ε of the synthesized nanocomplex was calculated, which was approximately 3.7 × 1011 M−1 cm−1. Such a high extinction coefficient at 808 nm indicates that NIR light could induce an efficient thermal effect in the presence of the synthesized nanocomplex.
Moreover, shows the peak at 280 nm for FA–PEG–Au@IONP nanocomplex, confirming the attachment of FA to the core-shell nanocomplex [Citation23,Citation24].
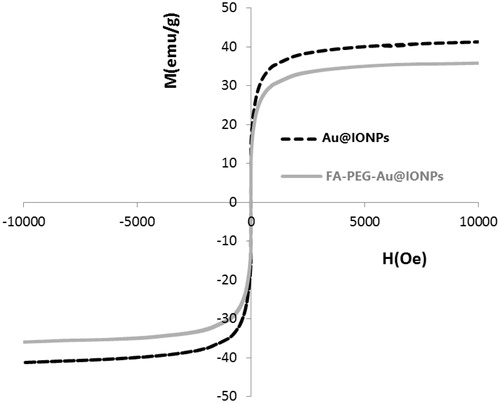
The magnetic properties of the nanocomplex were determined using VSM. For superparamagnetic NPs, the M–H curve should not have a hysteresis when measured above the blocking temperature. For the synthesized Au@IONP and FA–PEG–Au@IONP nanocomplex, no hysteresis was detected as shown in .
To determine the Au and Fe contents, 1 ml of the synthesized nanocomplex was dissolved in 4 ml of aqua regia, and the resultant mixture was analysed by ICP-OES. The Fe and Au contents in the synthesized nanocomplex were 120 and 47 µg/ml, respectively.
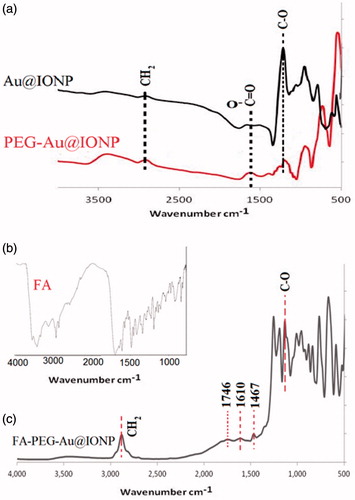
Fourier transform infrared spectroscopy is a powerful tool for identifying functional groups present on the surface of a nanocomplex. FTIR spectra of samples in KBr at room temperature in the spectral region of 500–4000 cm−1 were determined. shows the FTIR spectra from Au@IONPs before and after PEGylation. Also, the figure shows FTIR spectrum of FA conjugated PEGylated Au@IONP in comparison with FA alone, confirming the successful attachment of FA to the nanocomplex.
Finally, the number of folate groups per nanoparticle was estimated using data obtained from TEM, UV–visible spectroscopy and ICP-OES studies. The FA–PEG–Au@IONP nanocomplex showed absorbance peak at 280 nm (). The folate content can be conveniently obtained from the absorbance of 280 nm (the specific absorption attributed to folate, as stated earlier) [Citation23,Citation24]. A calibration curve was developed by measuring the intensity of 280 nm absorbance as a function of folate concentration. Using the calibration curve, the folate content of the nanocomplex was estimated to be 2.6 × 10−10 mol. It may be equivalently stated that the number of folate molecules in each millilitre of sample was 16 × 1013. On the other hand, using the measured particle size (based on TEM results) and considering the governing relation between volume and density, the mass of each nanoparticle was calculated to be 3.4 × 10−16 g. Then, the number of nanoparticles per ml was calculated using the data obtained from ICP-OES studies (49 × 1010/ml). Accordingly, the number of folate molecules divided by the number of nanoparticles in the sample determines the average number of folates on each nanoparticle which was about 326.
In vitro experiments results
MTT assay
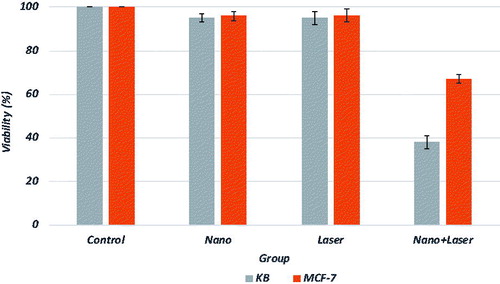
As shown in , the synthesized nanocomplex showed appropriate absorption in the NIR region and can potentially be used for photothermal therapeutic applications of cancer cells using NIR laser irradiation. To demonstrate the selective photothermal effects in vitro, the KB and MCF-7 cell lines were treated with nanocomplex and laser irradiation. It has been demonstrated that KB cells overexpress folate receptors on its membrane and can be selected as a good model to study the effects of a new synthesized folate conjugated nanomaterial [Citation24]. In contrast, MCF-7 has lower level of folate receptor expression and can be considered as a control model [Citation23]. In other words and according to the literature [Citation25], the level of folate receptor expressed on these two cell lines is in the following order: MCF7 ≪ KB. The proportions of viable cells in different groups for both cell lines were quantified through MTT assay. shows the percentages of viable cells with and without receiving nano-photo-thermal treatments. Viability of KB and MCF-7 cells incubated with 50 µg/ml of nanocomplex for 12 h decreased to 94 ± 2% and 96 ± 2%, respectively. After laser exposure to both cell lines, in the absence of nanocomplex, no significant change in cell viability was observed. Photothermal destruction was observed for both cell lines in the presence of nanocomplex. Viability of KB cells that received both nanocomplex and laser irradiation decreased to 38 ± 3%. Also, combination of nanocomplex and laser induced significant damages for MCF-7 cells and their viability decreased to 67 ± 2%.
Flow cytometry results
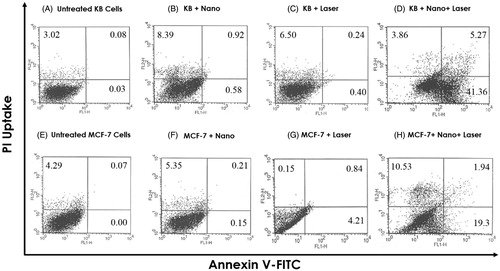
Flow cytometric analysis was performed to measure the percentage of live, apoptotic and necrotic cells. The effect of various treatments on both cell lines was investigated by measuring the binding of Annexin V-FITC and inclusion/exclusion of PI. The cytograms obtained for both KB and MCF-7 cells are presented in , showing the bivariate PI/Annexin V analysis of untreated and treated cells. Viable cells are negative for both PI and Annexin V, apoptotic cells are PI negative and Annexin V positive, while necrotic cells are positive for PI and negative for AnnexinV [Citation26]. As compared to treatment with nanocomplex or laser alone, it was found that apoptosis level is increased following nano-photo-thermal treatment. In addition, it is worthy to mention that apoptosis level for KB cells was significantly higher than for MCF-7 cells.
Discussion
Presently, several novel nanotechnology based methods for cancer diagnosis and therapy are under investigation and synthesis of various nanoparticles with particular properties has been the focus of extensive research over the past decade [Citation27–31]. Among these nanoparticles, IONPs and AuNPs are of major interests for developing unique systems with high potentials in cancer diagnosis and therapy [Citation24,Citation30]. In this study, the synthesis of folate conjugated PEG coated Au@IONP core–shell nanocomplex was reported. The resultant nanocomplex was examined by various characterization methods. The particles shown in are monodispersed and DLS studies confirmed this fact by a polydispersity index (PdI) of 0.24. According to , it is also obvious that nanoparticles are spherical in shape and they have core-shell structure. In addition, the zeta potential of the Au@IONP nanoshells changed from −7.82 to −22.74 mV after being coated with PEG, which demonstrated that the PEGylated nanoshells were more stable than the free nanoshells in water.
Optical absorption profile of nanocomplex were characterized by UV–vis spectroscopy (), showing that nanocomplex has an appropriate absorption in NIR region. It is worthy to note that absorption intensity of the nanocomplex in NIR region is highly stronger than that of AuNPs or IONPs alone. As shown in , FA–PEG–Au@IONPs nanocomplex possesses a superb magnetic response with a saturation magnetization of approximately 35 emu/g, suggesting that the nanocomplex enabled attraction in response to the extra magnetic field.
shows the FTIR spectra of Au@IONPs, PEG–Au@IONPs, FA and FA–PEG–Au@IONPs. In , the peaks at 1133 and 2882 cm−1 are attributed to stretching vibrations of C–O and CH2 groups in chemical structure of PEG. Stretching absorbance peaks at 1467 and 1610 cm−1 pertaining to aromatic rings of FA are visible in . A peak related to the carbonyl stretching vibration is visible at 1746 cm−1 (), which is related to carboxylic groups of FA. In , peaks of nitryl group (in structure of cyanogen bromide) between 2240–2260 cm−1 and twin peaks of amine group (3300–3500 cm−1) were deleted, confirming the attachment of FA to the nanocomplex.
The cytotoxicity of the nanocomplex was firstly determined by evaluating the livability of cancer cells (KB and MCF-7) using a MTT test, which is based on colorimetric determination of MTT being converted to formazan by viable cells. At a given concentration of the nanocomplex (50 µg/ml), both KB and MCF-7 presented a high cell viability (almost 95%), which suggested that the nanocomplex has a high biocompatibility and a low cytotoxicity (). Another method of determining the cytotoxicity of the nanocomplex is flow cytometry. As shown in ), the nanocomplex did not induce high level of toxicity in KB and MCF-7 cells. Such a low level of cytotoxicity may be related to PEGylation of the nanocomplex [Citation32]. The results of the current study are consistent with those of other publications reporting no significant cytotoxicity for gold nanoshell. Liu et al. [Citation33] reported on the synthesis of gold nanoshells functionalized with a small peptide as a targeting agent designed for photothermal therapy of hepatocarcinoma. The functionalized gold nanoshells synthesized by Liu et al. showed good targeting ability to liver cancer cells, had a low cytotoxic activity, and caused death to the liver cancer cells efficiently after being treated with a NIR light in vitro.
The in vitro effects of photothermal therapy using the synthesized nanocomplex were also evaluated by MTT assay and flow cytometry. and show the results of photothermal study of the nanocomplex or laser irradiation alone and their combinatorial effects. The ability of nanocomplex to target and cause selective death of highly overexpressed folate receptors cancer cells (KB) under a NIR laser irradiation was demonstrated ( and . This selective effect, due to conjugation of FA to nanoparticles, was also observed by other researchers. Mehdizadeh et al. reported on the synthesis and photothermal effects of folate conjugated gold nanorods (F-AuNRs) on KB cells. They reported no cell damage or cytotoxicity from the individual treatment of laser light (755 nm; 40 J/cm2; 7 pulses; pulse duration: 3 min) or F-AuNRs alone. They reported 56% cell lethality for KB cells using combined plasmonic photothermal therapy of 20 μM F-AuNRs.
In the field of multifunctional nanoparticles, photothermal effects of other shapes of AuNPs like gold nanostars in combination with chemotherapy were studied by Chen et al. [Citation6]. They synthesized gold nanostars and then conjugated them to doxorubicin as an anti-cancer drug. Then, they studied the combinatorial effects of nanocomplex on MDA-MB-231 cell line. They observed 60% cell lethality when both nanocomplex (12.5 μg/mg; 4 h incubation time) and laser (λ = 765 nm; 1 W/cm2; 10 min) were used. They also reported that majority of the cell deaths were related to the apoptosis process.
There is much attention on combining magnetic components with gold nanostructures and several preparation routes have been developed. Wang et al. [Citation34] recently reported a method for the synthesis of a new five-component hybrid nanocomposite made of a superparamagnetic iron oxide (SPIO) core and gold nanorods (AuNRs) in the mesoporous silica shells functionalized with FA (Fe3O4@SiO2@AuNRs@mSiO2–FA). They reported insignificant cytotoxicity when the synthesized nanocomposite was incubated with KB cells. Higher cellular uptake efficiency was observed for FA conjugated nanocomposite in comparison with non-targeted nanoparticles. Consequently, they observed selective photothermal effects on KB cells when FA conjugated nanocomposite was used as the photosensitizer.
For better comparison between the results of the current study and other related publications, was prepared, in which, type of nanomaterials and their size, concentration and incubation time with cancer cells were focused on. Laser exposure condition in each study is another subject which must be considered. There are some important laser beam parameters which must be reported for a repeatable scientific study. They include wavelength, irradiation time, laser mode (pulse or continuous), laser power and beam area on the skin or culture surface which may be stated as power density (W/cm2) [Citation35]. Accordingly, in , the laser beam parameters used in the current study and those reported in other publications were explained and compared. Finally, in , cell death and the percentage of apoptosis reported in each study were summarized and compared with results of the current study.
Table 1. Comparison between the results of this study and other publications related to nano-photo-thermal therapy of cancer cells.
Magnetic gold nanoshells may be used as a thermosensitizer in radiofrequency (RF) hyperthermia, and also in laser hyperthermia [Citation36]. The application of laser in hyperthermia is a promising strategy in tumour treatment. However, the therapeutic effect was extremely troubled by the low tissue penetration of laser. Therefore, radiofrequency (RF) with better tissue penetration has been recently used for tumour hyperthermia.
The synthesized nanocomplex may have some additional biomedical applications and it may be used as a:
Radiosenstizer [Citation37],
Sonosensitizer [Citation38],
Drug carrier [Citation36],
Computed tomography (CT) contrast agent [Citation39],
Magnetic resonance imaging (MRI) contrast agent [Citation8].
Conclusions
In summary, it was demonstrated that FA–PEG–Au@IONP nanocomplex can be used as an effective photothermal therapeutic agent for cancer treatment. The synthesized nanocomplex is a hybrid system with both magnetic and plasmonic properties. This hybrid nanocomplex was PEGylated so that it can be biocompatible and used in vivo with longer circulation time [Citation19]. Finally, the prepared nanocomplex was functionalized with FA to target the cancer cells which overexpress folate receptors on their surfaces.
The current study showed that the viability of KB or MCF-7 cells was not highly changed after an 808 nm laser irradiation (6 W/cm2, 10 min). This may imply that such an irradiation of NIR laser alone without any photosensitizer could not induce obvious cell death. The underlying mechanisms for cell death induced by nano-photo-thermal therapy were also elucidated to be mainly related to apoptosis. In addition, it was demonstrated that conjugation of FA to the nanocomplex increases the selectivity of photothermal therapy technique. In vivo experiments must be performed to further demonstrate how the synthesized nanocomplex affects the rate of tumour growth inhibition and tumour destruction.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Shakeri-Zadeh A, Kamrava SK, Farhadi M, et al. A scientific paradigm for targeted nanophotothermolysis; the potential for nanosurgery of cancer. Lasers Med Sci. 2014;29:847–853.
- Kamrava K, Behtaj M, Ghavami Y, et al. Evaluation of diagnostic values of photodynamic diagnosis in identifying the dermal and mucosal squamous cell carcinoma. Photodiagnosis Photodyn Ther. 2012;9:293–298.
- Mehdizadeh A, Pandesh S, Shakeri-Zadeh A, et al. The effects of folate-conjugated gold nanorods in combination with plasmonic photothermal therapy on mouth epidermal carcinoma cells. Lasers Med Sci. 2014;29:939–948.
- Samadian H, Hosseini-Nami S, Kamrava S, et al. Folate-conjugated gold nanoparticle as a new nanoplatform for targeted cancer therapy. J Cancer Res Clin Oncol. 2016;142:2217–2229.
- Beik J, Abed Z, Ghoreishi FS, et al. Nanotechnology in hyperthermia cancer therapy: from fundamental principles to advanced applications. J Control Release. 2016;235:205–221.
- Chen H, Zhang X, Dai S, et al. Multifunctional gold nanostar conjugates for tumor imaging and combined photothermal and chemo-therapy. Theranostics. 2013;3:633–649.
- Lim YT, Cho MY, Choi BS, et al. Paramagnetic gold nanostructures for dual modal bioimaging and phototherapy of cancer cells. Chem Commun. 2008;40:4930–4932.
- Montazerabadi AR, Oghabian MA, Irajirad R, et al. Development of gold-coated magnetic nanoparticles as a potential MRI contrast agent. Nano. 2015;10:1550048.
- Dong W, Li Y, Niu D, et al. Facile synthesis of monodisperse superparamagnetic Fe3O4 core@ hybrid@ Au shell nanocomposite for bimodal imaging and photothermal therapy. Adv Mater. 2011;23:5392–5397.
- KimPark JS, Lee JE, Jin SM, et al. Designed fabrication of multifunctional magnetic gold nanoshells and their application to magnetic resonance imaging and photothermal therapy. Angewandte Chem. 2006;118:7918–7922.
- Ji X, Shao R, Elliott AM, et al. Bifunctional gold nanoshells with a superparamagnetic iron oxide-silica core suitable for both MR imaging and photothermal therapy. J Phys Chem C. 2007;111:6245–6251.
- LeeYang J, Ko J, Oh H, et al. Multifunctional magnetic gold nanocomposites: human epithelial cancer detection via magnetic resonance imaging and localized synchronous therapy. Adv Functional Mater. 2008;18:258–264.
- Salgueiriño-Maceira V, Correa-Duarte M, Farle A, et al. Bifunctional gold-coated magnetic silica spheres. Chem Mater. 2006;18:2701–2706.
- Huang WC, Tsai PJ, Chen YC. Multifunctional Fe3O4@Au nanoeggs as photothermal agents for selective killing of nosocomial and antibiotic‐resistant bacteria. Small. 2009;5:51–56.
- Geszke M, Murias M, Balan L, et al. Folic acid-conjugated core/shell ZnS: Mn/ZnS quantum dots as targeted probes for two photon fluorescence imaging of cancer cells. Acta Biomater. 2011;7:1327–1338.
- Li K, Jiang Y, Ding D, et al. Folic acid-functionalized two-photon absorbing nanoparticles for targeted MCF-7 cancer cell imaging. Chem Commun. 2011;47:7323–7325.
- Stella B, Arpicco S, Peracchia MT, et al. Design of folic acid‐conjugated nanoparticles for drug targeting. J Pharm Sci. 2000;89:1452–1464.
- Beik J, Khademi S, Attaran N, et al. A nanotechnology based strategy to increase the efficiency of cancer diagnosis and therapy: folate conjugated gold nanoparticles. Curr Med Chem. 2017 [cited Aug 10]. DOI:10.2174/0929867324666170810154917.
- Sheng Y, Chang L, Kuang T, et al. PEG/heparin-decorated lipid–polymer hybrid nanoparticles for long-circulating drug delivery. RSC Adv. 2016;6:23279–23287.
- Massart R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans Magnet. 1981;17:1247–1248.
- Jana NR, Gearheart L, Murphy CJ. Seeding growth for size control of 5–40 nm diameter gold nanoparticles. Langmuir. 2001;17:6782–6786.
- TianJiang Q, Zou F, Liu R, et al. Hydrophilic Cu9S5 nanocrystals: a photothermal agent with a 25.7% heat conversion efficiency for photothermal ablation of cancer cells in vivo. ACS Nano. 2011;5:9761–9771.
- Shakeri-Zadeh A, Mansoori GA, Hashemian A, et al. Cancerous cells targeting and destruction using folate conjugated gold nanoparticles. Dyn Biochem Process Biotechnol Mol Biol. 2010;4:6–12.
- Mansoori GA, Brandenburg KS, Shakeri-Zadeh A. A comparative study of two folate-conjugated gold nanoparticles for cancer nanotechnology applications. Cancers. 2010;2:1911–1928.
- Sonvico F, Mornet S, Vasseur S, et al. Folate-conjugated iron oxide nanoparticles for solid tumor targeting as potential specific magnetic hyperthermia mediators: synthesis, physicochemical characterization, and in vitro experiments. Bioconjugate Chem. 2005;16:1181–1188.
- Kirui DK, Rey DA, Batt CA. Gold hybrid nanoparticles for targeted phototherapy and cancer imaging. Nanotechnology. 2010;21:105105.
- Peer D, Karp JM, Hong S, et al. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760.
- Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5:161–171.
- Shakeri-Zadeh A, Khoei S, Khoee S, et al. Combination of ultrasound and newly synthesized magnetic nanocapsules affects the temperature profile of CT26 tumors in BALB/c mice. J Med Ultrasonics. 2015;42:9–16.
- Shakeri-Zadeh A, Shiran MB, Khoee S, et al. A new magnetic nanocapsule containing 5-fluorouracil: In vivo drug release, anti-tumor, and pro-apoptotic effects on CT26 cells allograft model. J Biomater Appl. 2014;29:548–556.
- Shakeri-Zadeh A, Khoee S, Shiran M-B, et al. Synergistic effects of magnetic drug targeting using a newly developed nanocapsule and tumor irradiation by ultrasound on CT26 tumors in BALB/c mice. J Mater Chem B. 2015;3:1879–1887.
- Bhattacharya R, Patra CR, Earl A, et al. Attaching folic acid on gold nanoparticles using noncovalent interaction via different polyethylene glycol backbones and targeting of cancer cells. Nanomed: Nanotechnol, Biol Med. 2007;3:224–238.
- Liu S-Y, Liang Z-S, Gao F, et al. In vitro photothermal study of gold nanoshells functionalized with small targeting peptides to liver cancer cells. J Mater Sci: Mater Med. 2010;21:665–674.
- Wang D-W, Zhu X-M, Lee S-F, et al. Folate-conjugated Fe3O4@SiO2@ gold nanorods@ mesoporous SiO2 hybrid nanomaterial: a theranostic agent for magnetic resonance imaging and photothermal therapy. J Mater Chem B. 2013;1:2934–2942.
- Neshastehriz A, Tabei M, Maleki S, et al. Photothermal therapy using folate conjugated gold nanoparticles enhances the effects of 6MV X-ray on mouth epidermal carcinoma cells. J Photochem Photobiol B: Biol. 2017;172:52–60.
- Wang L, Li D, Hao Y, et al. Gold nanorod-based poly (lactic-co-glycolic acid) with manganese dioxide core–shell structured multifunctional nanoplatform for cancer theranostic applications. Int J Nanomed. 2017;12:3059.
- Hu R, Zheng M, Wu J, et al. Core–shell magnetic gold nanoparticles for magnetic field-enhanced radio-photothermal therapy in cervical cancer. Nanomaterials. 2017;7:111.
- Beik J, Abed Z, Shakeri-Zadeh A, et al. Evaluation of the sonosensitizing properties of nano-graphene oxide in comparison with iron oxide and gold nanoparticles. Physica E: Low-Dimen Syst Nanostruct. 2016;81:308–314.
- Huang P, Bao L, Zhang C, et al. Folic acid-conjugated silica-modified gold nanorods for X-ray/CT imaging-guided dual-mode radiation and photo-thermal therapy. Biomaterials. 2011;32:9796–9809.
- Hu Y, Wang R, Wang S, et al. Multifunctional Fe3O4@Au core/shell nanostars: a unique platform for multimode imaging and photothermal therapy of tumors. Sci Rep. 2016;6:28325.
- Ma N, Jiang Y-W, Zhang X, et al. Enhanced radiosensitization of gold nanospikes via hyperthermia in combined cancer radiation and photothermal therapy. ACS Appl Mater Interf. 2016;8:28480–28494.
- Li P, Shi Y-w, Li B-x, et al. Photo-thermal effect enhances the efficiency of radiotherapy using Arg-Gly-Asp peptides-conjugated gold nanorods that target αvβ3 in melanoma cancer cells. J Nanobiotechnol. 2015;13:1.