Abstract
We describe the results of adding a new biological agent HEMO2life® to a standard preservation solution for hypothermic static lung preservation aiming to improve early functional parameters after lung transplantation. HEMO2life® is a natural oxygen carrier extracted from Arenicola marina with high oxygen affinity developed as an additive to standard organ preservation solutions. Standard preservation solution (Perfadex®) was compared with Perfadex® associated with HEMO2life® and with sham animals after 24 h of hypothermic preservation followed by lung transplantation. During five hours of lung reperfusion, functional parameters and biomarkers expression in serum and in bronchoalveolar lavage fluid (BALF) were measured. After five hours of reperfusion, HEMO2life® group led to significant improvement in functional parameters: reduction of graft vascular resistance (p < .05) and increase in graft oxygenation ratio (p < .05). Several ischemia-reperfusion related biomarkers showed positive trends in the HEMO2life® group: expression of HMG B1 in serum tended to be lower in comparison (2.1 ± 0.8 vs. 4.6 ± 1.5) with Perfadex® group, TNF-α and IL-8 in BALF were significantly higher in the two experimental groups compared to control (p < .05). During cold ischemia, expression of HIF1α and histology remained unchanged and similar to control. Supplementation of the Perfadex® solution by an innovative oxygen carrier HEMO2life® during hypothermic static preservation improves early graft function after prolonged cold ischemia in lung transplantation.
Introduction
Since 1983, lung transplantation has become the mainstay of therapy for most end-stage lung diseases. In the last years, approximately 4000 double or single lung transplants were performed annually [Citation1].
Despite advances in organ preservation and surgical care, outcomes after lung transplantation remain poor. The primary graft dysfunction (PGD) after transplantation continues to be a common and significant cause of failure in the early postoperative period (within 72 h after the surgery) occurring in 20 to 35% of cases [Citation2–4]. Main features are: acute pulmonary edema, increased pulmonary vascular resistance and decreased airway compliance inducing an alteration of the PaO2/FiO2 ratio. The severity of PDG is assessed by the degree of hypoxemia based on PaO2/FiO2 ratio. Development of PDG is a predictor of poor outcomes and a major risk factor for development of bronchiolitis obliterans syndrome (BOS) or chronic lung allograft rejection (CLAD) [Citation5,Citation6]. Grade 3 PGD are associated with a mortality of nearly 50% in the first 30 days post-transplantation [Citation3] and an increased risk of developing BOS [Citation7,Citation8]. PGD is described as a multifactorial disease in which ischemia-reperfusion injury (IRI) has a pivotal role.
Solid organ graft procedure involves unavoidable steps of ischemia and reperfusion (I/R) sequences. A variety of pathophysiological pathways are activated during this period, such as oxidative stress, mitochondrial uncoupling and inflammation [Citation9,Citation10]. IRI thus plays a central role in determining grafted organ quality and is strongly correlated with early graft nonfunctioning or delayed graft function [Citation4,Citation11], as well as chronic graft failure and late graft loss [Citation8,Citation12]. It is thus of paramount importance to design strategies to better preserve organs in order to limit IRI and substantially improve graft quality as well as the post-transplant outcomes.
The use of HEMO2life® (Hemarina SA, France) in cold static preservation before transplantation, was previously reported. It significantly improved graft quality and outcome at different doses in a large animal preclinical model of renal transplantation [Citation13,Citation14]. The active principle of HEMO2life® is M101, an extracellular hemoglobin obtained from a marine invertebrate, Arenicola marina [Citation15–17], a worm living in sandy beaches and constantly subjected to environmental changes from normoxia to hypoxia at different temperatures. HEMO2life® is formulated as a GMP compliant commercial class III medical device intended as an additive to existing organ preservation solutions.
HEMO2life® was already tested in several preclinical models for solid organ transplantation and it is currently being tested in clinical trials in organ preservation before kidney transplant.
We aimed to evaluate the benefit of supplementation by HEMO2life® to the most common lung preservation solution Perfadex® in a well-recognized single-lung allo-transplantation model in pig [Citation18].
Material and methods
HEMO2life® was stored at −20 °C and brought to 4 °C just before adding it to standard preservation solution Perfadex® in a quantity corresponding to 2 g per liter of standard solution. The standard preservation solution used in this study was Perfadex® (Xvivo, Sweden), a colloid based “extracellular” low potassium electrolyte solution for rapid cooling, perfusion and storage of lungs in case of transplantation, stored at room temperature and brought to 4 °C before use.
Experimental groups and animals
Thirty three large white pig weighing 20–25 kg were used with 14 serving as donors, 14 as recipients and five as sham. Each donor and recipients pair came from the same sibling and they were randomly included in Perfadex® Group 1 (n = 7) or HEMO2life® + Perfadex® Group 2 (n = 7). Both lungs were retrieved from donors and were flushed and preserved for 24 h at 4 °C in the same solution according to their group. The right lung was used to study cold ischemia parameters and the left was transplanted into the recipients. Recipient animals had their left lung removed and replaced with the preserved lung from their corresponding donor pair. A sham group was also used (n = 5) where the animals were treated similarly to the recipient animals, but without undergoing lung transplantation. The experimental protocol was reviewed and approved by the Committee on Research Animal Care in compliance with the “Principles of Laboratory and Animal Care” and the Guide for the Care and Use of Laboratory Animals, prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH Publication No. 85–23, revised 1985).
Surgical procedure
All animals were anesthetized. After orotracheal intubation, mechanical ventilation was performed in a volume-controlled mode with a ventilation rate of 15 breaths/min and a tidal volume of 8 ml/kg at inspiratory oxygen-tension (FiO2) of 1.0. Positive end-expiratory pressure was adjusted to 5 mm Hg.
Donor operation: After median sternotomy a flushing tube was inserted into the main pulmonary artery. Systemic anticoagulation was performed with 3 mg/kg heparin. Lungs were flushed with 1.5 l anterograde and 0.5 l retrograde of 4° to 6 °C preservation solution and stored mildly inflated at 4° to 6 °C in the preservation solution for 24 h.
Recipient operation: In addition to the standard monitoring, a catheter was placed in the carotid artery and a Swan-Ganz catheter (Baxter, Healthcare Corp., Irvine, CA) was placed through a jugular vein introducer sheath for complete haemodynamic monitoring.
After left-sided lateral thoracotomy, pneumonectomy was performed. The donor lung was implanted in a typical manner with an end-to-end anastomosis of the left bronchus, followed by the atrial cuff and left pulmonary artery. A chest tube was inserted and the chest was closed. Animals were placed in supine position, medial sternotomy was performed. After pericardium opening, right pulmonary artery was accessed to allow clamping. Then the evaluation period started, 10 min before each physiological analysis the right pulmonary artery was clamped to evaluate only the function of the transplanted left lung.
In the sham group same procedure was performed as for all receivers except the transplantation. The native left lung was left in place to allow observation.
Each experiment was terminated after an observation period of five hours At the end of graft evaluation period, all the anastomosis were controlled. All animals with anastomoses troubles were excluded.
Monitoring and measurements
Hypoxia-inducible factor 1-alpha (HIF1α study during cold ischemia)
Lung samples were taken at 0, 1, 3, 6, 12 and 24 h of cold ischemia from the right lung. The samples were immediately frozen in liquid nitrogen. For the determination of tissue HIF1α level as markers of hypoxia, frozen lung tissue was homogenized by slicing. Total fraction protein was extracted and measured. Immediately after extraction proteins were separated by Western Blot and labelling was performed by Anti HIF1α pig antibody. Revelation was performed by ECL.
Hemodynamics and lung function after reperfusion
Hemodynamic data were monitored continuously (SC9000 [Siemens] and Swan–Ganz catheter [Edwards Lifesciences®, Irvine, CA]). Results were reported at baseline and at 2 and 5 h after reperfusion. Arterial blood gas analysis (Rapid Lab, Bayer, Siemens, Germany) and assessment of circulatory parameters (mean pulmonary arterial pressure [MPAP], pulmonary vascular resistance [PVR]) were performed at each time-point. Oxygenation values were presented as the PO2/FiO2 ratio. The circulatory parameters were calculated by the monitoring system after calibration by injection of cold saline solution via the Swan-Ganz catheter.
Lung water content after reperfusion
Five hours after reperfusion, grafted lower lobe was harvested and used to evaluate extra pulmonary lung water with a gravimetric technique already described by our group [Citation19].
Lung histology after reperfusion
Lung samples were taken five hours after reperfusion from the left lung. Then specimens were fixed in buffered 4.5% formaldehyde and paraffin-embedded and 2-μm-thick sections were stained with Hematoxylin Eosin Saffron (HES) according to standard techniques. Between 2 and 5 slides from each animal were investigated at magnification 200 x to assess the amount of interstitial neutrophil infiltrate, interstitial lymphocyte infiltrate, alveolar edema, intra-parenchymal hemorrhage, intra-alveolar cells and hyaline membranes indicating ischemia-reperfusion damage. The histo-morphologic changes were assessed using a 3-step grading score (0 = absent, 1 = mild, 2 = moderate and 3 = severe) inspired from literature [Citation20].
Circulating biomarkers of ischemia reperfusion
At H0, H0.5, H2, H3.5 and H5, blood samples were taken by arterial line on dry tube. After centrifugation supernatant serum was collected and stored frozen at −80 °C. ELISA quantification of high mobility group box 1 protein (HMGB1), tumour necrosis factor alpha (TNFα) and nitric oxide (NO) was performed using commercially available porcine cytokine multiplex immunoassay kit (HMGB1 porcine (Euromedex®) and Porcine TNFα DuoSet (R&Dsystem®)). For NO, a Nitrate/Nitrite ColorimetricAssay Kit (Caymanchem®) was used. Each value was reported to baseline value for each animals.
Bronchoalveolar lavage fluid (BALF) cytokine five hours after reperfusion
BALF of the upper lobe of the left lung in all groups was immediately performed after explantation using 40 ml of normal saline. BALF samples underwent centrifugation at 10,000 rpm at 4 °C for 20 min to remove cells and debris and the supernatant was collected and stored frozen at −80 °C. Quantification of cytokine levels in BALF was assessed using a commercially available porcine cytokine multiplex immunoassay kit: Interleukine 8 Elisa Kit (Euromedex®) and Porcine TNFα DuoSet (R&Dsystem®) according to the manufacturer’s protocol. All BALF samples were tested in duplicate and averaged. Cytokine concentrations are expressed in pico-grams per millilitre.
Statistical analysis
Data are expressed as mean ± standard error of the mean (SEM). Data were analysed by repeated-measures analysis of variance (ANOVA) and Bonferroni post-test. Data without repeated measurements were analysed with a Mann-Whitney U-test was performed. p values less than .05 were considered statistically significant. All data were analysed using Graph pad Prism, version 5.0a (Systat Software GmbH, Erkrath, Germany).
Results
Groups
Thirty-three animals were used. Two recipients in Group 1 and two recipients in Group 2 were excluded because of arterial stenosis or venous thrombosis based on examination after animals sacrifice. At the end, the study considered five recipients in Group 1, five in Group 2 and five sham animals.
HIF1α study during ischemia
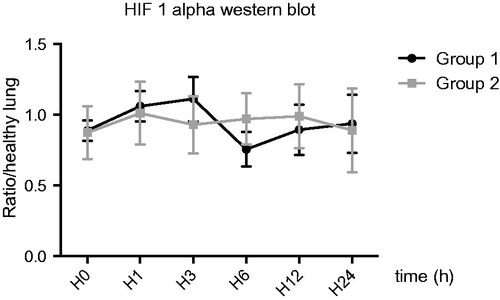
During preservation period, HIF1α western blot study showed no changes in HIF1α expression between groups ().
Hemodynamics and lung function after reperfusion
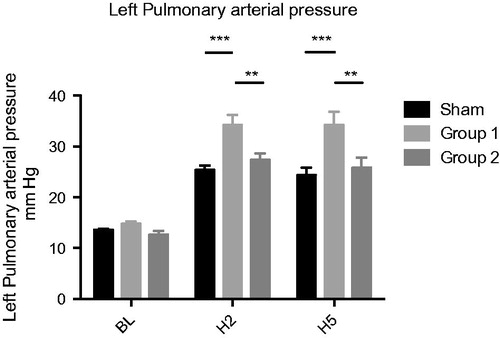
The left lung MPAP showed a significant increase in Group 1 compared to Sham at two hours (H2) (34.2 ± 2.059 vs. 25.4 ± 0812 mmHg (p < .001)) and after five hours (H5) (34.2 ± 2.653 vs. 24.4 ± 1.435 mmHg (p < .001)). In Group 2, we observed a statistically significant reduction of MPAP compared to Group 1 both at H2 (27.4 ± 1.208 vs. 34.2 ± 2.059 mmHg (p < .01)) and at H5 (25.8 ± 1.96 vs. 34.2 ± 2.653 mmHg (p < .01)) and no significant difference as compared to Sham ().
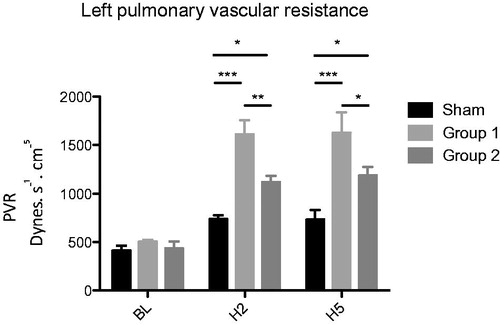
Left lung PVR showed a similar course with significant increasing values in Group 1 compared to Sham at both H2 (1612.4 ± 147.1 vs. 738.7 ± 37.2 dynes. s−1.cm−5 (p < .001)) and H5 (1627.9 ± 211.8 vs. 732.0 ± 100.9 dynes. s− 1.cm−5 (p < .001)). A significant reduction in Group 2 compared to Group 1 at both H2 (1121.3 ± 60.6 vs. 1612.4 ± 147.1 dynes .s − 1.cm−5 (p < .01)) and H5 (1187.4 ± 86.5 vs. 1627.9 ± 211.8 dynes. s− 1.cm−5 ( p < .01)) was observed ().
Figure 3. Left pulmonary vascular resistance PVR (dynes.s− 1.cm −5 ) *p < .05; **p < .01; ***p < .001.
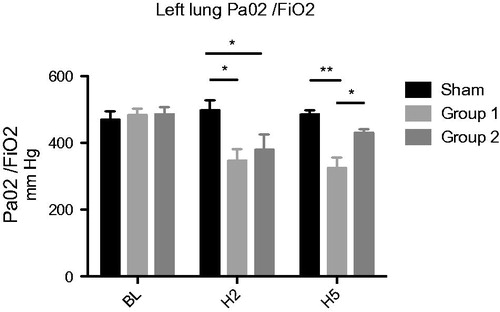
After two hours of reperfusion, PaO2/FiO2 ratio was reduced in both transplanted group compared to sham, but after five hours there is a significant improvement in Group 2 compared to Group 1 (430.4 ± 10.5 vs. 324.5 ± 32.3 mmHg (p < .05)) (). The difference between Group 2 and sham at H5 was not significant.
Lung water content
No difference between groups was observed for extrapulmonary lung water content determined by gravimetric method after termination of the experiment in inferior left lobe.
Lung histology after reperfusion
After five hours of reperfusion, the samples from Groups 1 and 2 showed interstitial oedema, perivascular, peribronchial and interstitial lymphocytic infiltrates. The histomorphologic score was significantly higher in Groups 1 and 2 compared to sham (4.0 ± 1.4 vs. 0.8 ± 0.3; p < .05) and (4.0 ± 1.8 vs 0.8 ± 0.3; p < .05), respectively. No difference between Groups 1 and 2 was observed.
Circulating biomarkers of ischemia reperfusion
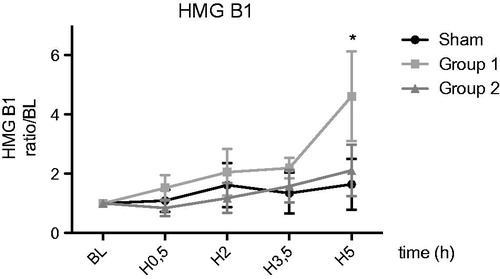
We observed a significant increase of HMGB1 at five hours in Group 1 comparing to sham (4.6 ± 1.5 vs. 1.6 ± 0.8; p < .05), as opposed to no difference between Group 2 and sham (2.1 ± 0.8 vs. 1.6 ± 0.8 p = ns) ().
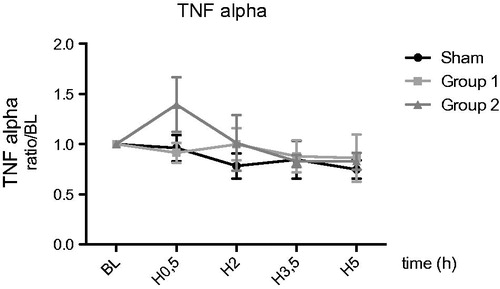
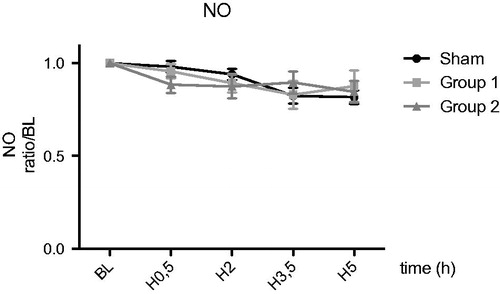
For TNFα and NO, no difference were observed between the groups during reperfusion ( and ).
BALF cytokines levels five hours post-reperfusion
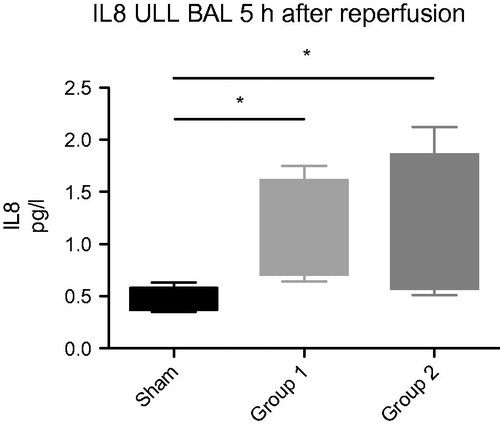
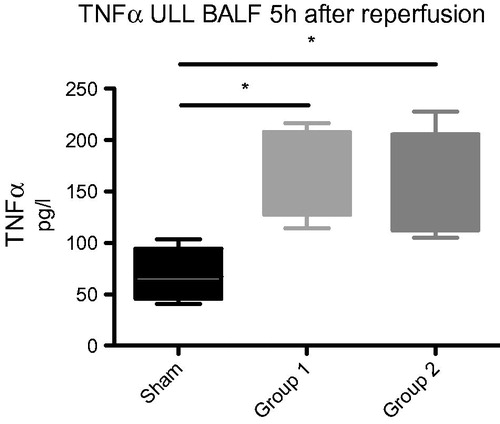
TNFα and IL-8 in BALF were significantly higher in the two experimental groups compared to Sham. TNFα: Group 1: 164 ± 18 pg/ml, Group 2: 151 ± 20 pg/ml vs. sham: 69 ± 18 pg/ml, p < .05. IL-8: Group 1: 1.14 ± 0.21 pg/ml, Group 2: 1.12 ± 0.26 pg/ml vs. sham: 0.5 ± 0.2 pg/ml, p < .05, respectively ( and ).
Discussion
Herein, we investigated the benefits of HEMO2life® against IRI during cold organ preservation before lung transplantation. HEMO2life® used as an additive to preservation solutions during 24 h cold static preservation improved early graft function recovery.
Current organ preservation protocols involve cold storage at 4 °C [Citation21], with the goal of reducing metabolism and thus the organ’s requirement of oxygen. However, even when the metabolism is slowed down, the cell still requires oxygen, making hypoxia one of the key hurdles to overcome on the way to improved organ quality. Several avenues of research are being currently pursued, some involving technological approaches through gaseous oxygen perfusion (PSF, persufflation or direct oxygenation with perfusion machines [Citation22], others involving the use of a pharmacological approach using a transporter to deliver oxygen into the tissue.
This latter concept of supplementing classical organ preservation protocols with adapted pharmaceuticals is rapidly gaining interest [Citation23,Citation24]. Indeed, the simple addition of an easy-to use universal additive to an established surgical protocol presents the potential of a rapid translation to the clinic, compared with the more cumbersome approach of changing the whole protocol with different solutions and machines. Several classes of materials have been studied as potential oxygen carriers, for instance perfluorocarbons [Citation25–27], polymerized hemoglobin solutions of human or bovine origin [Citation28,Citation29] and even non-protein oxygen carriers (liposomal nanoparticles-Lifor) [Citation30]. However, although the latter two solutions have shown promise in several animal models, they are designed for use in normothermic conditions. Perfluorocarbons have also the drawbacks of being hydrophobic, lipophilic and difficult to sterilize [Citation31]. Thus, a relevant strategy that can easily be translated to the clinic with minimal change in current transplantation protocols still remains to be found.
M101, the active compound of HEMO2life® is a hexagonal-bilayer extracellular hemoglobin active at a large range of temperatures (4–37 °C) and composed of 156 globin and 44 non-globin linker chains, carrying up to 156 O2 molecules when saturated (P50 = 7 mmHg; Bohr coefficient = 0.5 under human physiological conditions). M101 releases O2 according to a simple gradient without requiring any allosteric effector, providing the right amount of O2 to hypoxic tissues. This non-glycosylated protein induces no immunogenic or allergenic response upon intravenous injection in mice, nor does it induce significant vasoconstriction or impact on heart rate after intravenous injection in rodents [Citation32].
Benefits of HEMO2life® in addition to preservation solution in kidney transplantation was already demonstrated in preclinical models and its clinical use is being tested. We intended to evaluate its effect for lung preservation.
Lung ischemia is different from that of other solid organs because the lung contains gas in the alveoli. Although the cold-preserved lungs require oxygen, hyperoxygenation induced mitochondrial dysfunction and increased lipid peroxidation that led to deleterious lung function after reperfusion [Citation33]. Usually lung was preserved mildly inflated with FiO2 of 50% [Citation10].
To evaluate the benefit of adding HEMO2life® in preserving liquid, we used a pig model of left lung allo-transplantation which is widely used [Citation18,Citation20,Citation34–37] and has the distinction of mimicking the situation found in human clinical setting. To study IRI, we have chosen, as previous researches, to prolong cold ischemia time to 24 h [Citation20,Citation34] followed by a five hour follow-up post reperfusion sufficient to capture development of PDG an early critical event occurring in the first few hours after transplant and the one that dictates the major complications of transplant [Citation38]. The fact that the dominant right lung is still in place disrupts the evaluation of the physiological parameters of the graft, especially as graft vascular resistance are high, as this causes a preferential distribution of cardiac blood flow to the right lung. To overcome this problem, a sequential clamping of the right pulmonary artery was performed, allowing the isolated graft assessment considering that it reaches stability after 15 min.
In terms of mechanistic exploration we hypothesized that IRI induced an intricate immune response ultimately responsible for organ rejection. I/R is associated with increased generation of reactive oxygen species (ROS) and cellular necrosis that can further lead to activation of multiple complex inflammatory pathways and accentuate damage of both epithelial and endothelial cells in the lung [Citation39].
We hypothesized that, HEMO2life® plays a crucial role during the ischemic period by reducing tissue hypoxia. In order to determine the cellular suffering related to hypoxia, we studied in our model the kinetics of expression of HIF1α during the conservation of the graft for 24 h comparing the two experimental groups. This study in the right lung could not find any significant changes for HIF1α rate during storage. However, we have measured, for each experimental condition and each time-point, the expression levels of HIF1α and compared to those found in non-hypoxic lung witness. The results suggest that the healthy lung could express HIF1α rate even in the absence of hypoxia, a phenomenon already described [Citation40] for pulmonary artery smooth muscle cells.
After reperfusion we analyzed lung functional parameters. The study of PaO2/FiO2 ratio is the cornerstone of the functional assessment of lung grafts, this is the only criterion used to define the primary graft failure in clinical practice after 72 h of reperfusion [Citation3]. In both transplant groups, there is a decrease in this ratio after two hours of reperfusion. This may be related to described acute reperfusion injury after 30 min [Citation41]. However, after five hours of reperfusion, this value was increased only in HEMO2life® Group 2 while the values of PaO2 Perfadex® Group 1 were comparable with those found in recent literature [Citation24].
PVR measurements for grafts are accessible via clinically validated techniques, the increase in PVR values is a direct result of ischemia reperfusion. We note that the values observed in our study are higher than in the study of Pizani et al.’s [Citation20], but comparable with that of Sommer et al. [Citation35]. We believe this is due to the fact that, as in the latter, we have not used pulmonary artery vasodilators used in clinical practice. This was a voluntary choice to eliminate other treatments that may affect the value of resistance.
In our study, the addition of HEMO2life® induce a smaller PVR after graft reperfusion compared to Group 1 (Perfadex® alone). It can therefore be assumed that the addition of HEMO2life® during the preservation period decreases the functional consequences of the second phase of reperfusion (between 2 and 4 h) [Citation32,Citation33,Citation41,Citation42].
HMGB1 is a nuclear non-histone protein secreted early in case of cell necrosis, it plays the role of an “alarmine” or damage associated molecular pattern (DAMPS) that can turn into pro-inflammatory mediators once extracellularly released [Citation43,Citation44] activating the innate immune system via the Toll-like receptor pathway (TLR) . Its role as a mediator of pulmonary inflammation is well described [Citation45] and its expression during I/R renal [Citation46] and lung [Citation47] transplantation was also suggested. In a recent work [Citation48] in lung transplant it was suggested that extracellular HMGB1 promotes innate immune and autoimmune responses via Toll-like receptors or RAGE [Citation49–52], which may contribute to restrictive allograft syndrome (RAS) development - a biologically different chronic lung allograft dysfunction (CLAD) subtype featuring a rapid progression with pathological diagnoses of diffuse alveolar damage and pleuroparenchymal fibroelastosis. RAS shares radiological and pathological features with acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) [Citation53,Citation54] to which HMGB1 and its receptor for advanced glycation end products (RAGE) have also been demonstrated to be associated with [Citation55]. Specifically, diffuse alveolar damage that is the pathologic counterpart of ALI/ARDS is commonly observed in RAS [Citation52]. Thus, RAS and ALI/ARDS may share common biological pathways such as the RAGE-RAGE ligand axis involving HMGB1.
Activation of invariant natural killer T (iNKT) cells and signalling through receptor for advanced glycation end products (RAGE) are known to independently mediate lung IRI [Citation56]. HMGB1 has a sevenfold higher affinity for RAGE than other ligands and the HMGB1/RAGE axis has been implicated in the pathogenesis of various inflammatory processes [Citation45,Citation57–59].
Recently the role of HMGB1/RAGE axis as a new signalling paradigm in the context of lung IRI has been demonstrated [Citation57] confirming the role of the HMGB1 as a potent inflammatory molecule that leads to RAGE-mediated activation of iNKT cells. Furthermore the HMGB1/RAGE axis induces IL-17 and TNF-α production leading to infiltration of neutrophils and it is involved in the crosstalk between alveolar macrophages and iNKT cells.
Previous researches [Citation57] were conducted in a mouse hilar clamp model of lung IRI that does not truly represent a clinical lung transplant scenario, but allows detailed analysis of the molecular and cellular mechanisms and innate immune responses that occur in response to I/R, as a model of primary graft dysfunction. Our study complete the existing evidences with a transplantation model who approaches this newly proposed paradigm to the demonstrated role of HMGB1 in post-transplant lung RAS development.
Despite a high inter-individual variability, in our study there is an increase in the alarmine (HMGB1) level in Perfadex® group. This may induce an increased cellular damage after five hour reperfusion in this group and the activation of the HMGB1/RAGE axis could explain the inflammatory response leading to PDG. It comes here to the ultimate point of our study, which encourage us to extend for a more detailed analysis of the kinetics of this marker in relation with RAGE and cellular necrosis. In a recent review [Citation58], the most consistent and repeated finding associated with PGD was an elevation of RAGE in the recipient blood compartment during the early postoperative period. It was suggested that during the early postoperative period, RAGE acts as a marker of alveolar epithelial injury and epithelial barrier breakdown with subsequent pulmonary oedema as in PGD.
Finally, bronchoalveolar lavage is a simple way to access the deep lung study. Increase in inflammatory cytokines in BALF grafts after several hours of reperfusion, on the model of a “acute lung injury” has already been described [Citation37] We hypothesized that the inflammatory cytokine levels in BALF could be modified in the group HEMO2life®. We find a modulation of TNF-α and IL8 levels in both groups transplanted, although no significant difference between the two groups was detected. The finding are consistent with previous work [Citation59], showing an increase in IL-6 12 h post-transplant. Cytokine profile after lung transplantation correlates with allograft injury. However concentration of most studied inflammatory markers (TNFα, IL-6, IL-8 and IL-10) at different time-points suggest that kinetic of each inflammation biomarker is different and assessing the maximal excursion time-point could be a challenging issue.
What may be the most important finding of this study is that we demonstrated that protection from IRI with the addition of HEMO2life® results in protection against PGD in the early post-transplant period. This increases the ability of the recipient to recover better from various insults to pulmonary function up to this point and to propose a mechanistic explanation linked to inhibition of significant triggers for further deleterious inflammation pathway activation.
Conclusion
Herein, we first describe the use of HEMO2life® an innovative oxygen carrier as additive to standard preservation solution for lung cold static preservation as protection against IRI. In the HEMO2life® group we observed an improvement in terms of functional parameters: haematosis and pulmonary resistances and we identified important biomarkers that could help bringing a mechanistic explanation to this finding. Interesting results of this study grants further explorations.
These findings need to be confirmed on a larger study. The development of a more comprehensive model of prolonged reperfusion after lung transplantation study seems to be a key to progress in the evaluation and development of preservation techniques in lung transplantation.
Disclosure statement
FZ, is a founder and holds stocks in Hemarina SA, which produces the substance being investigated. VP is employee of Hemarina SA and does not hold any stocks.
Materials for the studies were provided by Hemarina SA. All other authors declare no conflict of interest.
References
- ISHLT: The International Society for Heart & Lung Transplantation [Internet]; 2017. [cited 2017 Jun 6]. Available from: https://www.ishlt.org/registries/slides.asp?slides=heartLungRegistry
- Christie JD, Carby M, Bag R, et al. Report of the ISHLT working group on primary lung graft dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2005;24:1454–1459.
- Lee JC, Christie JD. Primary graft dysfunction. Clin Chest Med. 2011;32:279–293.
- Ailawadi G, Lau CL, Smith PW, et al. Does reperfusion injury still cause significant mortality after lung transplantation? J Thorac Cardiovasc Surg. 2009;137:688–694.
- Kreisel D, Krupnick AS, Puri V, et al. Short- and long-term outcomes of 1000 adult lung transplant recipients at a single center. J Thorac Cardiovasc Surg. 2011;141:215–222.
- Whitson BA, Prekker ME, Herrington CS, et al. Primary graft dysfunction and long-term pulmonary function after lung transplantation. J Heart Lung Transplant. 2007;26:1004–1011.
- Daud SA, Yusen RD, Meyers BF, et al. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2007;175:507–513.
- Fiser SM, Tribble CG, Long SM, et al. Ischemia-reperfusion injury after lung transplantation increases risk of late bronchiolitis obliterans syndrome. Ann Thorac Surg. 2002;73:1041–1048.
- Kosieradzki M, Rowiński W. Ischemia/reperfusion injury in kidney transplantation: mechanisms and prevention. Transplant Proc. 2008;40:3279–3288.
- de Perrot M, Liu M, Waddell TK, et al. Ischemia-reperfusion-induced lung injury. Am J Respir Crit Care Med. 2003;167:490–511.
- Salahudeen AK. Cold ischemic injury of transplanted kidneys: new insights from experimental studies. Am J Physiol Renal Physiol. 2004;287:F181–F187.
- Jayle C, Favreau F, Zhang K, et al. Comparison of protective effects of trimetazidine against experimental warm ischemia of different durations: early and long-term effects in a pig kidney model. Am J Physiol Renal Physiol. 2007;292:F1082–F1093.
- Thuillier R, Dutheil D, Trieu MTN, et al. Supplementation With a new therapeutic oxygen carrier reduces chronic fibrosis and organ dysfunction in kidney static preservation: a new O2 therapeutic molecule improves static kidney preservation. Am J Transplant. 2011;11:1845–1860.
- Mallet V, Dutheil D, Polard V, et al. Dose-ranging study of the performance of the natural oxygen transporter HEMO2life® in organ preservation. Artif Organs. 2014;38:691–701.
- Rousselot M, Delpy E, Drieu La Rochelle CL, et al. Arenicola marina extracellular hemoglobin: a new promising blood substitute. Biotechnol J. 2006;1:333–345.
- Rousselot M, Le Guen D, Chabasse C, et al. Novel dissociation mechanism of a polychaetous annelid extracellular haemoglobin. FEBS J. 2006;273:1582–1596.
- Zal F, Green BN, Lallier FH, et al. Quaternary structure of the extracellular haemoglobin of the lugworm Arenicola marina: a multi-angle-laser-light-scattering and electrospray-ionisation-mass-spectrometry analysis. Eur J Biochem. 1997;243:85–92.
- Lama VN, Belperio JA, Christie JD, et al. Models of lung transplant research: a consensus statement from the National Heart, Lung, and Blood Institute workshop. JCI Insight. 2017;2(9):93121.
- Trebbia G, Sage E, Fadel E, et al. Ex vivo assessment of extravascular lung water with transpumonary thermodilution. J Heart Lung Transplant. 2013;32:840–842.
- Pizanis N, Petrov A, Heckmann J, et al. A new preservation solution for lung transplantation: evaluation in a porcine transplantation model. J Heart Lung Transplant. 2012;31:310–317.
- Belzer FO, Southard JH. Principles of solid-organ preservation by cold storage. Transplantation. 1988;45:673–676.
- Hosgood SA, Nicholson HFL, Nicholson ML. Oxygenated kidney preservation techniques. Transplantation. 2012;93:455–459.
- Favreau F, Thuillier R, Cau J, et al. Anti-thrombin therapy during warm ischemia and cold preservation prevents chronic kidney graft fibrosis in a DCD model. Am J Transplant off J Am Soc Transplant Am Soc Transpl Surg. 2010;10:30–39.
- Kumar S, Allen DA, Kieswich JE, et al. Dexamethasone ameliorates renal ischemia-reperfusion injury. J Am Soc Nephrol JASN. 2009;20:2412–2425.
- Maathuis M-HJ, Leuvenink HGD, Ploeg RJ. Perspectives in organ preservation. Transplantation. 2007;83:1289–1298.
- Matsumoto S, Kuroda Y. Perfluorocarbon for organ preservation before transplantation. Transplantation. 2002;74:1804–1809.
- Kuroda Y, Kawamura T, Suzuki Y, et al. A new, simple method for cold storage of the pancreas using perfluorochemical. Transplantation. 1988;46:457–460.
- Kakehata J, Yamaguchi T, Togashi H, et al. Therapeutic potentials of an artificial oxygen-carrier, liposome-encapsulated hemoglobin, for ischemia/reperfusion-induced cerebral dysfunction in rats. J Pharmacol Sci. 2010;114:189–197.
- Spahn DR, Kocian R. Artificial O2 carriers: status in 2005. Curr Pharm Des. 2005;11:4099–4114.
- Regner KR, Nilakantan V, Ryan RP, et al. Protective effect of Lifor solution in experimental renal ischemia-reperfusion injury. J Surg Res. 2010;164:e291–e297.
- Farrar D, Grocott M. Intravenous artificial oxygen carriers. Hosp Med. 2003;64:352–356.
- Tsai AG, Intaglietta M, Sakai H, et al. Microcirculation and NO-CO studies of a natural extracellular hemoglobin developed for an oxygen therapeutic carrier. Curr Drug Discov Technol. 2012;9:166–172.
- Fukuse T, Hirata T, Ishikawa S, et al. Optimal alveolar oxygen concentration for cold storage of the lung. Transplantation. 2001;72:300–304.
- Bacha EA, Hervé P, Murakami S, et al. Lasting beneficial effect of short-term inhaled nitric oxide on graft function after lung transplantation. Paris-Sud University Lung Transplantation Group. J Thorac Cardiovasc Surg. 1996;112:590–598.
- Sommer S, Warnecke G, Hohlfeld J, et al. Pulmonary preservation with LPD and celsior solution in porcine lung transplantation after 24 h of cold ischemia. Eur J Cardiothorac Surg. 2004;l26:151–157.
- Inci I, Erne B, Arni S, et al. Prevention of primary graft dysfunction in lung transplantation by N-acetylcysteine after prolonged cold ischemia. J Heart Lung Transplant off Publ Int Soc Heart Transplant. 2010;29:1293–1301.
- LaPar DJ, Laubach VE, Emaminia A, et al. Pretreatment strategy with adenosine A2A receptor agonist attenuates reperfusion injury in a preclinical porcine lung transplantation model. J Thorac Cardiovasc Surg. 2011;142:887–894.
- Shaver CM, Ware LB. Primary graft dysfunction: pathophysiology to guide new preventive therapies. Expert Rev Respir Med. 2017;11:119–128.
- Laubach VE, Sharma AK. Mechanisms of lung ischemia-reperfusion injury. Curr Opin Organ Transplant. 2016;21:246–252.
- Yu AY, Frid MG, Shimoda LA, et al. Temporal, spatial, and oxygen-regulated expression of hypoxia-inducible factor-1 in the lung. Am J Physiol. 1998;275:L818–L826.
- Carter YM, Gelman AE, Kreisel D. Pathogenesis, management, and consequences of primary graft dysfunction. Semin Thorac Cardiovasc Surg. 2008;20:165–172.
- Fiser SM, Tribble CG, Long SM, et al. Lung transplant reperfusion injury involves pulmonary macrophages and circulating leukocytes in a biphasic response. J Thorac Cardiovasc Surg. 2001;121:1069–1075.
- Guo WA, Knight PR, Raghavendran K. The receptor for advanced glycation end products and acute lung injury/acute respiratory distress syndrome. Intensive Care Med. 2012;38:1588–1598.
- Chan JK, Roth J, Oppenheim JJ, et al. Alarmins: awaiting a clinical response. J Clin Invest. 2012;122:2711–2719.
- Abraham E, Arcaroli J, Carmody A, et al. HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000;165:2950–2954.
- Gallinat A, Paul A, Efferz P, et al. Hypothermic reconditioning of porcine kidney grafts by short-term preimplantation machine perfusion. Transplantation. 2012;93:787–793.
- Goto T, Ishizaka A, Kobayashi F, et al. Importance of tumor necrosis factor-alpha cleavage process in post-transplantation lung injury in rats. Am J Respir Crit Care Med. 2004;170:1239–1246.
- Saito T, Liu M, Binnie M, et al. Distinct expression patterns of alveolar “ alarmins” in subtypes of chronic lung allograft dysfunction”. Am J Transplant. 2014;14:1425–1432.
- Todd JL, Palmer SM. Bronchiolitis obliterans syndrome: the final frontier for lung transplantation. Chest. 2011;140:502–508.
- Verleden SE, Vandermeulen E, Ruttens D, et al. Neutrophilic reversible allograft dysfunction (NRAD) and restrictive allograft syndrome (RAS). Semin Respir Crit Care Med. 2013;34:352–360.
- Li G, Liang X, Lotze MT. HMGB1: the central cytokine for all lymphoid cells. Front Immunol. 2013;4:68.
- Nakagiri T, Inoue M, Morii E, et al. Local IL-17 production and a decrease in peripheral blood regulatory T Cells in an animal model of bronchiolitis obliterans. Transplantation. 2010;89:1312–1319.
- Sato M, Waddell TK, Wagnetz U, et al. Restrictive allograft syndrome (RAS): a novel form of chronic lung allograft dysfunction. J Heart Lung Transplant. 2011;30:735–742.
- Sato M, Hwang DM, Waddell TK, et al. Progression pattern of restrictive allograft syndrome after lung transplantation. J Heart Lung Transplant. 2013;32:23–30.
- Ueno H, Matsuda T, Hashimoto S, et al. Contributions of high mobility group box protein in experimental and clinical acute lung injury. Am J Respir Crit Care Med. 2004;170:1310–1316.
- Sharma AK, LaPar DJ, Zhao Y, et al. Natural killer T cell-derived IL-17 mediates lung ischemia-reperfusion injury. Am J Respir Crit Care Med. 2011;183:1539–1549.
- Sharma AK, LaPar DJ, Stone ML, Z, et al. Receptor for advanced glycation end products (RAGE) on iNKT cells mediates lung ischemia-reperfusion injury: RAGE and NKT Cells inlung reperfusion injury. Am J Transplant. 2013;13:2255–2267.
- Hamilton BCS, Kukreja J, Ware LB, et al. Protein biomarkers associated with primary graft dysfunction following lung transplantation. Am J Physiol Lung Cell Mol Physiol. 2017;312:L531–L541.
- Moreno I, Vicente R, Ramos F, et al. Determination of Interleukin-6 in lung transplantation: association with primary graft dysfunction. Transplant Proc. 2007;39:2425–2426.