?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Alzheimer’s disease (AD) is an unfavourable neurological condition of the brain leading to the loss of behavioural and cognitive skills of the aging population. At present, drugs representing cholinesterase inhibitors provide lateral side effects to AD patients. Hence, there is a need for improved fabrication of drugs without side effects, for which nanoencapsulated bioactive compounds that can cross the blood–brain barrier offer new hope as novel alternative treatment strategy for AD. This study involved synthesis of phytol loaded poly(lactic-co-glycolic acid) (PLGA) nanoparticles by solvent evaporation method. Physico-chemical characterization of phytol-PLGA NPs through the field emission scanning electron microscope, dynamic laser scattering (DLS) measurement revealed that the particles were nanosize range with smooth surface and spherical morphology. Furthermore, the biocompatibility of drug/polymer ratio was investigated by power X-ray diffraction (PXRD) and Fourier-transform infrared spectroscopic (FT-IR) analysis. The in vitro drug release study showed that the phytol was released in a sustained manner. Moreover, phytol-PLGA NPs were able to disrupt amyloid aggregates, exhibit anti-cholinesterase and anti-oxidative property and are non-cytotoxic in Neuro2a cells.
Introduction
Alzheimer’s disease (AD) is an acquired neurological disorder, which leads to cognitive impairment and behavioural functions in the ageing people. The major neuropathological hallmark of AD comprises formation of senile plaques and neurofibrillary tangles. The plaques are extracellular deposits mainly composed of fibrillar beta-amyloid (Aβ) peptide, which are possibly linked to the modulation of synaptic activity of aging brain [Citation1]. In addition, intracellular fibrillar aggregates of the microtubule-associated protein tau, which is also called as neurofibrillary tangles exhibit hyper-phosphorylation in AD cases [Citation2]. Another major pathological event is the disruption of the cholinergic system, which plays a decisive role in learning and memory, and the impairment of memory in AD is mainly linked with cholinergic dysfunction. Apart from these major hallmarks, oxidative stress and metal-mediated toxicity also play a role in mediating AD [Citation3].
At present, drugs such as donepezil, rivastigmine and galantamine are approved clinically for treating the cognitive impairments of AD brain. However, the usage of these drugs only gives symptomatic relief to AD patients. Moreover, the major limitation to the development of drugs against the central nervous system (CNS)-related diseases is the presence of stringent blood-brain barrier (BBB), which acts as a formidable porter towards the entry of exogenous substances into the brain [Citation4]. Consequently, there is a need for an alternative approach to block the disease-inducing mechanism by identifying novel therapeutic agents with BBB permeability and lesser side effects, as exhibited by natural drugs to combat AD.
Natural products are potentially fruitful source of highly bioactive secondary metabolites with its significant therapeutic values, which opens new and alternative avenues in the phase of drug discovery and development [Citation5]. Among the various bioactive compounds, phytol is one of the broadly investigated applicants because of its profuse availability in nature and has been reported to hold many bioprotective efficacies ranging from anti-inflammatory to anti-cancer properties [Citation6]. Phytol is a class of terpenoid which comes under the category of acyclic monounsaturated diterpene alcohol and is a part of chlorophyll molecule [Citation7]. Regarding the neuroprotective potential, phytol has been reported to possess anti-convulsant effect in response to pilocarpine induced seizures in mice [Citation8]. In addition, phytol also exhibits excellent neuroprotective effect on human neuroblastoma cells (SH-SY5Y) in which a simulated ischemia model was used [Citation9]. In spite of its effectiveness, phytol has not yet been accepted as a pharmaceutical agent due to its poor solubility and low bioavailability owing to the hydrophobic nature of the molecule [Citation10]. In this regard, scientific studies have suggested that nanoparticle-based drug delivery system possibly will be able to enhance the solubility and stability of the encapsulated drugs and also maintain their therapeutic concentration in addition to inducing less toxic effect to the brain cells [Citation11]. The polymeric nanoparticles appear to be one of the promising candidates as they are capable of opening the tight junctions and successfully cross the BBB. Nanoparticles of different compositions are available; however, the advantages of using synthetic polymeric nanoparticles are their higher purity and reproducibility over natural polymers [Citation12] and it is well suited for drug delivery system because they have inherent structure and surface adjustable properties. Various biodegradable polymers such as poly lactic acid (PLA) poly(D,L-lactic acid-co-glycolide) (PLG) and poly(lactic-co-glycolic acid) (PLGA) are widely used for biomedical applications [Citation13]. Among them, PLGA is considered as good candidates to deliver drugs due to its BBB penetrability, biocompatibility, biodegradability, sustained release, low toxicity, ease of administration and patient compliance [Citation14,Citation15], and has been approved by the Food and Drug Administration (FDA) and the European Medicine Agency (EMA) as good drug delivery system. In addition, the PLGA NPs can effectively encapsulate hydrophobic and hydrophilic drugs. For instance, PLGA encapsulated curcumin cross the BBB effectively and exhibited beneficial therapeutic effect against AD compared with free curcumin [Citation16]. Based on these existing evidences, the aim of the present study was designed to synthesize and characterize phytol-PLGA NPs and evaluate its anti-cholinesterase, anti-oxidant and anti-amyloidogenic properties.
Experimental
Materials
PLGA (50:50), thioflavin T (Th-T) and phytol were purchased from Sigma-Aldrich (St. Louis, MO, USA). Polyvinyl alcohol (PVA) and 5,5′-dithiobis(2-nitrobenzoic) acid (DTNB) were purchased from Himedia Laboratories (Mumbai, India). Acetylcholinesterase (AChE) from electric eel (Type-VI-S, EC 3.1.1.7, Sigma) and butyrylcholinesterase (BuChE) from horse serum (EC 3.1.1.8, MP Biomedicals), Santa Ana, CA, USA were used as enzyme. Acetylthiocholine iodide (ATCI) and butyrylthiocholine iodide (BTCI) (Himedia Laboratories, West Chester, PA, USA) were used as substrates. Aβ25–35 peptides were purchased from GenScript (Piscataway, NJ, USA). All the other chemicals and solvents used in the experiment are of high grade and purity.
Preparation of phytol loaded PLGA nanoparticles
Phytol-loaded PLGA nanoparticles were obtained by oil in water emulsion-solvent evaporation method [Citation16]. Briefly, 40 mg of PLGA (50:50, MW: 30,000–60,000) was dissolved in 3 mL of dichloromethane. Furthermore, 10 mg of phytol was added to this mixture and allowed to dissolve by keeping on the magnetic stirrer for 10 min. This mixture was further drop wise added into 20 mL of PVA (2%), which acts as a surfactant. The complete mixture of the PLGA loaded phytol suspension was sonicated at 40% amplitude for 5 min to form a fine emulsion. The emulsion was kept on the stirrer overnight for solvent evaporation. The nanoparticles were collected by centrifugation at 13,000 rpm for 15 min at 4 °C. After this process, the nanoparticle pellets were washed thrice with water then freeze dried and stored at −20 °C for further use. The blank nanoparticles were prepared by following the same procedure excluding the addition of phytol. For all the analyses, the nanoparticles were dispersed in milli-Q water.
Characterization of phytol loaded PLGA nanoparticles
Determination of encapsulation efficiency and drug loading (DL) capacity
After the nanoparticles were synthesized, the amount of phytol in the supernatant was measured by centrifugation at 13,000 rpm at 4 °C for 15 min. Encapsulation efficiency (EE), DL capacity and yield of the nanoparticle were quantified by UV-spectrophotometric analysis [Citation16]. Standard calibration curve was plotted using standard concentrations of phytol (1–5 mg/mL) dissolved in dichloromethane. The quantification was measured by taking the absorbance at 250 nm. The following formulae were used to calculate the EE, DL and yield of the nanoparticles, respectively:
Particle size and zeta potential
Particle size and zeta potential of the phytol loaded PLGA nanoparticles were analyzed by dynamic laser scattering (DLS) using zetasizer (Malvern Zetasizer nano-ZS90, UK). The samples (1 mg/mL) were suspended in milli-Q, then vortexed and sonicated for a few minutes. Subsequently, the samples were placed in a zetasizer for the measurement of size distribution and zeta potential of the phytol loaded PLGA nanoparticles. The mean diameter for each sample was measured in triplicate and the results were uttered as mean size ± SD.
Field emission scanning electron microscope (FESEM) analysis
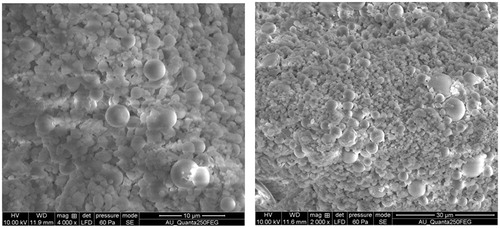
The surface morphology and size distribution were observed by FESEM (Quanta FEG 250, FEI, Eindhoven, The Netherlands). For FESEM analysis, freeze-dried nanoparticles were mounted on aluminium stubs with dual sided tape and coated with a thin layer of gold. The coated samples were then scanned and photographed under the microscope with an accelerating voltage of 5 kV.
Fourier transform infrared spectroscopic (FT-IR) study
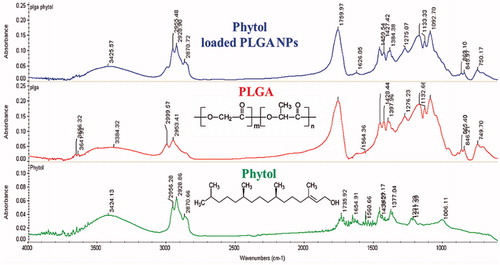
FT-IR spectroscopy study was performed to identify the presence of functional groups that are bound characteristically. In this analysis, the sample transmittance and reflectance of the infrared rays at various frequencies were observed as IR absorption plot which shows characteristic peaks. For FT-IR (Thermo Scientific, Marietta, GA, USA) spectroscopic study, the Phytol, PLGA alone and encapsulated phytol were mixed with potassium bromide to get pellet and was analyzed from the range of 400–4000 cm−1 using OMNIC software (Nicolet iS5 FT-IR Spectrometer Thermo Scientific, Marietta, GA, USA).
Power X-ray diffraction (PXRD) analysis
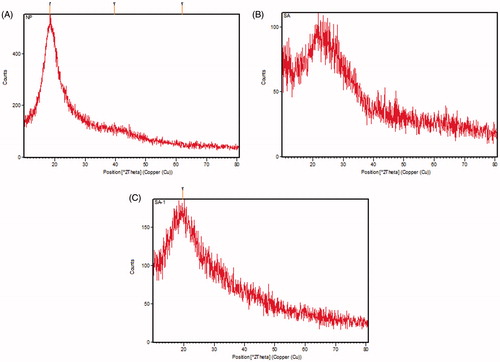
PXRD analysis was used to assess the crystalline nature of the pure compound phytol, encapsulated phytol and PLGA was analyzed by PXRD spectrophotometer measurement (X’ pert Pro, PANalytical, Almelo, The Netherlands) using Cu–Ka radiation generated at 40 kV and 30 mA over a 2θ scan range of 10–80 °C with scan step time of 10 s at 25 °C.
In vitro drug release kinetics
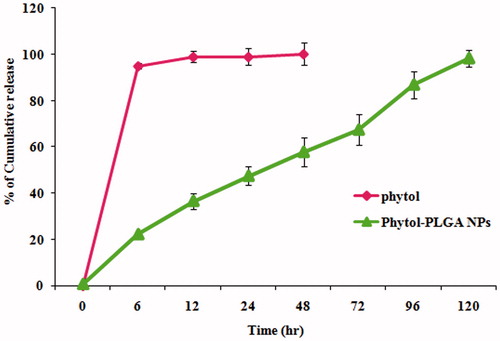
The in vitro drug release profile of phytol loaded PLGA nanoparticle was studied by dialysis method. Briefly, 1 mg of nanoparticles suspension was placed in a dialysis bag (molecular weight cut-off 10,000–12,000, Himedia, Mumbai, India), which was then sealed at both the ends. The dialysis bag was soaked in 0.1 M of 5 mL of phosphate buffer saline (pH 7.4) which was stirred endlessly at 100 rpm and maintained at 37 °C in a shaker. The volumes of the samples were withdrawn at various time intervals, and the same volume of fresh dissolution medium was replaced in a beaker. The absorbance of the solutions was determined in triplicates using UV spectrophotometer at 250 nm [Citation17].
Cholinesterase inhibitory assay
Determination of AChE and BuChE
Anti-cholinesterase activities were evaluated by previous reported method with slight modifications [Citation3]. Briefly, AChE/BuChE (10 µL/12 µL at 10 U/mL) in 50 mM Tris-HCl buffer (pH 8.0/7.4) solution with various concentrations (10–50 μg/mL) of phytol, phytol-PLGA NPs and PLGA NPs alone was incubated in a 96-well cell culture plate for 45 min at room temperature. After incubation of the reaction mixture, 125 µL of 3 mM DTNB was added and the total volume was made upto 300 µL with 50 mM Tris-HCl buffer (pH 8.0/7.4). Finally, enzyme activity was initiated by the addition of 50 µL of 15 mM ATCI/BTCI for AChE/BuChE, respectively. The hydrolysis of ATCI and BTCI was assessed by the formation of the yellow 5-thio-2-nitrobenzoateanion at 405 nm for 3 min using UV-visible spectrophotometer (U-2800, Hitachi, Japan). The standard anti-cholinesterase drug donepezil (10–50 µg/mL) was used as a positive control. Percentage of inhibition of AChE/BuChE was determined by comparing the rate of reaction of samples with control using the below formula:
Free radical scavenging assay
Free radical scavenging activity was quantified by standard method [Citation18]. In brief, 1 mL of DPPH (0.1 mM) in methanol was added to different concentrations (5–25 μg/mL) of phytol and phytol loaded PLGA nanoparticles. The mixture was shaken vigorously, allowed to stand in dark at room temperature for 4 h and the absorbance was taken at 517 nm in a UV-visible spectrophotometer (U-2800 Model, Hitachi, Japan). Butylated hydroxyl toluene (BHT) (10–50 µg/mL) was used as standard. The scavenging effect was calculated as follows:
Anti-aggregation and disaggregation assays
The anti-aggregation and disaggregation property of phytol and encapsulated phytol were evaluated against the amyloidogenic activity of Aβ peptide in two different phases described by previous reports [Citation1]. The effect of phytol and encapsulated phytol on the aggregation pattern of Aβ peptide was assessed in phase I; parallely, the disaggregation of mature Aβ fibrils by phytol and encapsulated phytol was verified in phase II. Freshly prepared 100 μM of Aβ25–35 monomer was incubated in Tris-HCl buffer pH 7.4 at 37 °C for 24 h to form oligomers. After the formation of Aβ25–35 oligomer, it was co-incubated with/without of phytol and encapsulated phytol (10 μg/mL) for phase I (24 and 48 h). Whereas, for phase II, the freshly prepared Aβ25–35 (100 μM) was incubated for 96 h for the formation of pre-formed Aβ25–35 fibrils. After that, phytol and encapsulated phytol (10 μg/mL) were co-incubated with the pre-formed fibrils, and the disaggregation pattern was assessed in 96 h and 9 days. The structural variation of Aβ peptide was evaluated by Th-T assay with the aliquots of 20 µL drawn from the composite mixture of incubated sample at different time interval from phases I and II. Galantamine (50 µg/mL) was used as a positive control. Microfluorescence assay and FT-IR analysis were also executed for additional verification of the anti-aggregation and disaggregation potential of phytol and encapsulated phytol.
Th-T assay
The amyloid fibril formation and disaggregation were quantified by Th-T induced fluorescence changes [Citation1]. To determine the amyloid fibril formation, the mixture containing Aβ25–35 with/without phytol and encapsulated phytol (10 µg/mL) were added to 50 mM glycine-NaOH buffer, pH 8.5, containing 5 μM Th-T to a final volume of 300 μL. The assay was measured in triplicate and fluorescence intensities were measured at 450 nm (excitation) and 485 nm (emission) (Molecular Device Spectramax M3, Sunnyvale, CA, USA). The background Th-T fluorescence intensity was subtracted from the experimental values.
Microfluorescence assay
About 2.5 μL of aliquot of the fibrillated Aβ25–35 peptide (100 μM) sample was diluted with 5 μM Th-T in 50 mM glycine–NaOH buffer (pH 8.5) and transferred to a glass slide. The fluorescent signals (488 nm) were observed by the Confocal Laser Scanning Electron Microscope System (CLSM FV300, Olympus, Tokyo, Japan).
FT-IR analysis
Alterations in the structural characteristics of Aβ25–35 peptides were analyzed through FT-IR by OMNIC software [Citation19]. The Aβ25–35 peptides (100 μM) were incubated with/without phytol and encapsulated phytol (10 μg/mL) for 24 h, 48 h, 96 h and 9 days. For the analysis, a small ration of the incubated sample was mixed with KBr and then scanned from 1400–1800 cm−1 with a spectral resolution of 4 cm−1.
Neuroprotective effect of phytol and phytol-PLGA NPs against Aβ25–35 induced toxicity in Neuro2a cells
The neuroprotective competences of phytol, encapsulated phytol and PLGA NPs alone against Aβ25–35 induced neurotoxicity were assessed in Neuro2a cells by MTT assay [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazoliumbromide] [Citation19]. Briefly, Neuro2a cells were seeded in a 96-well cell culture plate at a density of 2 × 105 cells per well and allowed to adhere on the surface of the plate for overnight. Simultaneously, the cells were pretreated with different concentrations of phytol and phytol-PLGA NPs (5 and 10 µg/mL) for 2 h, and then exposed to 50 μM of Aβ25–35 for 24 h. Afterwards, the medium was completely removed and MTT was added to each well (1.2 mM) and incubated at 37 °C for 4 h. The dimethyl sulfoxide (DMSO) was added as 200 µL/well in order to solubilize the formazan crystals and the absorbance was read at 570 nm in an UV-visible spectrophotometer (U-2800, Hitachi, Japan). The cell morphology was observed by light microscope and cell viability was expressed as percentage of control.
Statistical analysis
Statistical analysis was performed using SPSS 17.0 software package (SPSS Inc., Chicago, IL). All the experiments were performed in triplicate and the results were expressed as mean ± SD. Statistical analysis of variance between control and treated groups were done by one-way ANOVA. Duncan’s multiple range tests were evaluated to analyze the significance and the p values < .05 were observed as significant. IC50 values were further estimated by Probit analysis.
Results and discussion
Preparation and characterization of phytol loaded PLGA nanoparticles
Phytol is a natural compound with numerous pharmacological properties; however, the utility of phytol is greatly restricted because of its hydrophobic nature of this compound and as a result it is poorly soluble in biological fluids. However, this problem was overcome through an encapsulation process. In the present study, phytol was successfully encapsulated into PLGA nanoparticle by solvent evaporation method. The results showed that the drug/polymer ratio 1:4 has nearly 92% of EE, which could be explicated that the encapsulation process improved the solubility of phytol and thereby influenced its physicochemical and pharmacokinetic properties [Citation20]. The high entrapment efficiency of phytol loaded PLGA NPs observed in the current study could be attributed to the ionic interactions between the phytol and PLGA NPs and it also reduces the wastage of phytol molecules during the encapsulation process.
Furthermore, the DL capacity of phytol-PLGA NPs was 56% depending upon the drug/polymer ratio (1:4). During formulations process, it was observed that increase in concentration of drug, increases the DL capacity, which might be due to the ability of the polymer matrix to accommodate more amount of phytol molecules in the polymeric matrix. It also ensures the ability of polymeric matrix to release higher drug concentration at the specific target site. The yield of the nanoparticles was around 80%. Notably, the high EE, DL capacity and yield of the nanoparticles achieved in our work () are very similar as reported in galantamine-PLGA NPs [Citation21].
Table 1. Parameters of the entrapment efficiency, drug loading (DL) capacity and yield of phytol-PLGA NPs.
Particle size and zeta potential analysis
The size of nanoparticles is the most significant aspect to be controlled in the development of new formulations, because it has direct relevance with the stability, surface charge, bio-distribution, cellular uptake and drug release of nanomedicine [Citation22]. Mean size of phytol-PLGA NPs and PLGA NPs alone were found to be 177.4 ± 5.9 and 91.1 ± 1.9 nm respectively as confirmed by DLS measurements (). The particle size which is above 100 nm is ideal for intravenous route injection and also control their bio-distribution to an increase in their capture by kupffer cells or other phagocytic cells [Citation23]. Particularly, smaller size nanoparticle (<100 nm) can easily move across the blood vessels as well as prolong circulation in the blood [Citation24]. Hence, the size of nanoparticles which is in nanometer range can possibly enhance the delivery of phytol into CNS by increasing the concentration of phytol in specific site of brain or at the BBB cell luminal surface.
Table 2. Mean particle size, polydispersity index (PDI) and zeta potential for phytol-PLGA NPs.
The surface charge of the particles is the vital characteristic which could control the stability of the particles during formulation through strong electrostatic interactions with the cell membrane and it can also ameliorate the pharmacokinetics of the drug [Citation25]. Positively charged particles have a higher affinity to attach to cell membrane and internalize the drugs when compared to negatively charged particles. The mean zeta potential of phytol-PLGA NPs was determined by Malvern zeta-sizer and it was found to be −32.8 ± 2.2 mV, whereas the PLGA nanoparticles were found at −29.1 ± 2.4 mV (). The zeta potential values of nanoparticles above +30 mV to −30 mV are normally considered stable for a longer period in suspended state [Citation26]. The negative value of zeta potential represents the presence of carboxyl groups present on the surface of the PLGA polymer, which will prevent their aggregation and thereby stabilize the nanoparticulate dispersion, which specifically permit them to be in the blood circulation for a longer period.
Surface morphology of NPs
Characterization of surface morphology of the nanoparticles was performed using field emission scanning electron microscopy. It has been reported in earlier report that the hydrochloric norvancomycin loaded PLGA nanoparticles which are spherical in shape, non-porous and have a smooth surface are efficient carriers for the supply of therapeutic drug at specific target [Citation27]. Similar results were observed in phytol-PLGA NPs formulation which suggested that the NPs are used as competent drug delivery carrier for therapeutic molecules ().
FT-IR analysis
FT-IR analysis of NPs was performed to confirm the interaction between the functional groups of drug and polymer. FT-IR spectrum of phytol-PLGA NPs was compared with phytol and PLGA NPs alone (). The FT-IR spectra of nanoparticle were recorded over the range of 400–4000cm−1. A characteristic peak of OH stretching (3200–3600 cm−1), CH stretching (2850–3000 cm−1), carbonyl C=O stretching (1700–1800 per cm) and C–O stretching (1050–1250 cm−1) absorption band was observed for PLGA polymer. However, the spectrum for phytol showed the corresponding bands of OH-stretching was observed at 3000–3700 per cm. The bands of C=O absorption (1735 cm−1), C–C stretching (1654 cm−1), C–H bending (1438 and 1377 cm−1) and a band region attributed to the C–O stretching (1231 and 1006 cm−1) were also observed. In the case of FT-IR spectrum of phytol-PLGA NPs showed the characteristic peaks of OH stretching band (3000–3500 cm−1) are slightly shifted were observed. This fact confirmed that interactions between the hydroxyl and carboxyl groups of both phytol and PLGA polymeric nanoparticles.
PXRD analysis
PXRD analysis was used to predict the crystalline nature of the pure compound, PLGA NPs alone and nanoformulation. The PXRD pattern of phytol showed a characteristic diffraction of sharp peaks indicating the crystalline structure. Whereas, PLGA polymer did not show any diffraction peaks indicating its amorphous nature and pure phytol revealed the characteristic peak of their crystalline structures. Conversely, the nanoformulation of phytol-PLGA NPs also exhibited a number of diffraction peak. The results noticeably proved that the diffraction peak of phytol is slightly shifted and other peaks were disappeared during formulation with the PLGA polymer. Earlier study suggested that quercetin and catechin loaded PLGA NPs displayed the crystalline nature of each compounds and also other diffraction peaks were dispersed in a noncrystalline state of PLGA polymer [Citation28]. Similar PXRD pattern was noticed and shown in .
In vitro drug release kinetics
The in vitro drug release profile of phytol loaded PLGA NPs was evaluated by dialysis method. The percentage of phytol discharged from the PLGA nanoparticles at various time intervals was analyzed. The release rate of a drug from nanocarrier depends on several factors such as, PLGA concentration, size of the nanoparticle, solubility, molecular weight and biodegradable properties of polymer matrix [Citation29]. The resultant of phytol-PLGA NPs showed a burst release of up to 22% within the initial 6 h, followed by sustained release. After 6 h, A slow cumulative drug release that reached up to 98% from the NPs (drug:polymer ratio, 1:4) was observed (). The initial burst release is usually attributed to the tightly adsorbed surface obstructed fraction of phytol on the PLGA nanoparticles. This may be due to the diffusion of phytol entrapped within the polymer matrix after which, phytol is dissolved by the entry of dissolution medium into the particle. Finally, the encapsulated nanoparticles are completely released within 120 h. Similar biphasic release pattern with an initial burst effect followed by release of drug in a sustained manner has been also found in dexamethasone loaded PLGA NPs [Citation30]. The in vitro drug release study clearly reveals that the deliverance action of PLGA NPs sustained release of phytol molecule for the competence of AD treatment.
ChE inhibitory efficacy of phytol and phytol loaded PLGA nanoparticles
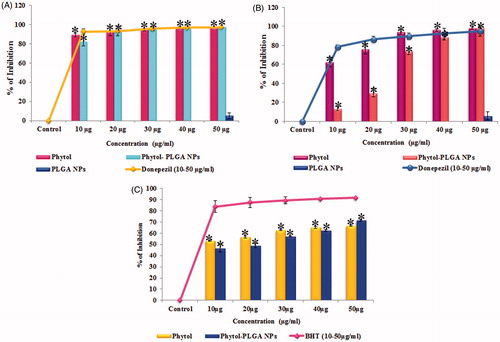
Cholinergic dysfunction has been considered as one of the major pathogenesis of AD which occurs in the cortex and hippo campal regions of the brain and is liable for learning, memory and behavioural symptoms. AChE is a key enzyme that plays a chief role in cholinergic neurotransmission by regulating the ACh levels in brain neurons. Several studies reported that ChEI’s act as the most promising therapeutic agents for AD. The inhibition of cholinesterase enzymes is responsible for blocking the hydrolysis of ACh at the synaptic cleft and also improving the cognitive deficit in AD patients. BuChE is another ACh degrading enzyme mainly found in the glial cells and subcortical region of neurons and promotes the neurotoxic effect of amyloid plaques in AD brain [Citation3]. Inhibition of BuChE may also boost the cognitive deficit. In this background, the present study was carried out to evaluate the dual cholinesterase inhibitory effect of phytol and phytol-PLGA NPs. The results suggest that AChE activity of the phytol and encapsulated phytol (10–50 μg/mL) achieved a significant inhibition (p < .05) of around 97.29 ± 0.22% (IC 50 value <10 μg/mL) and 96.93 ± 0.72% (IC 50 value <10 μg/mL), respectively, while PLGA NPs alone exhibited very less inhibition of 5.21 ± 2.97% are shown in . Similarly, phytol and encapsulated phytol were also able to inhibit BuChE with significant (p < .05) inhibitory potential of 97.92 ± 1.39% (IC 50 value <10 μg/mL) and 97.94 ± 0.58% (IC 50 value <30 μg/mL) in a dose dependent manner and PLGA NPs alone showed slight inhibition of 5.51 ± 4.23% (), when compared to standard drug donepezil (96 ± 0.48% and 94 ± 5.56%), respectively. Earlier report suggested that inhibition of both AChE and BuChE not only enhances the therapeutic effects and also reduces the aggregation form of β-amyloid peptide, thereby protecting the neurons from neurodegeneration [Citation31]. The present work evidently illustrates that phytol and encapsulated phytol could act as cholinesterase inhibitors to combat AD.
Figure 5. (A) Acetylcholinesterase, (B) butyrylcholinesterase inhibitory activity of phytol, phytol-PLGA NPs, PLGA NPs alone (10–50 μg/mL) compared with standard drug donepezil. (C) DPPH radical scavenging activity of phytol, phytol-PLGA NPs and PLGA NPs alone compared with standard BHT (10–50 μg/mL). Astersik (*) denotes statistically significant (p < .05) as compared drug versus control. Values are expressed as mean ± SD (n = 3).
Assessment of anti-oxidant potential of phytol and encapsulated phytol
In AD, the Aβ peptide accumulation results in a burst of oxidative stress, which leads to neurotoxicity. Hence, neuroprotective agents which can act as anti-oxidants can protect the neuronal cells by attenuating the oxidative stress [Citation32]. The anti-oxidant potential of phytol and encapsulated phytol were evaluated through DPPH radical scavenging assay. The results obviously proved that phytol and phytol-PLGA NPs possess significant (p < .05) DPPH scavenging activity (IC 50 value <30 μg/mL) at the maximum concentration of 50 μg/mL has 66 ± 0.64% and 71 ± 1.01% of inhibition, respectively, which was observed and compared to standard anti-oxidant BHT (91 ± 0.66%) are shown in . Since, anti-oxidants act as effective therapeutic agents in delaying the disease progression and appearance of AD symptoms, from the results of the present study it can be understood that the high anti-oxidant potential of phytol and phytol loaded PLGA NPs may act as effective therapeutic agent for AD.
Anti-aggregation and disaggregation property of Aβ25–35 by phytol and encapsulated phytol
The prevention of the formation of oligomers and the fibrils from soluble monomers is a therapeutic significance for AD drug discovery. Several reports show that the bioactive compounds from plants rich in polyphenols such as curcumin, rosmarinic acid, tannic acid, catechin and quercetin have the ability to inhibit the formation of oligomer and mature fibrillar assembly in vitro and the associated cytotoxicity [Citation33]. Based on these observations, the present study was carried out for the first time to investigate the effect of phytol and phytol-PLGA NPs on amyloid toxicity under in vitro conditions using Th-T flurosecence, confocal microscopy and FT-IR analysis.
Th-T assay
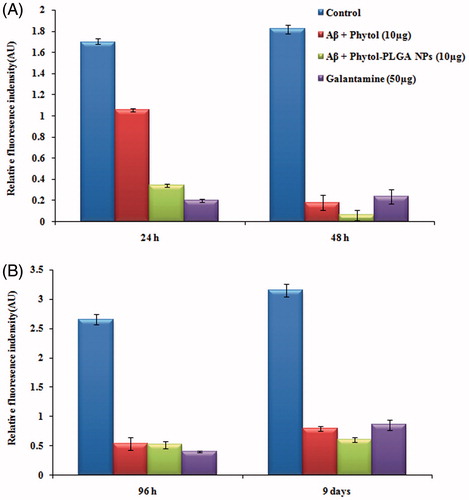
Th-T fluorescence assay was performed to evaluate the anti-amyloidogenic property of Aβ peptide. The results of Th-T assay data which represent with and without phytol and phytol loaded PLGA NPs in two different phases at various time intervals (24 h, 48 h, 96 h and 9 days) are shown in . In phase I, incubation of Aβ25–35 peptide resulted in the increase fluorescence intensity from 1.69 ± 0.14 AU and 1.8 ± 0.43 AU at 24 h and 48 h respectively, which represent the formation of oligomer due to aggregation of Aβ peptide. Interestingly, during Aβ25–35 peptide co-treatment with phytol (1.05 ± 0.01 AU) and phytol loaded PLGA NPs (0.3 ± 0.12 AU) at 10 µg/mL, an extensive decrease in the fluorescence intensity was observed at 24 h. However at 48 h, a noticeably reduced fluorescence intensity of 0.1 ± 0.07 AU and 0.06 ± 0.04 AU was observed in phytol and encapsulated phytol treated group respectively, when compared to treatment with 50 μg/mL of the positive control galantamine (0.2 ± 0.15 AU and 0.2 ± 0.06 AU). This observation shows that the phytol and phytol loaded PLGA NPs efficiently decreased the formation of β-sheet pattern thereby preventing the formation of oligomers from fibrils in phase I. In phase II study, the disaggregation ability on pre-formed mature fibrils by phytol and phytol loaded PLGA NPs was evaluated. An increase in fluorescence intensity was observed from 96 h to 9 days in Aβ25–35 (2.6 ± 0.09 AU and 3.1 ± 0.11 AU) treated groups, while the significant reduction in fluorescence intensity was observed in phytol (0.5 ± 0.11 AU and 0.7 ± 0.04 AU), phytol-PLGA NPs (0.5 ± 0.06 AU and 0.6 ± 0.04 AU) and galantamine (0.4 ± 0.17 AU and 1.4 ± 0.12 AU) treated group. It clearly indicates that at different time intervals (96 h and 9 days), Aβ co-treated with phytol and phytol-PLGA NPs expeditiously disaggregate the pre-formed mature fibrillation of Aβ peptide and could be used as an anti-amyloidogenic agent for AD treatment.
Microfluorescence assay
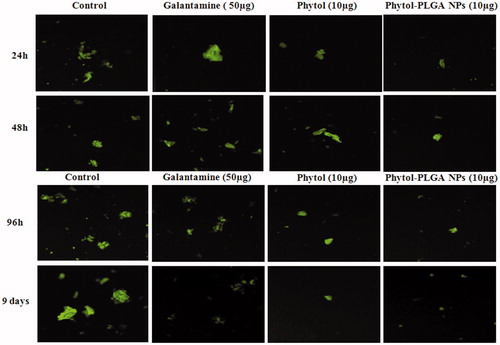
The results of Th-T assay were further confirmed through confocal microscopy. Consistent with the fluorimetry assay, CLSM images also clearly demonstrate the initiation of Aβ25–35 peptide aggregation at 24 h and the rapid enhancement of the green fluorescence intensity at 48 h. However, during co-treatment with phytol and encapsulated phytol, the green fluorescence intensity decreased considerably, which suggests the inhibition of Aβ25–35 aggregates from oligomers by phytol and encapsulated phytol in phase I. A similar inhibitory effect was evidenced in phase II experiments revealing that the phytol and phytol-PLGA NPs have the ability to disaggregate the formation of mature fibrils in 96 h and 9 days. The results of both the phases were compared with control (Aβ25–35 alone) and standard drug galantamine (). Consequently, the outcome of these experiments demonstrated that the phytol and encapsulated phytol acts as multi-targeted agent exhibiting anti-aggregation and disaggregation of Aβ fibrils.
FT-IR spectroscopic analysis of phytol and encapsulated phytol Aβ25–35 peptide
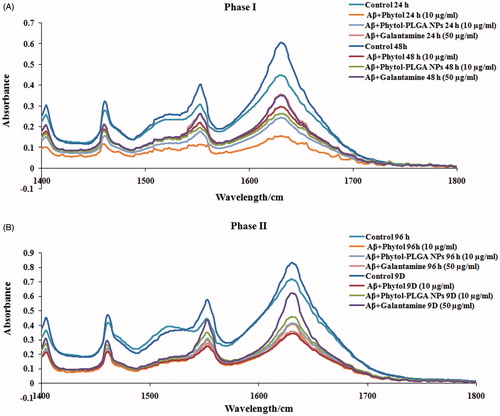
The anti-aggregation and disaggregation property of phytol and phytol loaded PLGA NPs was confirmed through FT-IR analysis. IR spectroscopy has been widely used to assess the structural conformation of proteins.1 Despite the presence of various IR absorption bands, the amide I (1600–1690 cm−1 C=O stretching) and amide II (1480–1575 cm−1 CN stretching, NH bending) are the familiar vibrational IR bands, predominantly used to reveal the conformational changes of peptides [Citation34]. The FT-IR spectrum of Aβ25–35 peptide showed a major peak at 1600 cm−1, which is attributed to the presence of Aβ-aggregates. The phytol and encapsulated phytol treated groups showed reduction in absorbance similar to standard drug galantamine, which demonstrates that phytol and encapsulated phytol prevents the aggregation of Aβ oligomers to mature fibrils in phase I. In phase II, an increase in absorbance was observed at 1600 cm−1 in Aβ control groups at 96 h and 9 days illustrating the formation of mature plaques. Whereas in phytol and encapsulated phytol treated group showed decrease in absorbance illustrating that the phytol and encapsulated phytol effectively disaggregates the mature fibrils. Results of FT-IR analysis () proposed that phytol and encapsulated phytol restores the modification occurring in the spectral pattern of aggregated Aβ25–35 in 24 h, 48 h, 96 h and 9 days.
In vitro cell survival and protective effect of phytol and phytol-PLGA NPs against Aβ25–35 induced toxicity in Neuro2a cells
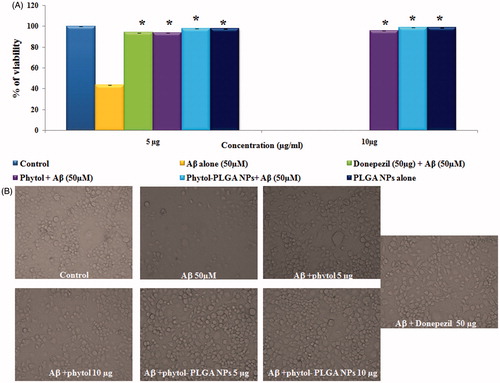
The neuroprotective prospective of phytol and phytol-PLGA NPs were investigated in Neuro2a cells treated with 50 µM of Aβ25–35 peptide by MTT assay. The statistical data obviously proved that the viability of cells was significantly decreased in 50 µM of Aβ25–35 (45%) treated group. But, significant (p < .05) protection was observed in the presence of phytol and phytol-PLGA NPs increased the viability of the cells above 95% when compared to standard drug donepezil (93%) were shown in . The increase in cell viability may be owing to phytol and phytol-PLGA NPs has great anti-oxidant potential ability to reduce the oxidative stress induced by Aβ peptide. Finally, the light microscopic image confirmed that phytol and encapsulated phytol are nontoxic and can protect the Neuro2a cells against Aβ induced neurotoxicity are shown in .
Figure 9. (A) Neuroprotective effect of phytol and phytol-PLGA NPs (5 and 10 mg/mL) against Aβ25–35 induced neurotoxicity on Neuro 2a cells. Astersik (*) denotes statistically significant (p < .05) as compared drug versus Aβ25–35 treated group. (B) Microscopic images exhibiting the protective effect of phytol, phytol-PLGA NPs and donepezil against Aβ25–35 neurotoxicity.
Conclusion
To the best of our knowledge, this is the first report regarding the synthesis of phytol-PLGA NPs enhances the solubility of phytol, may possibly aid in permeation through the BBB, resulting in sustained release of phytol to the brain, which may be beneficial for long-term therapeutic effects. Overall, the results conclude that phytol and phytol-PLGA NPs can be proposed as novel therapeutic strategies for AD treatment.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Shanmuganathan B, Malar DS, Sathya S, et al. Antiaggregation potential of Padina gymnospora against the toxic Alzheimer’s beta-amyloid peptide 25–35 and cholinesterase inhibitory property of its bioactive compounds. PLoS One. 2015;10:e0141708.
- Pryor NE, Moss MA, Hestekin CN. Unraveling the early events of amyloid-β protein (Aβ) aggregation: techniques for the determination of Aβ aggregate size. IJMS. 2012;13:3038–3072.
- Shanmuganathan B, Pandima Devi K. Evaluation of the nutritional profile and antioxidant and anti-cholinesterase activities of Padina gymnospora (Phaeophyceae). Eur J Phycol. 2016;51:482–490.
- Chen Y, Liu L. Modern methods for delivery of drugs across the blood–brain barrier. Adv Drug Deliv Rev. 2012;64:640–665.
- Choi EY, Hwang HJ, Kim IH, et al. Protective effects of a polysaccharide from Hizikia fusiformis against ethanol toxicity in rats. Food Chem Toxicol. 2009;47:134–139.
- Silva RO, Sousa FB, Damasceno SR, et al. Phytol, a diterpene alcohol, inhibits the inflammatory response by reducing cytokine production and oxidative stress. Fundam Clin Pharmacol. 2014;28:455–464.
- Yoo KY, Park SY. Terpenoids as potential anti-Alzheimer’s disease therapeutics. Molecules. 2012;17:3524–3538.
- Costa JP, Ferreira PB, De Sousa DP, et al. Anticonvulsant effect of phytol in a pilocarpine model in mice. Neurosci Lett. 2012;523:115–118.
- Chang HJ, Kim HJ, Chun HS. Quantitative structure − activity relationship (QSAR) for neuroprotective activity of terpenoids. Life Sci. 2007;80:835–841.
- Anand P, Kunnumakkara AB, Newman RA, et al. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818.
- Fonseca-Santos B, Gremião MP, Chorilli M. Nanotechnology-based drug delivery systems for the treatment of Alzheimer’s disease. Int J Nanomedicine. 2015;10:4981.
- Soppimath KS, Aminabhavi TM, Kulkarni AR, et al. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release. 2001;70:1–20.
- Nicolas J, Mura S, Brambilla D, et al. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem Soc Rev. 2013;42:1147–1235.
- Sahni JK, Doggui S, Ali J, et al. Neurotherapeutic applications of nanoparticles in Alzheimer’s disease. J Control Release. 2011; 152:208–231.
- Stevanovic M, Uskokovic D. Poly (lactide-co-glycolide)-based micro and nanoparticles for the controlled drug delivery of vitamins. Curr Nanosci. 2009;5:1–4.
- Mathew A, Fukuda T, Nagaoka Y, et al. Curcumin loaded-PLGA nanoparticles conjugated with Tet-1 peptide for potential use in Alzheimer’s disease. PLoS One. 2012;7:e32616.
- Ray S, Ghosh S, Mandal S. Development of bicalutamide-loaded PLGA nanoparticles: preparation, characterization and in-vitro evaluation for the treatment of prostate cancer. Artif Cells Nanomed Biotechnol. 2017;45:944–954.
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200.
- Hafezi Ghahestani Z, Alebooye Langroodi F, Mokhtarzadeh A, et al. Evaluation of anti-cancer activity of PLGA nanoparticles containing crocetin. Artif Cells Nanomed Biotechnol. 2017;45:955–960.
- Sah AK, Suresh PK, Verma VK. PLGA nanoparticles for ocular delivery of loteprednol etabonate: a corneal penetration study. Artif Cells Nanomed Biotechnol. 2017;45:1156–1164.
- Fornaguera C, Feiner-Gracia N, Calderó G, et al. Galantamine-loaded PLGA nanoparticles, from nano-emulsion templating, as novel advanced drug delivery systems to treat neurodegenerative diseases. Nanoscale. 2015;7:12076–12084.
- Lamprecht A, Schäfer U, Lehr CM. Size-dependent bioadhesion of micro-and nanoparticulate carriers to the inflamed colonic mucosa. Pharm Res. 2001;18:788–793.
- Wisse E, De Leeuw AM. Structural elements determining transport and exchange processes in the liver. In: Daviss SS, Illum L, McVie JG, Tomlinson E, editors. Microspheres and drug therapy, pharmaceutical, immunological and medical aspects. Amsterdam (The Netherlands): Elsevier; 1984 . p. 1–23.
- Allemann E, Gurny R, Doelker E. Drug-loaded nanoparticles: preparation methods and drug targeting issues. Eur J Pharm Biopharm. 1993;39:173–191.
- He C, Hu Y, Yin L, et al. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials. 2010;31:3657–3666.
- Yue PF, Yuan HL, Yang M, et al. Preparation, characterization, and pharmacokinetic evaluation of puerarin submicron emulsion. PDA J Pharm Sci Technol. 2008;62:32–45.
- Yang H, Hao Y, Liu Q, et al. Preparation and in vitro study of hydrochloric norvancomycin encapsulated poly (d,l-lactide-co-glycolide, PLGA) microspheres for potential use in osteomyelitis. Artif Cells Nanomed Biotechnol. 2017;45:1326–1330.
- Pool H, Quintanar D, d Dios Figueroa J, et al. Antioxidant effects of quercetin and catechin encapsulated into PLGA nanoparticles. J Nanomater. 2012;2012:86.
- Lee JH, Yeo Y. Controlled drug release from pharmaceutical nanocarriers. Chem Eng Sci. 2015;125:75–84.
- Fornaguera C, Llinàs M, Solans C, et al. Design and in vitro evaluation of biocompatible dexamethasone-loaded nanoparticle dispersions, obtained from nano-emulsions, for inhalatory therapy. Colloid Surface B. 2015;125:58–64.
- Hodges JR. Alzheimer’s centennial legacy: origins, landmarks and the current status of knowledge concerning cognitive aspects. Brain. 2006;129:2811–2822.
- Jiang T, Sun Q, Chen S. Oxidative stress: a major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson’s disease and Alzheimer’s disease. Prog Neurobiol. 2016;147:1–19.
- Vauzour D. Dietary polyphenols as modulators of brain functions: biological actions and molecular mechanisms underpinning their beneficial effects. Oxid Med Cell Longev. 2012;2012:914273.
- Adochitei A, Drochioiu G. Rapid characterization of peptide secondary structure by FT-IR spectroscopy. Rev Roum Chim. 2011;56:783–791.