Abstract
Chronic Hepatitis B Virus (HBV) infections are severe with weak antiviral immune responses. The lack of an appropriate small animal model for chronic hepatitis, a major hurdle for studying the immunotolerance and immunopathogenesis induced by hepatitis B viral (HBV) infection. In this study, for enhancing the antibody production efficiency the prepared polymeric HBsAg-loaded nanoparticles (nanovaccine) will be tested in immune-deficit mice, which suffer from chronic Hepatitis B virus. Vaccination of Balb/c mice by this prepared nanoparticles that were engrafted with peripheral blood mononuclear cells (PBMCs), which was already lethally irradiated and transplanted by the bone marrow of NOD (knockout mice) mice. In the present study, after the vaccination detected the high frequencies of immunoglobulin G (IgG)-secreting B cells and mitogen-responsive interferon-Y (IFN-Y) secreting T cells in serum, determined by specific ELISA technique. During the entire observation period, unvaccinated animals showed lower concentration of specific IgG secreting B cells and IFN-Y secreting T cells found in comparison to vaccinated mice group. Chronic HBV carrier PBMCs transplanted into the chimera failed to produce antigen and increased the antibodies production due to vaccination. Furthermore, another advantage was that the viral gene expression and viral DNA replication was no longer observed in vaccinated group. This prepared nanovaccine formulations is better for the cure of Hepatitis B viral infection carrier. Therefore, specific memory responses were elicited by vaccination with Hepatitis B virus surface (HBsAg) antigen of chimeric mice transplanted with PBMCs derived from HBV donors.
Introduction
Hepatitis B viral infection is a worldwide problem nowadays. When immune system is weak or unable to remove HBV (hepatitis B virus), infection with this virus leads to disorders. HBV is a non-cytoptahic viral infection and causes liver injury, cirrhosis, hepatocellular carcinoma and finally chronic Hepatitis B occurs [Citation1]. Therefore, infection mainly occurs in immunocompromised people specially infants (less than 1 year) either by direct contact or by indirect contact of virus [Citation2,Citation3]. HBV such as hepatitis B core antigen (HBcAg), hepatitis B pre-core/envelop antigen (HBeAg) and viral DNA causes acute as well as chronic hepatitis [Citation4]. Persistence of HBV infection leads to increased amount of viral particles or HBsAg in blood and liver cells [Citation5]. Consequently, in chronic condition, immune tolerance develops in patients [Citation6]. Previous research indicates that only therapeutic vaccine is unable to control the HBV infection. Therefore, better strategy includes pre-treatment with nucleos(t)ide analogues like lamivudine, adefovir, entecavir, telbivudine and tenofovir. It is mainly increased the CD8 + T-cell response [Citation6,Citation7]. These analogs are potent inhibitors of HBV polymerase/reverse transcriptase and effective in the suppression of HBV replication, but not eliminate the virus [Citation8,Citation9]. Further, the DNA vaccines have quality to increase therapeutic efficacy and indicate that increase activity of surface antigen specific B and T cells, which play a major role in chronic HBV infection [Citation10,Citation11]. Strong HBV core/pre-core specific T helper (Th) cell activities are detectable in acute condition and during exacerbation of disease i.e. chronic condition T cells activity diminishes [Citation11–13].
The study of the vaccine over the immunological system, an appropriate mouse models is essentially to explain the mechanism of HBV elimination and antibody production. It is also necessary to explain targets for antiviral therapies [Citation14,Citation15]. But in vivo direct evaluation of anti-HBV immunity in the liver/immunological system is currently not possible due to the absence of appropriate mice model [Citation16]. For this purpose, chimera mice had been developed, which stimulate human serum antibody responses by vaccination with the help of recall the antigens [Citation17]. In chimera mice after vaccination, HBsAg was complexed with human anti-HBs, this immunogenic complex (IC) increases HBsAg processing and presenting by enhancing T-cell activity through increased uptake of HBsAg which are present on receptors of antigen-presenting cells (APCs) [Citation18,Citation19]. B cells stimulation occurs in the secondary lymphoid organs, such as the spleen and lymph nodes and its activation is enhanced through the activity of CD21 [Citation20,Citation21].
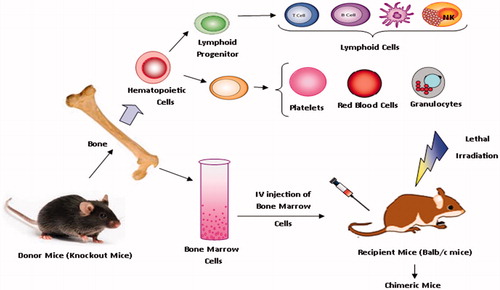
The purpose of this study was to administer prepared polymeric HBsAg-loaded nanoparticles (nanovaccine) in immune deficit mice which suffer from chronic Hepatitis B for enhancing the antibody production efficiency and elimination of viral. For this study, human/mouse radiation chimera xenograft mice model had been developed. It is also known as Trimera or humanized mice. The chimera mice has engrafted with peripheral blood mononuclear cells (PBMCs) in Balb/c mice, which have already lethally irradiated and transplanted by the bone marrow of NOD (knockout mice) mice [Citation22–24].
Human PBMC have been obtained from chronic HBV carriers or from HBV immunized donors [Citation25,Citation26]. Chimera mice having more production of human antibody as well as T-cell responses in vivo. This model might results in enhancement of viral-specific immune response in comparison to other ones [Citation27,Citation28]. Therefore, strong viral-specific mononuclear cell activity could be shown in vivo along with antibody responses of PBMCs after revival from HBV infection. However, in our model, PBMCs obtained from chronic hepatitis B carriers was unable to produce viral antigen or replicate (anti-HBs antibodies) in response to nano vaccination. Reason of this failure might be due to reduced activity of viral-specific Th1 cells not due to lack of HBs-specific B cells. When a viral replication was not detected that means influence of HBsAg or viraemia on immune response was excluded.
Materials and methods
Mice
Balb/c mice, 6–12 weeks of age, were obtained from Zoology Department, Banaras Hindu University, Uttar-Pradesh, India. These mice were lethally irradiated by specific protocol. Bone marrow was obtained from 4 to 8 weeks old NOD (knockout) mice (obtained from Centre of Cellular and Molecular Biology, India) and transplanted into recipient mice though intravenous injection with phosphate-buffered saline (PBS). The main region of selection of NOD mice is, as they exhibit not only a lack of functional B cells and T cells but also a reduced natural killer (NK) cell and low macrophage activity [Citation28,Citation29]. The Schematic representation of bone marrow component is show on . All mice were kept under animal ethical committee protocol like free pathogen conditions, fed sterile food, and given acid water containing ciprofloxacin (20 μg/mL). Ciprofloxacin is an antibiotic in a group of drugs called fluoroquinolones. Ciprofloxacin fights pathogens and protect body from bacterial infections.
Irradiation
The successful survival of a bone marrow graft, depends upon suppression of the host’s immune system. Irradiation process causes depletion of the bone marrow function of the host progenitor cells. It provides a complete space for the engraftment of donor stem cells. For this purpose, ionization radiation was used for suppression of the host immune system. Ionizing radiation causes break in DNA double-strand at multiple sites [Citation30,Citation31]. For the ionization process, gamma irradiation is mostly used. The whole-body gamma irradiation is commonly accomplished in small animals. The amount of irritation is limited (300–700 cGy) for mice at very short period around seven minutes. The irradiator chamber is small size, which holds only a few mice at a time [Citation32].
Isolation of bone marrow from NOD mice
Bone marrow was isolated from femur and tibia bone cavities. The donor mice were euthanized and pull both halves of the skin away by incision. During overall procedure, 70% ethanol was sprayed periodically in mice, to maintain the aseptic condition in experiment. Legs were taken out and remove too much hair. Thoroughly scrap the tibia and femur of all fascia, muscle and connective tissue. Epiphysis and distal ends of each bone was clipped. Bone marrow was flushed in 2 ml cells media with a 27-gauge needle in to the culture dish. Rinse and wash the culture dish and collect the cells [Citation28,Citation33].
Human donors and patients
Human peripheral blood mononuclear cells (PBMCs) were obtained from HBV infected/immunized human donors (blood sample obtained from Parul Patholab, Varanasi, Uttar Pradesh, India), which having more than six month after spontaneous clearance of HBV infection, that is donors were serologically positive for HBsAg, HBcAg and anti-HBe antibodies. Donors used in experiments were completely healthy and tested negative for anti-hepatitis C virus (HCV) or anti-human immunodeficiency viral (HIV)-1/2 antibodies.
Table 1. Chimera mice groups and vaccination schedule.
Isolation and transplantation of human PBMCs
After bone marrow transplantation (BMT) of recipients Balb/c mice, the human peripheral nlood mononucleolus cell (PBMCs) was collected by leukapheresis, isolated by Ficoll density gradient centrifugation. Separated cells were cultured in RPMI-1640 medium containing autologous serum. Finally, 8 to 10 × 107 PBMC cells were injected Intra-Peritoneally (IP) in recipient mice [Citation34].
Formulation of nanoparticles
A central composite experimental design and screening was applied for the formulation of nanoparticles to obtained minimum particles size and maximum entrapment efficiency. Some factor like amount of polymer, concentration of stabilizer and aqueous organic phase ratio and speed of homogenizer significantly influence on both the particle size and entrapment efficiency of nanoparticles. The antigen loaded PLGA nanoparticles (NPs) were prepared by a water–oil–water (W/O/W) emulsion solvent evaporation method (). Briefly, 10 µL of phosphate buffer saline–(pH 7.4) containing antigen (17.4 µg) was emulsified in 2.5 ml of dichloromethane (DCM), an organic phase, containing PLGA (35 mg), by means of sonication using a water bath sonicator (PCI Analytics Pvt. Ltd, Mumbai, Maharashtra) for 2 min, using span 80 as emulsifier, to form a primary W/O emulsion. The primary emulsion was further emulsified in 6 ml of PVA solution (1%, w/v) and continuously homogenized for 20 min and then sonicated for 2 min, to obtain a double W/O/W emulsion. The resultant double emulsion was then stirred at a certain rate (300 or 600 rpm) for 4 h at room temperature (25 °C) on a magnetic stirring plate to evaporate the organic solvent. NPs were recovered by ultracentrifugation (Beckman Coulter India Pvt. Ltd, Andheri (East) Mumbai, Maharashtra) at 15,000 rpm for 20 min at 4 °C. The supernatant was removed and NPs’ sediments were washed twice with Milli-Q water to remove free drug and excess surfactant and then lyophilized [Citation35–37].
Study design and vaccination
After the successful transplantation and irradiation of Balb/c mice, vaccination was given. The mice were divided into three groups, each group having six mice. On the same day of experiments, 1 group of mice (having bone marrow transplant and received human blood mononuclear cells) was vaccinated with 5 μg per mouse of prepared polymeric nanoparticles and toxoid injection through I.M. (Intra Muscular) route (). Whereas the control group or second group received only saline solution. The third group of chimeric Balb/c mice received vaccination having only bone marrow transplant by I.M. route. In experiment using tetanus toxoid, it protects against this life-threatening disease and provides protection against tetanus in chimeric mice.
Collection of cells and serum from human-mouse chimera
The PBMCs transplanted chimeric mice were checked by routinely isolation cells and serum test. All vaccinated and control mice were bled from the retro-orbital puncher and scarified by cervical dislocation at time points (1, 6, 12, 18 days). Spleens were removed aseptically and homogenized by pressing the fragment of spleen and single-cell suspension was prepared. Lymphocytes were separated by Ficoll density centrifugation and the isolated cells were cultured on RPMI-1640 medium containing autologous serum. Finally, the three colour flow cytometer CD45-fluoroscein isothiocyanate (FITC), CD4-phycoerythrin (PE) and CD3-peridinin chlorophyll protein were performed. Also at the indicated time points HBV surface antigen (HBsAg), HBV antigen (HBeAg), HBs antibody (HBsAb) and HBV genomic DNA in serum were monitored. Immunohistochemistry was also performed to observe the infection of HBV in mice [Citation12,Citation16].
(i) Analysis of human antigen–specific B and T cells
Quantification of anti-HBs blood T and B cells was determined by using commercially available solid-phase enzyme-linked immunoassay kit [Citation38]. The detection of anti-HBs immunoglobulin G (IgG)- secreting B cells, microtitre plates (Nunc-Immuno 96 well plate) were coated with 100 μL solution of HBsAg (10 μg/mL in PBS, pH 7.4) and incubated overnight at 4 °C. Plate was then washed three times with PBS-T (0.05% v/v Tween 20 in PBS). Serial dilutions of samples in PBS–BSA (1% (w/v) were added in each well then plate was incubated for 2 h at room temperature and washed three times with PBS-T followed by 100 μL of diluted horseradish peroxidase conjugated goat antimouse-IgG and incubated for 2 h. Further, the plate was again washed three times with PBS-T and then 100 μL of substrate solution (3, 3, 5, 5-tetramethyl benzidine containing hydrogen peroxide) was added to each well, incubated under dark at room temperature for 15 min. Colour reaction was stopped by adding 50 μL of H2SO4 (2 M) in each well. The absorbance was measured at 491 nm using a microplate ELISA reader (Novex ELISA Kits, Thermo-Fisher Scientific, Waltham, MA). The actual antibody concentration is found in mIU/mL, a standard curve of anti-hepatitis B was also prepared. Antibody response was plotted as log of anti-HBsAg antibody titres (mIU/mL) versus time in days [Citation39,Citation40].
(ii) Estimation of cytokines levels
The detection of cytokine-secreting T cells was done by specific ELISA kits. Coating and detection were performed by using monoclonal antibody against the determination of cytokines endogenous levels like interferon-Y (IFN-Y). Blood serum was used for cytokines estimation by selected ELISA method.
Detection of immunoglobulin was assessed routinely by a standard quantitative sandwich enzyme linked immunosorbent assay (ELISA) technique (Biomedicals). Tetanus toxoid antigen was bound on the surface of the microtitre plate. Diluted patient serum was pipetted into the wells of microtiter plate. Binding between the serum antibodies and immobilized tetanus toxoid antigen took place. After one-hour incubation, plate was rinsed and washed, and unbound material was removed. Then anti-human-immunoglobulin peroxidase conjugate was added and incubated for an hour. The substrate (TMB) solution was pipetted and further incubated for 30 min. The reaction was stopped and spectrophotometrically measured at the wavelength of 450 nm. The concentration of the antibodies was directly proportional to the intensity of the colour [Citation15].
Measurement of AAV/HBV infection in mice
(i) Detection of HBsAg/HBcAg (ELISA assay)
In vitro qualitative detection of serum hepatitis B surface antigen (HBsAg) was measured by ELISA according to the manufacturer’s protocol. HBsAg is a lipoprotein polypeptide which constitutes the external envelope of the HB virus. The detection of HBsAg in human serum or plasma indicates an ongoing HBV infection, either acute or chronic state of hepatitis. It not only diagnoses the HBV infections but also monitors the course of the disease as well as efficacy of antiviral therapy. In ELISA (Abbott India Ltd) the microtitre plate of solid phase is made of polystyrene and wells coated with mouse monoclonal antibodies (HBsAb) specific for HBsAg. HBsAb was analysed on a precoated HBsAg plate and developed by HRP-labelled (horseradish conjugate) anti-mouse IgG. The lower limit of detection of HBsAg was 0.5 ng/mL. The different serum dilutions were prepared to obtain the values within the linear range. Serum containing HBsAg is added to the anti-HBs antibody-coated wells together with peroxidise conjugated anti-HBs antibody and incubated. An antibody HBsAg-antibody–peroxidase complex will form on the wells. Wash the microtiter plate to remove unbound material, a solution of TMB (3,3′,5,5′-tetramethylbenzidine) substrate is added to the wells and incubated (37 ± 0.5 °C) for 15 min. A development of the colour is proportion to the amount of HBsAg bound to anti-HBs. The reaction is stopped by addition of sulphuric acid. The optical density of developed colour is read with a suitable photometer at 450 nm with a selected reference wavelength.
HBV core protein (HBcAg) was detection and visualized by immmuno histochemical Staining. Liver tissues were fixed with 10% neutral buffered formalin and embedded in paraffin. After washing or deparaffinization, the sections were incubated with primary antibodies (polyclonal rabbit anti-HBcAg; DAKO, Glostrup, Denmark) Sections were also counter stained with hematoxylin and visualized [Citation41,Citation42].
(ii) Quantitation of viraemia (real-time PCR)
Viremia (HBV-DNA) was measured by purification and quantitation of encapsidated viral DNA by real-time PCR (QIAGEN, Hilden, Germany) method. The lower limit of detection is 1X 105 genome equivalents/mL [Citation13]. HBV DNA was extracted from 100 μL of serum and a mixture containing 10 pmol of each oligonucleotide primer and 0.5 U of Taq polymerase in reaction buffer (10 mmol/L Tris-HCl [pH 8.3], 50 mmol/L KCl, 2.5 mmol/L MgCl2, 0.01% [wt/vol] gelatin, 500 μmol/L [each] dATP, dGTP, dCTP and dTTP).
Primers and probe design
The PCR primers and probe were designed in order to equally amplify all known HBV genotypes. In this way, an alignment with sequences of all HBV genotypes and sequences from Gen Bank was carried out. After this, PCR primers and Taq probe sequences were checked concerning base composition, melting temperatures, GC content, internal folding using Primer Express software.
The PCR primers and probe were designed in order to equally amplify all known HBV genotypes. In this way, HBV-specific primers oligonucleotide 1, sense (5′-GGA-GGC-TGT-AGG-CAT-AAA-TTG-GTC-TGC-GC-3′) was used. After this, PCR primers and probe sequences were checked concerning base composition and oligonucleotide 2, antisense (5′-CCC-GAG-ATTGAG-ATC-TTC-TGC-GAC-GCG-GCG-ATT-GAG-ACC-3′) was used. HBV DNA assay was performed in accordance with the manufacturer’s protocol. The numbering starts from the EcoRI site and PCR products were analysed on a 1.5% agarose gel [Citation21].
DNA extraction
For the isolation of HBV DNA from serum, the QIAamp DNA mini kit (QIAGEN, Hilden, Germany) was used, following manufacturer’s protocol. DNA was extracted from 200 μL serum with 1 μL of the internal control (1X IPC DNA; Applied Biosystems, Foster City, CA) and eluted in 50 μL buffer.
HBV quantification by versant HBV DNA assay
The versant HBV DNA Assay version 3.0 was performed in accordance with the manufacturer’s protocol.
Statistical analysis
Statistical analysis was performed on the data obtained by in vivo studies. One-way analysis of variance (ANOVA) was accomplished of different variance. All through, the level of significance was chosen as less than .05 (i.e. p < .05).
Results and discussion
Human donors and patients
Peripheral blood mononuclear cells (PBMCs) were obtained from more than six month of HBV-infected/immunized human donors. HBV infection has serologically positive for HBsAg, HBcAg and anti-HBe antibodies (). Anti-HBs–secreting B cells and HBs specific IFN-Y secreting T cells were found in the PBMC of donor blood in high frequencies. The aspartate aminotransferase and alanine aminotransferase were also measured in donor’s blood. The ratio of AST (aspartate aminotransferase) and ALT (alanine aminotransferase) was found to be less than one; it indicates non-alcoholic fatty liver disease and viral hepatitis.
Table 2. Serological data of PBMCs donors/patients suffering from hepatitis B virus.
Characterization of functional human B and T cells at different time points in the human/mouse radiation chimera
When a chimeric Balb/c mice, (transplanted with 8 to 10 × 107 infected PBMC), were vaccinated either with prepared polymeric HBsAg nanoparticles, with or without tetanus vaccine or normal saline. The mice were divided into three groups, each group having six animals. The first group of mice given by the same protocol condition was vaccinated with antigens vaccine but did not receive TT. The second group of mice received only tetanus toxoid vaccine. A third group of mice given by the same protocol condition was vaccinated with both antigens vaccine as well as receive TT. The vaccinated mice shows strongest specific immune response is observed in the human/mouse radiation chimera mice during the around two weeks of post-transplantation. The blood serum and cells were collected approximately in 1, 6, 12 and 18 days after PBMC transplantation and vaccination. The estimation of B cells secreting immunoglobulins IgG and T cells specific IFN-Y was measured in ELISA technique.
(i) Examination of IgG-secreting B cells
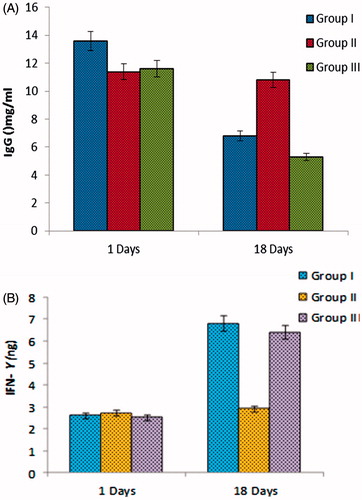
The human/mouse radiation chimera mice exhibit high levels of IgG, acquired on some days of post-transplant. The amounts of such B cells were studied in the serum of chimeric mice. The challenge is recovery of the mice cells after being transplanted with PBMCs of HBV donors. HBsAg vaccine mice exhibited substantially greater frequencies of anti-HBs as compared with unvaccinated control mice (). It was found that immunoglobulin in PBMCs transplanted chimera mice of chronic HBV carriers was highest on starting day of post-transplantation. The excess production of the immunoglobulin in chimera mice was also observed. Levels are again measured on last days of experiment. The immunoglobulin level is approximately maintained in group treated with vaccine. The group receives only saline/TT not significant change observed, that is, the level of immunoglobulin was higher. The first group that receives only vaccine but not taken tetanus toxoid, the level of IgG was decreased but in comparison to vaccine group (Group III) less amounts was observed. The results indicate that TT vaccine was also helps in increasing the activity of the polymeric nanoparticles vaccine.
Figure 3. (A) Levels of human immunoglobulins (IgG) and (B) cytokine (IFN-Y) in sera of chimeric mice transplanted with PBMCs of HBV-immunized carriers. Balb/c chimeric mice were transplanted with PBMCs and vaccinated with HBsAg loaded polymeric particles or TT. Sera were collected at day first and 18 days.
(ii) Examination of secreting T cells
Antigen-specific T cell is need of B cells it helps for proliferation, differentiation and effective production of specific type of antibodies. Determination of the total number of functional T cells, measure the frequencies of T cells secreting response interferon Y (IFN- Y). All PBMCs transplanted mice found HBs-specific less amount of IFN-Y secreting activity on starting days. After vaccination on 18th day, the frequency of IFN-Y-secreting cells reached its normal level in both group I and III (). The group II showed hyper immune response (more interferon levels), which receive tetanus toxoids and normal saline. TT vaccination of control and test mice of the same donors were examined for TT-specific T cells response. It also observed that after vaccination with TT not only higher serum levels of anti-TT antibodies, also high frequencies of TT-specific IFN-Y was found in mice transplanted with PBMCs.
Measurement of AAV/HBV infection in mice
(i) Detection of HBsAg/HBcAg (ELISA assay)
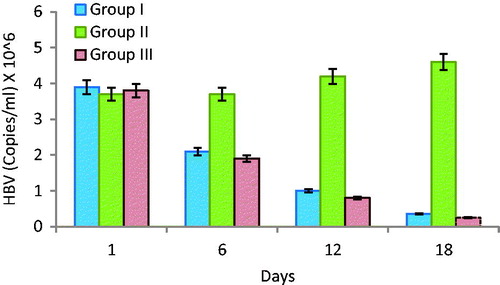
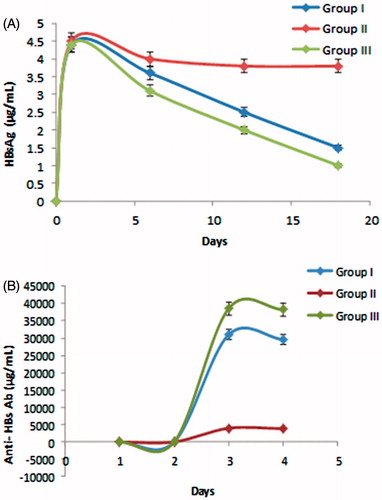
In chimera mice, having infected PBMC viral replication in the liver is common but due to presence of vaccination, not in sufficient/properly. Presented viral DNA in chimera mice was purified from blood, and viral genomes were measured by a quantitative PCR assay technique. Group II & III mice developed high-titre anti-HBs antibody (HBsAb), than group I. However, the amount of viral HBsAg was reduced. Large amount of viremia was detected on first day of experiment. Viraemia subsequently declined through day 6th and viral titres was decreases more in 18th day. The amount of HBV surface antigen (HBsAg) was higher on initial day, after that, serum HBsAg decline slowly and the concentration was reached lowest on day 18th.
The antibodies specific for HBsAg became detectable in the blood serum. The highest level of HBsAg antibody was found in group III. On initial day, HBsAg antibodies level was lowest (35.6 ± 3.5 mIU) and highest levels were 18th (35,621 ± 4.6 mIU). The group II, which received only saline did not showed significant increment of antibodies production. The group I had low antibody comparison to group III. The results indicated that TT is having quality to increase the activity of the hepatitis B polymeric vaccine activity.
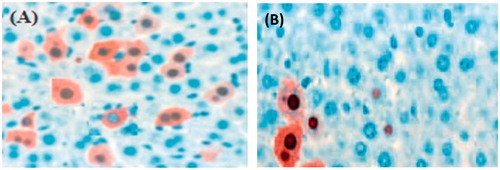
The hepatitis B viral core protein (HBcAg) expression was examined by immune histochemical staining liver cells on day 1st and 18th. Core proteins are mostly randomly distributed throughout the liver lobule (). HBcAg expression highest on first day and the frequency of HBcAg-positive hepatocytes decreased on day 18. HBcAg-positive hepatocytes were surrounded by inflammatory cells, therefore decreasing of hepatocytes increasing the inflammatory cells. ) are shows the vaccinated group chimera mice liver cells, which received the polymeric nanoparticles vaccine as well as TT.
Figure 4. (A) HBsAg and (B) Anti-HBs antibody levels in serum of chimeric mice transplanted with PBMCs of HBV-immunized carriers. Balb/c/scid chimeric mice were transplanted with PBMCs and vaccinated the same day with HBsAg loaded polymeric particles. Sera were collected at the indicated time points and assessed.
(ii) Quantitation of viraemia (real-time PCR)
When a HBV-infected PBMC was transferred in human/mouse radiation chimeras mice, large amounts of HB viral replicated in these mice and that viraemia as well as viral-like antigens particles was observed in blood. It is also supress/negative responses the chimera mice immune response after vaccination. The blood serums of all chimera mice were tested for the presence of HBsAg and HBcAg. HBV-DNA could be detected and assessed by PCR in given time period. All mice groups shows the average concentration of HBV surface antigen (HBsAg) is highest 3.8 × 106 ±2 copies/mL at the first day of the infection. After day by day the amount of viraemia was slowly decreases continuously in blood. The group which received polymeric vaccine as well as TT were 1.9 × 106 ±2.8 copies/mL and 0.8 × 106 ±8.9 copies/mL was found in days six and 12 () and last day of experiments minimum amount of viraemia was observed (0.25 × 106 ±3.6 copies/mL). The chimera mice group which received only vaccine showed comparatively high level of viremia in blood (0.35 × 106 ±3.5 copies/mL) in 18 days of experiments. The second group of mice found only saline/TT showed high levels of HBV in blood, and amount of virus was increases day by day [Citation27]. Results indicate the vaccinated group of mice having ability to production of antibodies as well as control the growth of viraemia in chimera mice transplanted with PBMCs.
Viral gene expression in chimera mice
The role of cellular and humoural immune response on the hepatitis B viral expression was checked by hydrodynamic transfer of human infected PBMCs cell in immunodeficient mice, which having lack functional B and T cells, as well as natural killer (NK) cell activity [Citation43]. After the transfer of PBMCs cells in mice, viral DNA replications and its intermediates were detected in the liver and blood of these mice in 6 days. The B cells were also studied in the serum of chimeric mice. HBs vaccinated group of mice exhibited substantially greater frequencies of anti-HBs as compared with unvaccinated control group mice. It was found that highest numbers of immunoglobulin in PBMC transplanted chimera mice of chronic HBV carriers on starting day of post transplantation. All PBMCs transplanted mice found HBs-specific more IFN-Y secreting activity on starting days. After the vaccination, the frequency of IFN-Y secreting cells was reach on normal state receiving polymeric vaccine. In starting large amounts of HB viral replicated in these mice and supress after vaccination. The blood serums of all chimera mice were tested for the presence of HBsAg and HBcAg. HBV-DNA could be detected and assessed. The expression of the HBcAg was examined by immunohistochemical staining of liver (). The disappearances of the HBcAg-positive hepatocytes from the liver of treated mice and development of HBV-specific T-cell response. The persistence of viral gene expression in irradiated and bone marrow transplanted Balb/c mice which received infected PBMCs for extended periods of time. In the absence of immune responses, vaccine helps for production of specific immune immunoglobulin and interferon. An immune response strongly suggests that, It play a central role in the elimination of HBV-positive hepatocytes in the immune competent mice [Citation44,Citation45].
Discussion
The development of a humanized or human/mouse chimeric mice model that would allow us to study the immune response to HBV in an immunologically small animal host exists in a model for HBV infection [Citation34]. In our model, there is possibility to increase B and T-cell responses, the model is already immune supressed and transplanted from bone marrow cells of knockout mice. This means the model completely lacks immune system. In this condition, the infected human PBSCs cells containing verioma was allowed to grow, but chances to severe condition or death. After the administration of specific HBsAg-loaded polymeric nanoparticles vaccination in vivo, production of antibody. It is the re-stimulation of immune system after nanoparticles vaccination. The ELISA analyses observed high frequency of HBs-specific T-cell response (interferon-Y) and B-cell-specific immunoglobulin activities.
An in vivo humanized model or human/mouse chimeric mode is an extremely valuable tool for the study of new vaccine strategies for the generation and detection of antigen-specific immunoglobulin G (IgG) secreting B cells and mitogen-responsive interferon-Y secreting T cells. Therefore, the occurrence of human immunoglobulin and specific antibodies in chimeric mouse sera indicated the presence of functional B and T cells in the mice model, direct detection of such cells which was impossible [Citation16].
In earlier study, viral persistence in chronic HBV carriers is associated with HBsAg-specific Th1 cell defect, which likely is responsible for the insufficient neutralizing anti-HBs-antibody response and is not reversed by HBs vaccination [Citation12,Citation46]. It is good approach to develop human monoclonal antibodies in a human/mouse radiation chimera specific for hepatitis B viral surface antigen, also having a strong memory response [Citation43]. Mice containing livers repopulated with human hepatocytes would provide another in vivo model for studies on human liver diseases and hepatotropic viruses, for which no permissive cell lines exist. It has studies on aetiology and therapy of viral and nonviral human liver diseases, as well as on hepatocyte biology and hepatocellular transplantation [Citation1].
In previous studies of human/mouse chimeric models, the detection of antigen-specific B-cell and T-cell responses has been difficult due to the lack of sensitive B-cell and T-cell assays. Also, when the chimera mice were transplanted with PBMCs of chronic HBV carriers, they did not show anti-HBs antibodies in serum. This defective problem of anti-HBs production was not protected by vaccination with a conventional recombinant HBs vaccine. Likewise, defect in anti-HBs antibody production of mice transplanted with PBMCs infected donors. Because the immune system having ability to exclude the HBsAg in blood through immune complexes with help of anti-HBs antibodies [Citation47]. A recent study developed the existence of antienvelope antibodies production in immune complexes system. The antienvelope antibody production was detected by these techniques might be only quantitatively reduced or be directed against HBs antigens [Citation48].
In present work, previously prepared HBsAg-loaded PLGA nanoparticles were studied for immunotherapy. Also analysed the reduction of hepatitis B virus in mice xenografts humanized or human/mouse chimeric model. Xenografts chimeric Balb/c mice engrafted with peripheral blood mononuclear cells (PBMCs) which was lethally irradiated and transplanted with bone marrow of NOD mice, allowing the in vivo induction of high frequencies of B-cell and T-cell responses. It is valuable tool for the study of new vaccine approaches and for the production of antigen-specific immunoglobulin and cytokinine. The present model is exciting for studying of HBV infection related immunology. The chimeric mice transplanted with PBMC from infected HBV donors spontaneously developed high levels of serum anti-HBs antibodies that were further vaccinated with HBsAg loaded polymeric nanoparticles. After the vaccination, high serum antibody responses were developed in chimeric mice. However, it also reduces the quantity of viraemia without interfering with translation or cross reactivity for anti-HBs antibodies production. After the vaccination, proliferative of HBcAg and HBeAg in liver was reduced that means decreases of HBV viraemia along with core antigen particles (HBcAg). The high HBV viraemia load suggesting that suppress of HBV-specific Th cell responses, because this core antigen–specific T cell was reversible. In this, all phenomena an early innate immune response, NK cells mediates and IFN-Y dependent down-regulation of viral replication. The successive virus-specific T-cell response led to the eventual clearance of HBV by cytolytic and noncytolytic mechanisms. The developed mouse immune system is capable of both modes of immune control system because they have both been induced in the transgenic mouse model [Citation49,Citation50]. The developed mice model show virus-specific CTL (Cytotoxic T Lymphocytes) response. The relationship between the HBV-specific CTL response and viral clearance remains to be determined, as do the presence and functional impact of any innate immune response. Generally, this manipulated xenograft mice are easy to analyse in comparison to chimpanzee. The specific cytotoxic T lymphocytes response IFN-Y and any innate immune response are important for presence in mice model. The cytotoxic T lymphocytes response is capable to remove virus and viral-like particles and explain the study of HBV gene regulation and replication. Therefore, the prepared formulation made by polymeric shell and having quality to sustain release of antigen, which maintain the properly production of antibody continuously in humanized model. This continuously production of antibody prevent the growth of HBcAg and clears varioma in blood.
Acknowledgements
Authors are thankful to institute for providing the necessary facilities. I would like to thank Central instrument facility, IIT (BHU) for characterization of synthesized compounds. Authors also acknowledge Prof. Arvindacharya, Department of Zoology, Banaras Hindu University, for his kind help in carrying out differential cell culture study. Authors also acknowledge Dr. Ashish Singhai, Assistant Professor, VNS Institute of Pharmcy, Bhopal for his kind support and provide ‘Sapience Lab’ facility. Authors also acknowledge Dr. Promod, Post Doctorate Fellow in Department of Zoology, Banaras Hindu University for their tireless assistance in carrying out the experiments.
Disclosure statement
The authors have no conflict of interest.
Additional information
Funding
References
- Dandri M, Burda MR, Török E, et al. Repopulation of mouse liver with human hepatocytes and in vivo infection with hepatitis B virus. Hepatology. 2001;33:981–988.
- Marrocco C, Rinalducci S, Mohamadkhani A, et al. Blood transfus plasma gelsolin protein: a candidate biomarker for hepatitis B-associated liver cirrhosis identified by proteomic approach. Blood Transfus. 2010;8:s105–s112.
- Norman G. Hepatitis B: diagnosis, prevention, and treatment. Clin Chem. 1997;43:1500–1506.
- Liang TJ. Hepatitis B: the virus and disease. Hepatology. 2009;49:S13–S21.
- Patterson LJ, Aberdeen A, Kone J, et al. Formation of HIV-1 envelope-hepatitis B core antigen hybrids with high affinity for CD4. Biochem Biophys Res Commun. 2001;285:639–643.
- Pol S. Immunotherapy of chronic hepatitis B by anti HBV vaccine. Biomed Pharmacother. 1995;49:105–109.
- Chien RN, Liaw YF. Nucleos(t)ide analogues for hepatitis B virus: strategies for long-term success. Best Pract Res Clin Gastroenterol. 2008;22:1081–1092.
- Bisceglie AMD. Combination therapy for hepatitis B. Gut. 2002;50:443–445.
- Pol S, Michel ML. Therapeutic vaccination in chronic hepatitis B virus carriers. Expert Rev Vaccines. 2006;5:707–716.
- Penna A, Artini M, Cavalli A, et al. Long-lasting memory T cell responses following self-limited acute hepatitis B. J Clin Invest. 1996;98:1185–1194.
- Rehermann B, Lau D, Hoofnagle JH, et al. Cytotoxic T lymphocyte responsiveness after resolution of chronic hepatitis B virus infection. J Clin Invest. 1996;97:1655–1665.
- Bocher WO, Galun E, Marcus H, et al. Reduced hepatitis B virus surface antigen- specific Th1 helper cell frequency of chronic HBV carriers is associated with a failure to produce antigen-specific antibodies in the trimera mouse. Hepatology. 2000;31:480–487.
- Huang LR, Wu HL, Chen PJ, et al. An immunocompetent mouse model for the tolerance of human chronic hepatitis B virus infection. Proc Natl Acad Sci USA. 2006;103:17862–17867.
- Wen YM, Wu XH, Hu DC, et al. Hepatitis B vaccine and anti-HBs complex as approach for vaccine therapy. Lancet. 1995;345:1575–1576.
- Liang S, Du J, Yan H, et al. A mouse model for studying the clearance of hepatitis B virus in-vivo using a luciferase reporter. PLoS One. 2013;8:495–500.
- Bocher WO, Marcus H, Shakarchy R, et al. Antigen-specific B and T cells in human/mouse radiation chimera following immunization in vivo. Immunology. 1999;96:634–641.
- Inuzuka T, Takahashi K, Chiba T, et al. Mouse models of hepatitis B virus infection comprising host-virus immunologic interactions. Pathogens. 2014;3:377–389.
- Lubin I, Segall H, Marcus H, et al. Engraftment of human peripheral blood lymphocytes in normal strains of mice. Blood. 1994;83:2368–2381.
- Yao X, Zheng B, Zhou J, et al. Therapeutic effect of hepatitis B surface antigen-antibody complex is associated with cytolytic and non-cytolytic immune responses in hepatitis B patients. Vaccine. 2007;25:1771–1779.
- Kosinska AD, Liu J, Lu M, et al. Therapeutic vaccination and immunomodulation in the treatment of chronic hepatitis B: preclinical studies in the woodchuck. Med Microbiol Immunol. 2015;204:103–114.
- Murphy K. Janeway’s Immunology. 8th ed. New York (NY): Garland Science. 2012.
- Marcus H, Burakova T, Shezen E, et al. Human->mouse radiation chimera do not develop Epstein-Barr virus lymphoma. Immunol Lett. 1996;49:155–161.
- Dion S, Bourgine M, Godon O, et al. Adenoassociated virus-mediated gene transfer leads to persistent hepatitis B virus replication in mice expressing HLA-A2 and HLADR1 molecules. J Virol. 2013; 87:5554–5563.
- Burakova T, Marcus H, Canaan A, et al. Engrafted human T and B lymphocytes form mixed follicles in lymphoid organs of human/mouse and human/rat chimera. Transplantation. 1997;63:1166–1171.
- Asokan R, Banda NK, Szakonyi G, et al. Human complement receptor 2 (CR2/CD21) as a receptor for DNA: implications for its roles in the immune response and the pathogenesis of systemic lupus erythematosus (SLE). Mol Immunology. 2013;53:99–110.
- Reisner Y, Dagan S. The Trimera mouse: a novel system for the generation of human monoclonal antibodies and models for human diseases. Trends Biotechnol. 1998;16:242–246.
- Segall H, Lubin I, Marcus H, et al. Generation of primary antigen-specific human cytotoxic T lymphocytes in human/mouse radiation chimera. Blood. 1996;88:721–730.
- Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol. 1995;13:29–60.
- Larsen SR, Kingham JA, Hayward MD, et al. Damage to incisors after nonmyeloablative total body irradiation may complicate NOD/SCID models of hemopoietic stem cell transplantation. Comp Med. 2006;56:209–214.
- Elshaikh M, Ljungman M, Ten Haken R, et al. Advances in radiation oncology. Annu Rev Med. 2006;57:19–31.
- Prise KM, Schettino G, Folkard M, et al. New insights on cell death from radiation exposure. Lancet Oncol. 2005;6:520–528.
- Duran-Struuck R, Dysko RC. Principles of bone marrow transplantation (BMT): providing optimal veterinary and husbandry care to irradiated mice in BMT studies. J Am Assoc Lab Anim Sci. 2009;48:11–22.
- Ikehara S. Bone marrow transplantation: a new strategy for intractable diseases. Drugs Today. 2002;38:103–111.
- Yang PL, Althage A, Chung J, et al. Hydrodynamic injection of viral DNA: a mouse model of acute hepatitis B virus infection. PNAS. 2002;99:13825–13830.
- Pankhurst QA, Connolly J, Jones SK, et al. Applications of magnetic nanoparticles in biomedicine. J Phys D: Appl Phys. 2003;36:R167–R181.
- Nellore RV, Pande PG, Young D, et al. Evaluation of biodegradable microspheres as vaccine adjuvant for hepatitis B surface antigen. J Parenter Sci Technol. 1992;46:176
- Singh M, Li XM, McGee JP, et al. Controlled release microparticles as a single dose hepatitis B vaccine: evaluation of immunogenicity in mice. Vaccine. 1997;15:475–481.
- Mishra D, Dubey V, Asthana A, et al. Elastic liposomes mediated transcutaneous immunization against hepatitis B. Vaccine. 2006;24:4847–4855.
- Debin A, Kravtzoff R, Santiago JV, et al. Intranasal immunization with recombinant antigens associated with new cationic particles induces strong mucosal as well as systemic antibody and CTL responses. Vaccine. 2002;20:2752–2763.
- Marcus H, David M, Canaan A, et al. Human/mouse radiation chimera are capable of mounting a human primary humoral response. Blood. 1995;86:398
- Raney AK, Eggers CM, Kline EF, et al. Nuclear covalently closed circular viral genomic DNA in the liver of hepatocyte nuclear factor 1α-null hepatitis B virus transgenic mice. J. Virol. 2001;75:2900–2911.
- Yant SR, Meuse L, Chiu W, et al. Somatic integration and long-term transgene expression in normal and haemophilic mice using a DNA transposon system. Nat Genet. 2000;25:35–41.
- Eren R, Lubin I, Terkieltab D, et al. Human monoclonal antibodies specific to hepatitis B virus generated in a human/mouse radiation chimera: the Trimera system. Inmunology. 1998;93:154–161.
- Liu F, Song Y, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;6:1258–1266.
- Chen DS. Toward elimination and eradication of hepatitis B. J Gastroenterol Hepatol. 2010;25:19–25.
- Guidotti LG, Matzke B, Schaller H, et al. High-level hepatitis B virus replication in transgenic mice. J Virol. 1995;69:6158–6169.
- Shultz LD, Schweitzer PA, Christianson SW, et al. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol. 1995;154:180–191.
- Budkowska A, Dubreuil P, Poynard T, et al. Anti-pre-S responses and viral clearance in chronic hepatitis B virus infection. Hepatology. 1992;15:26–31.
- Kakimi K, Guidotti LG, Koezuka Y, et al. Natural killer T cell activation inhibits hepatitis B virus replication in Vivo. J Exp Med. 2000;192:921–930.
- Guidotti LG, Ishikawa T, Hobbs MV, et al. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25–36.