Abstract
Human induced pluripotent stem cells (iPSCs) have been shown to have promising potential for regenerative medicine and tissue engineering applications. Chondrogenic differentiation of iPSCs is important for application in cartilage tissue engineering. In this study, we considered the effect of nanofibre-based polyethersulfone (PES) scaffold on the chondrogenesis of iPSCs. IPSC cells were cultured on the PES scaffold and scaffold free method. After 21 d, real-time PCR was performed to evaluate the cartilage-specific genes in the mRNA levels. For confirm our results, we have done immunocytochemistry and scanning electron microscopy (SEM) imaging. According to the results, higher significant expressions of common chondrogenic-related genes such as aggrecan, collagen type II and collagen type X were observed in PES seeded human iPSCs when compared to the mRNA levels measured in scaffold free method. Expression of collagen type I down regulated in both methods. Also, both methods were showed a similar pattern of expression of SOX9. Our results showed that nanofibre-based PES scaffold enhanced the chondrogenesis of iPSCs and the highest capacity for differentiation into chondrocyte-like cells. These cells and PES scaffold were demonstrated to have great efficiency for treatment of cartilage damages and lesions.
Introduction
Nowadays, tissue engineering has an important role in science and technology so that many studies have focused on this subject. According to the therapeutic potential of this technology, some diseases are anticipated to be treated by tissue engineering as one of the best therapeutic options, while many defects still do not have a definitive treatment. Recently tissue engineering techniques are used to repair tissues such as skin, bone, cartilage, liver, heart valve, bladder, ligament, spinal cord injury, etc., and great success has been obtained in these fields [Citation1–4].
Cartilage is made from distributed chondrocytes embedded within extracellular matrix (ECM). The ECM is created of mostly proteoglycans and type II collagen that make the cartilage tissue with sufficient mechanical properties for in vivo function [Citation5]. Cartilage defects have limited self-repair capacity according to the intrinsic biology of cartilage tissue, such as lack of vascular supply and low matrix turnover. The novel strategy for regeneration of cartilage defects involves cells seeded biomaterials with appropriate growth factors [Citation6,Citation7].
Nowadays, interest in pluripotent cells has increased. Human embryonic stem cells (hESCs) can be differentiated into chondrocytes using certain procedures and conditions, but there are concerns on their immunogenicity, potential for malignancy, ethical issues (for ESCs) and heterogeneous differentiation [Citation8,Citation9]. Since Yamanaka and colleagues retro differentiated somatic cells to an ESC-like state, namely iPSCs, numerous reports regarding safer and more efficient methods for generating iPSCs for clinical applications have been published [Citation10,Citation11]. iPSCs can also be induced into a variety of cell lineages, such as osteochondral lineage and studies using scaffolds to enhance the chondrogenesis of iPSCs have been performed [Citation11,Citation12].
Commercially available synthetic and natural matrix has been tested in animal models or clinical trials for repair of cartilage. So, tissue engineering is a promising option for the treatment of cartilage defects. Many forms of biomaterial scaffolds with different materials and properties have been developed for cartilage tissue engineering [Citation13]. In the past decade, nanofibrous scaffolds have attracted much interest as tissue engineered scaffolds because of their unique physical and topographical properties. The nanosized structure of a scaffold plays an important role to imitate the ECM Structure. Nanofibre scaffolds composed of ultra-fine biodegradable polymeric fibres morphologically similar to natural ECM have been widely emerged as potential scaffolds for cartilage tissue engineering [Citation14].
Different types of polymers have been used as scaffold in tissue engineering [Citation15]. Among polyethersulfone (PES) can be used in biomedical applications like haemodialysis, filtration and ultrafiltration due to its positive attributes of a biomaterial [Citation16]. Several studies showed that PES nanofibrous scaffolds fabricated by electrospinning could be an appropriate substrate to support proliferation and differentiation of different cells and stem cells. For example, these scaffolds have the potential for feeder-free culture of pluripotent stem cells because of 3 D structure and bioactivity that enhances proliferation, differentiation and infiltration of embryonic stem cells [Citation17,Citation18].
There are few studies on the biocompatibility and tissue engineering applications of PES scaffolds. Consequently, this polymer is receiving increasing attention for use in tissue engineering [Citation19–21]. Gelatine, glycosaminoglycan, chitosan and hyaluronic acid as specific natural ingredients can promote the hydrophilicity and cell affinity of the scaffold. These features enable electrospun nanofibrous scaffolds to imitate the ECM of cartilage [Citation22,Citation23].
The goal of this study was to evaluate the chondrocyte differentiation capacity of iPSCs on fabricated and modified PES nanofibres with plasma. Thus, we synthesized a PES scaffold and investigated its effect on the chondrogenic induction of iPSCs, via embryoid body (EB) formation in vitro.
Materials and methods
Cell culture
Human IPSCs were obtained from cell bank [a gift from Stem Cells Technology Research Center (Tehran, Iran)]. These cells were maintained on feeder layers of mouse embryo fibroblast MEF cells treated with mitomycin-C (Invitrogen Co., Carlsbad, CA) in 6-well culture plates (Orange Science, Braine-l'Alleud, Belgium), and were passaged every 3 d. In brief, human IPSC cells were maintained in DMEM/F12 culture medium supplemented with 15% knockout serum replacement (KSR) (Invitrogen Co., Carlsbad, CA), 0.1 mmol/L nonessential amino acids, 1 mmol/L L-glutamine (all from Invitrogen), 0.1 mmol/L b-mercaptoethanol (Sigma), penicillin/streptomycin (sigma) and 4 ng/mL of human fibroblast growth factor 2 (Invitrogen), and about 60% of the medium was replaced every day. Every 4–5 d, human IPSC colonies were detached with 0.1% collagenase IV, and seeded onto inactivated MEF for expansion. Prior to cell seeding, circular scaffolds were immersed overnight in the following solutions: (1) 70% ethanol for sterilization, (2) penicillin, streptomycin and amphotericin B to prevent from yeast growth and (3) culture medium to ensure sterilization and enhance cell attachment after seeding.
Embryoid body formation
For EB formation, IPSC colonies were detached with 0.1% collagenase IV, then transferred onto Non-treated six well plate (Jet Biofil, Kyoto, Japan) for 3–5 d in EB medium consisting of human IPSC medium without human fibroblast growth factor 2.
Fabrication of electrospun nanofibrous PES scaffolds
Nanofibrous PES scaffolds were fabricated and Produced by an electrospinning setup according to a protocol modified with Shabani et al. [Citation20].
Chondrogenic differentiation using EB-derived outgrowth cells in vitro
Nanofibrous membranes (scaffolds) were placed in a 24-well culture plate. Scaffolds were floated in 70% ethanol for 2 h, and washed with PBS three times. After that, scaffolds were pre-treated in basal medium (DMEM with 20% KSR) for 24 h to facilitate protein adsorption and cell attachment on to the scaffold surface. Finally, the scaffolds were seeded with 3 × 105 outgrowth cells derived from EBs (outgrowth cells derived from EBs were washed and detached from the dish).
In the control negative sample, the basal medium was replaced with chondrogenic medium. Chondrogenic medium was contain DMEM/F12, 20% knockout serum replacement, 1 × non-essential amino acids, 1 mM L-glutamine, 1% sodium pyruvate, 1% ITS + premix, 10–7 M dexamethasone, 50 mM ascorbic acid, 40 μg/mL L-proline supplemented with 50 ng/mL human bone morphogenetic protein 2 and 10 ng/mL human transforming growth factor beta 3 (TGFβ3). Cell seeded scaffolds were incubated up to 21 d and media was changed every other day.
Cell proliferation assay
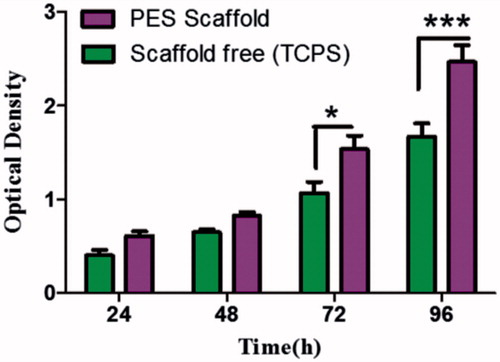
To assess cell proliferation rate of IPSCs on tissue culture polystyrene (TCPS) and PES scaffolds was evaluated via MTT assay. The scaffolds were first sterilized and prepared for cell seeding as mentioned above. Sterilized nanofibrous membranes were placed in a 24-well culture plate, seeded with a cell density of 4 × 103 cells per cm2, and incubated at 37 °C, 5% CO2. After 24, 48, 76 and 96 h of cell seeding, 50 μL of MTT solution (5 mg/mL in DMEM) was added to each well. For conversion of MTT to formazan crystals by mitochondrial dehydrogenases of living cells, the plate was incubated at 37 °C for 4 h. For dissolution of the dark-blue intracellular formazan, the supernatant was removed and constant amount of an appropriate solvent was added. The optical density (OD) was read at a wavelength of 570 nm in a micro-plate reader (BioTek Instruments, Winooski, VT). Scanning electron microscope (SEM) was investigated morphology of stem cells on scaffolds.
Scanning electron microscopy (SEM)
The cell-polymer constructs were fixed in 2.5% glutaraldehyde, dehydrated through a graded series of ethanol, vacuum dried, mounted onto aluminium stubs and sputter coated with gold. Samples were examined using a SEM (S-4500; Hitachi, Shiga, Japan) at an accelerating voltage of 20 kV.
Real-time PCR
Total RNA extraction performed using RNX reagent (Sinaclon Co., Tehran, Iran). Reverse transcriptase (Thermo Fisher Co., Waltham, MA) were used to prepare complementary DNA (cDNA) according to the instructions provided by Thermo Fisher Corporation. Expression levels were quantitatively determined with the Rotor Gene Q system using the SYBR Green I dye method (Thermo Fisher). The expressions of the following genes were examined: collagen type I (COL1A1), collagen type II (COL2A1), collagen type X (COL10A1), aggrecan and SOX-9. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a housekeeping gene. Primer sequences are listed in . The PCR reaction was performed for 1 min at 95 °C, followed by 39 amplification cycles (15 s at 95 °C, 15 s at 60 °C and 20 s at 72 °C). After the last cycle, a melt-curve was generated. Each PCR was processed in triplicate. The Ct value of GAPDH is subtracted from the Ct value of the interest gene (ΔCT). The average ΔCT value of the triplicates is taken. IPSCs culture in the pellet culture system is used as controls (ΔΔCT). Relative expression levels for each primer set are expressed as fold changes by the 2–ΔΔCT method [Citation24].
Table 1. Primers used for real-time PCR.
Immunostaining
After chondrogenic differentiation, we evaluated the production of collagen II and aggrecan markers. The cell-seeded scaffolds were fixed in 4% paraformaldehyde and then processed for immunofluorescence staining. After blocking with blocking solution (1% bovine serum albumin and 0.1% Triton X-100 in phosphate buffered saline), constructs were incubated at 4 °C overnight with primary antibodies [rabbit anti-human collagen type II (1:100) and rabbit anti-human aggrecan (1:200) (Abcam, Cambridge, MA)]. Constructs were then incubated with fluorescein isothiocyanate (FITC)-conjugated secondary antibody (Abcam, Cambridge, MA) for 1 h at room temperature. The cell nuclei were stained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI; 1:1000, Invitrogen, Carlsbad, CA) and then observed under florescence microscope.
Statistical analysis
All experiments were performed at least triplicate independently, and the data are reported as a mean ± standard deviation (SD). Results were analysed by one-way ANOVA, and Bonferroni’s post-hoc test was used for comparison of DWJS group with 2 D by Graphpad Prism 6 software (Graphpad Software Inc., La Jolla, CA). In between the compared groups, a value of p < .05 was considered statistically significant.
Results
EB formation and cultivation
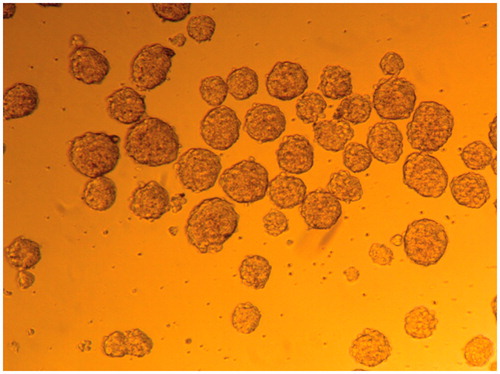
Spherical, uniformly sized EBs were harvested using the serial pipetting method after suspension-culture of identical numbers of cells for 2 d. A further 5 d of cultivation resulted in enlargement and maturation of EBs for the following differentiation ().
Morphology and biocompatibility of PES scaffold
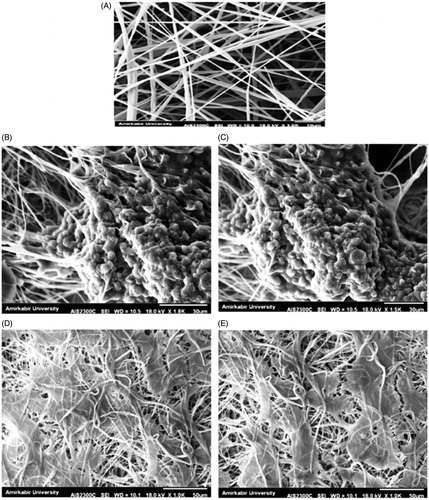
PES electrospun nanofibres were porous, beads-free and had a uniform and smooth morphology (). SEM images showed that most cells were attached to the surface of the scaffold and also penetrated between the fibres with increasing culture duration (). Morphology of chondrogenic differentiated IPSC on nanofibrous PES showed in . Biocompatibility of PES scaffold was investigated via MTT assay which revealed the proliferation rate and viability of IPSC on PES nanofibres (). MTT assay showed that PES scaffolds supported proliferation of cells.
Evaluation of chondrocyte differentiation
Immunological analysis
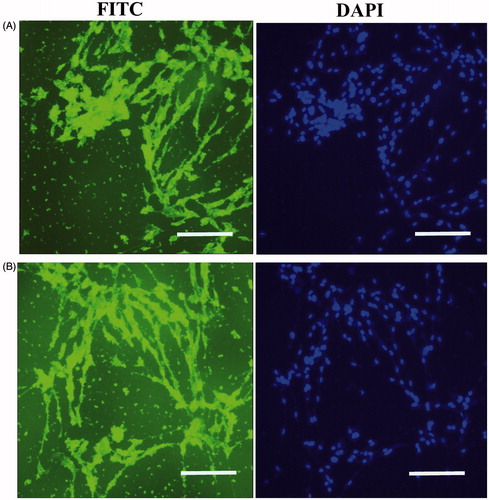
Chondrogenic differentiation to IPSCs can be assessed by immunological tests on the 21th day after induction with chondrogenic media. Immunocytochemistry (ICC) was performed for collagen type II and aggrecan markers in chondrogenic differentiated IPSC. Immunofluorescence analysis detected collagen type II and aggrecan in differentiated IPSC at day 21 in the PES scaffold ().
Figure 4. In vitro expression analysis of collagen type II and aggrecan markers on PES Scaffold. Immunofluorescence for collagen type II (A) and aggrecan markers (B) in chondrogenic differentiated IPSC after 21 d of in vitro differentiation. Staining of nucleus was performed by DAPI (blue), scale bars are 100 μm.
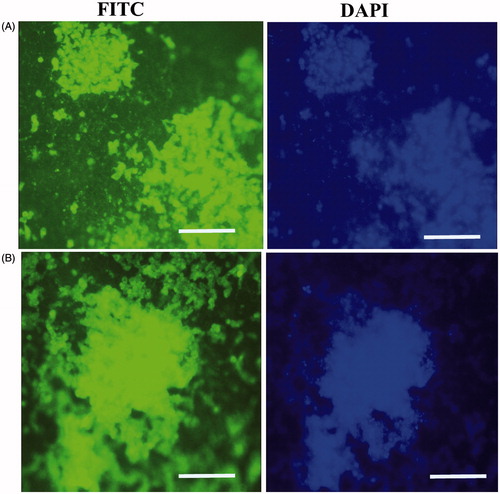
For chondrogenic differentiation with scaffold free method, we performed immunological analysis for collagen type II and aggrecan proteins after 21 d. Immunocytochemical analysis detected collagen type II and aggrecan in differentiated IPSC at day 21 in the scaffold free group (). Also, ICC test was performed for undifferentiated IPSC (data not shown).
Gene expression analysis
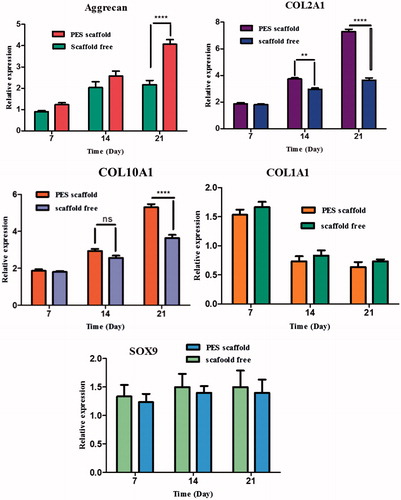
The PCR analysis showed that the expression levels of chondrogenic marker genes were elevated in both groups; moreover, expression levels were significantly higher in the scaffold group than the control group. Expressions of genes involved in chondrogenesis were analysed on day 7, 14 and 21 using real-time RT-PCR. Our results showed a significant increase in expression of collagen II on PES scaffolds on days 14 and 21. An increasing trend for the expression of aggrecan was observed on both methods during time, and a significantly higher expression of aggrecan was observed for PES scaffold at day 21. A higher expression of collagen type X was identified on PES scaffolds at day 21 compared to scaffold free. Collagen type I expression did not change significantly in either group but was lower at the end of cultivation in the scaffold group. Also, real time PCR analysis did not show a significant difference for SOX9 gene expression during chondrogenic differentiation of IPSC on both methods (). The bars represent means ± SD. Significant levels are **p < .01, ***p < .001 and ****p < .0001.
Discussion
In this study we demonstrated the effect of nanofibre-based PES scaffold on the chondrogenic induction of iPSCs in vitro. Our results showed that iPSC chondrogenesis were enhanced by culture on a PES scaffold.
Different strategies have been used to optimize the in vivo and in vitro chondrogenic induction, involving direct differentiation by EB formation, high cell density culture and co-culture with chondrocyte cells [Citation25,Citation26]. The high cell density system showed a chondrogenic efficacy superior to that of direct plating of EBs, which is in agreement with the pellet method for chondrogenic induction of MSC or expansion of chondrocytes in vitro [Citation25,Citation27]. These systems comfort interactions between cells and also cells and matrix [Citation28]. In this experiment, the scaffolds were seeded with 3 × 105 outgrowth cells derived from EBs and created a micromass with sufficiently high cell density [Citation24,Citation29].
Cell therapy for cartilage deficiency needs support from biomaterials or scaffolds. In the regeneration and restoration of tissue deficiency, scaffolds can surrender cells or growth factors, create a structure to which cells can attach and make tissue, and increase cell growth into the implant, both in vitro and in vivo [Citation30]. Nanofibrous scaffold imitating from biomechanical and biological structure of ECM which have a critical role in cell proliferation, adhesion and differentiation. These nanofibrous scaffolds are an effective strategy for the development of tissue engineering and regenerative medicine [Citation31,Citation32]. Several studies showed high potential application of electro-spun nanofibres fabricated from various polymers in combination with various types of cells including IPSCs, ESCs and MSCs in cartilage tissue engineering [Citation33]. The formation and designation of scaffolds are critical in differentiation and tissue engineering. A normal scaffold must imitate the role, structure and environment pattern of ECM [Citation34,Citation35]. Some researchers have found nanofibrous scaffolds could play a vital role in tissue engineering by providing a suitable matrix for proliferation, differentiation and attachment of stem cells [Citation36,Citation37]. Recently, electro-spinning method, a high electric field generated between a needle and a collector, has achieved popularity within the tissue engineering society as a potential mean for producing scaffolds [Citation38].
A type of polymers has been used as scaffold for tissue engineering. Among them, PES nanofibre can be used in biomedical applications such as haemodialysis, filtration and ultrafiltration due to its positive attributes as a biomaterial. This polymer has been considered by some researcher for use in tissue engineering. Also, minor efforts have been made to increase the infiltration of the cells into electro-spun nanofibrous scaffolds [Citation19,Citation21]. PES nanofibres have many fascinating properties including favourable mechanical strength, thermal and chemical resistance, and excellent biocompatibility [Citation20]. In this study surface modification of the PES nanofibres were performed with plasma treatment. Plasma surface treatment of scaffolds with N2, O2 and NH3 makes the polymer surface more hydrophilic, more polar and more bioadhesive [Citation20].
Electro-spun nanofibres have been widely studied for chondrocyte differentiation with different compositions and conditions. Chondrogenic differentiation of BMSCs has been extensively studied on electro-spun nanofibrous matrices using single polymer, such as PCL [Citation39,Citation40] and PLGA [Citation41,Citation42]. Wise et al. showed significant increase in the aggrecan content and expression of collagen type II when IPSC cells cultured in chondrogenic media (contain TGF-beta3) on nanofibres compared to culturing in normal growth media and on microfiber scaffolds [Citation40]. Alves da Silva et al. cultured BMSC on electro-spun PCL nanofibre network in a multi-chamber flow perfusion bioreactor to produce cartilage ECM. Statically cultured cells had a fibroblast-like morphology, while dynamic condition induced round-shaped morphology with increased amount of aggrecan and collagen type II [Citation39]. Dahl et al. investigated the potential of human umbilical cord mesenchymal stem cells (UCMSCs) for chondrogenic differentiation on PLGA and PCL electro-spun nanofibres [Citation43]. One study has been conducted on seeding BMSCs and fibrochondrocytes on PCL-PEO aligned nanofibrous networks. They demonstrated that topography of aligned nanofibrous could influence human BMSCs chondrogenesis by imitating ECM [Citation44]. Liu et al. showed that PCL/gelatine scaffold as nanofibrous scaffold enhanced the chondrogenesis of iPSCs in the in vitro and in vivo [Citation45]. Shafiee et al. several nanofibrous scaffolds were fabricated with electro-spinning and they showed that electro-spun constructs maintain NSP proliferation and enhance chondrogenic differentiation. They showed that NSPs have various behaviour in two scaffolds [Citation46].
In our study, we have demonstrated increasing chondrogenic differentiation of IPSC in vitro by using PES scaffold. This could be attributed to the unique properties of PES scaffolds. The three-dimensional IPSC seeded constructs display a cartilage-like morphology, containing chondrocyte-like cells surrounded by abundant cartilaginous matrix, as seen by SEM and ICC. Expressions of cartilage specific genes are detected at mRNA level by RT-PCR. The level of IPSC chondrogenesis in the PES scaffold appears to higher than scaffold free method. Taken together, these observations strongly suggest that the nanofibrous based scaffold (PES) effectively help to IPSC chondrogenesis. The most commonly used method to promote chondrogenesis of IPSC in vitro is to maintain them as a high cell-density culture system. In these systems, environment thus simulates the act of cellular condensation during embryonic limb bud development, where cell–cell interactions have been shown to be critical for the initiation of chondrogenesis [Citation47–49].
Four important cartilage-related genes were selected and their expression was evaluated for better comparison and understanding of the chondrogenic differentiation behaviour of PES scaffold and scaffold free methods. Aggrecan, the core protein of the large aggregating proteoglycan, is a major ECM component in hyaline cartilage [Citation50]. Our results also showed expression and production of aggrecan in both method during time, and significantly higher expression of aggrecan was observed for PES scaffold at day 21 (). In other study was also reported that MSCs undergoing chondrogenesis in 3 D, highly porous scaffolds synthesize more aggrecan than those in high cell-density culture systems (scaffold free), possibly because of the increased substrate surface for ECM accumulation in such matrixes [Citation51].
Between the various proteins existing in ECM, collagen II is the major component of ECM in hyaline-like cartilage [Citation24,Citation52]. It was demonstrated at 14 and 21 d after chondrogenic induction of cells that collagen II was secreted from chondrocyte-like cells on both methods. Based on immunostaining, this protein was expressed on a PES scaffold higher than scaffold free ( and ). Interestingly, this higher level of protein expression was also confirmed in the mRNA level by real-time RT-PCR (). These results showed that PES scaffold enhanced the chondrogenic differentiation of IPSC in vitro. Up-regulation and significantly higher levels of collagen X were observed in PES scaffold compared to scaffold free. Collagen type I expression was down regulated at the end of cultivation. Also it is likely that the expression of SOX-9 at low levels was already sufficient to support matrix production [Citation24,Citation52]. Both methods showed a similar pattern of expression of SOX9.
Figure 6. Expression of cartilage-specific genes. Relative expression of aggrecan, collagen I, collagen II, collagen X and SOX9 in IPSC cultured on PES scaffold during chondrogenic differentiation compared to scaffold free method. The expressions of collagen II, collagen X and aggrecan genes were significantly upregulated in PES scaffold compared to that in scaffold free method. Collagen type I expression was down regulated at the end of cultivation and also, SOX9 gene expression did not show a significant difference during chondrogenic differentiation of IPSC on both scaffolds. Results are presented as mean ± SD. Significant levels are **p < .01, ***p < .001 and ****p < .0001.
Conclusion
Our findings showed that the chondrogenic differentiation enhanced on PES scaffold when compared with scaffold free method. These results were supported by the higher expression of cartilage-specific markers in the protein and mRNA levels. According to these observations, the role of scaffolds has to be considered in the development of efficient stem cell-scaffold complexes for tissue engineering applications due to its nanofibrous structure and hydrophilicity. Our study identified, that IPSC have more significant differentiation potential into chondrocyte and also PES nanofibrous scaffold could be very useful for cartilage tissue engineering. So we described a new nanofibrous scaffold that may be applied for chondrogenesis differentiation.
Acknowledgements
This article was extracted from a thesis for the Ph.D. degree of Hossein Mahboudi and financially supported by the School of Advanced Technologies in Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran through grant no. 9693 and was conducted in Stem Cell Technology Research Center, Tehran, Iran.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Gomes ME, Reis RL. Tissue engineering: key elements and some trends. Macromol Biosci. 2004;4:737–742.
- Gunatillake PA, Adhikari R. Biodegradable synthetic polymers for tissue engineering. Eur Cell Mater. 2003;5:1–16.
- Shahrezaie M, Mansour RN, Nazari B, et al. Improved stem cell therapy of spinal cord injury using GDNF-overexpressed bone marrow stem cells in a rat model. Biologicals. 2017;S1045–S1056: 30104–30105.
- Soleimannejad M, Ebrahimi-Barough S, Soleimani M, et al. Fibrin gel as a scaffold for photoreceptor cells differentiation from conjunctiva mesenchymal stem cells in retina tissue engineering. Artif Cells Nanomed Biotechnol. 2017 [Jun 10]; [10]. DOI:10.1080/21691401.2017.1345922
- Chung C, Burdick JA. Engineering cartilage tissue. Adv Drug Deliv Rev. 2008;60:243–262.
- Kalson NS, Gikas PD, Briggs TW. Current strategies for knee cartilage repair. Int J Clin Pract. 2010;64:1444–1452.
- Kuo CK, Li WJ, Mauck RL, et al. Cartilage tissue engineering: its potential and uses. Curr Opin Rheumatol. 2006;18:64–73.
- Carr AJ, Vugler AA, Hikita ST, et al. Protective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic rat. PLoS One. 2009;4:e8152.
- Shafiee A, Kabiri M, Langroudi L, et al. Evaluation and comparison of the in vitro characteristics and chondrogenic capacity of four adult stem/progenitor cells for cartilage cell-based repair. J Biomed Mater Res A. 2016;104:600–610.
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676.
- zur Nieden NI, Kempka G, Rancourt DE, et al. Induction of chondro-, osteo- and adipogenesis in embryonic stem cells by bone morphogenetic protein-2: effect of cofactors on differentiating lineages. BMC Dev Biol. 2005;5:1.
- Kawaguchi J, Mee PJ, Smith AG. Osteogenic and chondrogenic differentiation of embryonic stem cells in response to specific growth factors. Bone. 2005;36:758–769.
- Zhang S, Chen L, Jiang Y, et al. Bi-layer collagen/microporous electrospun nanofiber scaffold improves the osteochondral regeneration. Acta Biomater. 2013;9:7236–7247.
- Shafiee A, Soleimani M, Chamheidari GA, et al. Electrospun nanofiber-based regeneration of cartilage enhanced by mesenchymal stem cells. J Biomed Mater Res A. 2011;99:467–478.
- Islami M, Mortazavi Y, Soleimani M, et al. In vitro expansion of CD 133+ cells derived from umbilical cord blood in poly-L-lactic acid (PLLA) scaffold coated with fibronectin and collagen. Artif Cells Nanomed Biotechnol. 2017 [Aug 6]; [9]. DOI:10.1080/21691401.2017.1358733
- Krieter DH, Lemke HD. Polyethersulfone as a high-performance membrane. Contrib Nephrol. 2011;173:130–136.
- Mahmoodinia Maymand M, Soleimanpour-Lichaei HR, Ardeshirylajimi A, et al. Improvement of hepatogenic differentiation of iPS cells on an aligned polyethersulfone compared to random nanofibers. Artif Cells Nanomed Biotechnol. 2017 [Jul 11]; [8]. DOI:10.1080/21691401.2017.1345929
- Shabani I, Haddadi-Asl V, Soleimani M, et al. Enhanced infiltration and biomineralization of stem cells on collagen-grafted three-dimensional nanofibers. Tissue Eng Part A. 2011;17:1209–1218.
- Christopherson GT, Song H, Mao HQ. The influence of fiber diameter of electrospun substrates on neural stem cell differentiation and proliferation. Biomaterials. 2009;30:556–564.
- Shabani I, Haddadi-Asl V, Seyedjafari E, et al. Improved infiltration of stem cells on electrospun nanofibers. Biochem Biophys Res Commun. 2009;382:129–133.
- Yoshimoto H, Shin YM, Terai H, et al. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomaterials. 2003;24:2077–2082.
- Kim MS, Jun I, Shin YM, et al. The development of genipin-crosslinked poly(caprolactone) (PCL)/gelatin nanofibers for tissue engineering applications. Macromol Biosci. 2010;10:91–100.
- Yang X, Yang F, Walboomers XF, et al. The performance of dental pulp stem cells on nanofibrous PCL/gelatin/nHA scaffolds. J Biomed Mater Res A. 2010;93:247–257.
- Zhang L, Su P, Xu C, et al. Chondrogenic differentiation of human mesenchymal stem cells: a comparison between micromass and pellet culture systems. Biotechnol Lett. 2010;32:1339–1346.
- Toh WS, Yang Z, Heng BC, et al. Differentiation of human embryonic stem cells toward the chondrogenic lineage. Methods Mol Biol. 2007;407:333–349.
- Vats A, Bielby RC, Tolley N, et al. Chondrogenic differentiation of human embryonic stem cells: the effect of the micro-environment. Tissue Eng. 2006;12:1687–1697.
- Toh WS, Yang Z, Liu H, et al. Effects of culture conditions and bone morphogenetic protein 2 on extent of chondrogenesis from human embryonic stem cells. Stem Cells. 2007;25:950–960.
- Jukes JM, Moroni L, van Blitterswijk CA, et al. Critical Steps toward a tissue-engineered cartilage implant using embryonic stem cells. Tissue Eng Part A. 2008;14:135–147.
- Tanaka H, Murphy CL, Murphy C, et al. Chondrogenic differentiation of murine embryonic stem cells: effects of culture conditions and dexamethasone. J Cell Biochem. 2004;93:454–462.
- Spector M. Biomaterials-based tissue engineering and regenerative medicine solutions to musculoskeletal problems. Swiss Med Wkly. 2006;136:293–301.
- Ardeshirylajimi A, Hosseinkhani S, Parivar K, et al. Nanofiber-based polyethersulfone scaffold and efficient differentiation of human induced pluripotent stem cells into osteoblastic lineage. Mol Biol Rep. 2013;40:4287–4294.
- Sun C, Jin X, Holzwarth JM, et al. Development of channeled nanofibrous scaffolds for oriented tissue engineering. Macromol Biosci. 2012;12:761–769.
- Kazemnejad S, Zarnani AH, Khanmohammadi M, et al. Chondrogenic differentiation of menstrual blood-derived stem cells on nanofibrous scaffolds. Methods Mol Biol. 2013;1058:149–169.
- Kim BS, Mooney DJ. Development of biocompatible synthetic extracellular matrices for tissue engineering. Trends Biotechnol. 1998;16:224–230.
- Smith LA, Ma PX. Nano-fibrous scaffolds for tissue engineering. Colloids Surf B Biointerfaces. 2004;39:125–131.
- Li M, Mondrinos MJ, Chen X, et al. Co-electrospun poly(lactide-co-glycolide), gelatin and elastin blends for tissue engineering scaffolds. J Biomed Mater Res A. 2006;79:963–973.
- Venugopal JR, Zhang Y, Ramakrishna S. In vitro culture of human dermal fibroblasts on electrospun polycaprolactone collagen nanofibrous membrane. Artif Organs. 2006;30:440–446.
- Schumann D, Ekaputra AK, Lam CX, et al. Biomaterials/scaffolds. Design of bioactive, multiphasic PCL/collagen type I and type II-PCL-TCP/collagen composite scaffolds for functional tissue engineering of osteochondral repair tissue by using electrospinning and FDM techniques. Methods Mol Med. 2007;140:101–124.
- Alves da Silva ML, Martins A, Costa-Pinto AR, et al. Cartilage tissue engineering using electrospun PCL nanofiber meshes and MSCs. Biomacromolecules. 2010;11:3228–3236.
- Wise JK, Yarin AL, Megaridis CM, et al. Chondrogenic differentiation of human mesenchymal stem cells on oriented nanofibrous scaffolds: engineering the superficial zone of articular cartilage. Tissue Eng Part A. 2009;15:913–921.
- Sonomoto K, Yamaoka K, Kaneko H, et al. Spontaneous differentiation of human mesenchymal stem cells on poly-lactic-co-glycolic acid nano-fiber scaffold. PLoS One. 2016;11:e0153231.
- Xin X, Hussain M, Mao JJ. Continuing differentiation of human mesenchymal stem cells and induced chondrogenic and osteogenic lineages in electrospun PLGA nanofiber scaffold. Biomaterials. 2007;28:316–325.
- Dahl JP, Caballero M, Pappa AK, et al. Analysis of human auricular cartilage to guide tissue-engineered nanofiber-based chondrogenesis: implications for microtia reconstruction. Otolaryngol Head Neck Surg. 2011;145:915–923.
- Baker BM, Nathan AS, Gee AO, et al. The influence of an aligned nanofibrous topography on human mesenchymal stem cell fibrochondrogenesis. Biomaterials. 2010;31:6190–6200.
- Liu J, Nie H, Xu Z, et al. The effect of 3D nanofibrous scaffolds on the chondrogenesis of induced pluripotent stem cells and their application in restoration of cartilage defects. PLoS One. 2014;9:e111566.
- Shafiee A, Seyedjafari E, Sadat Taherzadeh E, et al. Enhanced chondrogenesis of human nasal septum derived progenitors on nanofibrous scaffolds. Mater Sci Eng C Mater Biol Appl. 2014;40:445–454.
- Barry F, Boynton RE, Liu B, et al. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res. 2001;268:189–200.
- DeLise AM, Fischer L, Tuan RS. Cellular interactions and signaling in cartilage development. Osteoarthr Cartil. 2000;8:309–334.
- Nagase T, Muneta T, Ju YJ, et al. Analysis of the chondrogenic potential of human synovial stem cells according to harvest site and culture parameters in knees with medial compartment osteoarthritis. Arthritis Rheum. 2008;58:1389–1398.
- Knudson CB, Knudson W. Cartilage proteoglycans. Semin Cell Dev Biol. 2001;12:69–78.
- Aung T, Miyoshi H, Tun T, et al. Chondroinduction of mouse mesenchymal stem cells in three-dimensional highly porous matrix scaffolds. J Biomed Mater Res. 2002;61:75–82.
- Hossein M, Masoud S, Hana HA, et al. New approach for differentiation of bone marrow mesenchymal stem cells toward chondrocyte cells with overexpression of MicroRNA-140. ASAIO J. 2017 [Oct 16]. DOI:10.1097/MAT.0000000000000688