Abstract
The present study reports a simple and eco-friendly synthesis of silver nanoparticles (AgNPs) using leaf extract of Rhynchosia suaveolens. UV-Vis analysis of R. suaveolens synthesized AgNPs (RS-AgNPs) showed surface plasmon resonance (SPR) peak at 426 nm. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) analysis revealed that RS-AgNPs were 10–30 nm in size with spherical shape. X-ray diffraction (XRD) analysis of RS-AgNPs confirmed the crystalline nature with face-centered cubic (FCC) lattice. Fourier transform infrared (FTIR) interprets that polyphenols and proteins take part in bioreduction and capping of RS-AgNPs. RS-AgNPs exhibited dose-dependent inhibition of proliferation of different cancer cells including DU145 and PC-3(human prostate carcinoma cell lines), SKOV3 (human ovarian carcinoma) and A549 (human lung adenocarcinoma)with IC50 values of 4.35, 7.72, 4.2 and 24.7 μg/mL, respectively. The plausible reasons behind anticancer activity of RS-AgNPs were explained using different assays on the most susceptible SKOV3 cells. RS-AgNPs induced oxidative stress in SKOV3 cells by generating reactive oxygen species (ROS), enhancing lipid peroxidation (LPO) levels and decreasing glutathione (GSH) levels. RS-AgNPs induced the apoptosis of SKOV3 cells by up regulating the caspase-3, caspase -8, caspase -9, p53 and BAX and down regulating the antiapoptotic protein Bcl-2. Further, RS-AgNPs showed elevation of caspase 3/7 activity and also exhibited antimigratory effect by inhibiting the migration of SKOV3 cells into the wounded area. The findings suggested that biogenic RS-AgNPs provide an alternative approach to overcome several limitations of chemotherapy.
Introduction
Owing to their unique-physico chemical properties including large surface area to volume ratio, optical absorbance, surface plasmon resonance (SPR) phenomena and reduced imperfections, metal nanoparticles (NPs) have been found to possess wide-variety of applications in various disciplines including electronics, physics, chemistry, biotechnology and biomedical fields [Citation1–3]. Among the various metal NPs, silver nanoparticles (AgNPs) have been studied extensively due to different applications including optical receptors, polarizing filters, intercalating materials of batteries [Citation4,Citation5], catalysts, sensors, signal enhancers [Citation6–8], biolabelling materials, drug delivery, anticancer, anti-inflammatory, antioxidants and antimicrobial agents [Citation9–13]. Different chemical and physical synthesis methods of AgNPs have been reported. Well-known examples include electron and gamma irradiation, thermal decomposition, laser ablation, microwave processing [Citation14–18], ultrasonic irradiation, polyol method [Citation19], electrochemical assisted, polyaniline assisted [Citation20], photochemical and chemical reduction methods [Citation21,Citation22]. But all of these methods possess certain demerits including application of toxic radiations and hazardous chemicals, laborious, time consuming and expensive approaches, environmental and health risks. Further biocompatibility is in speculation, as the chemically synthesized AgNPs contains toxic chemicals on their surface (toxic chemicals used for the capping or stabilization purposes). Hence, the biological methods for the synthesis of AgNPs have become prime choice. The biological or biosynthesis methods of AgNPs including bacterial assisted [Citation23], fungal mediated [Citation24], actinomycetes mediated [Citation25] and plant assisted methods [Citation26–28] have been reported as simple, rapid, inexpensive and ecofriendly approaches which are alternative to physical and chemical methods. Different plant extracts including Anthemis atropatana [Citation29], Bauhinia variegata [Citation30], Caesalpinia pulcherrima [Citation31], Cassia fistula [Citation32], Euphrasia officinalis [Citation33], Glycyrrhiza uralensis [Citation34], Indigofera tinctoria [Citation35], Ipomoea batatas [Citation36], Prosopis juliflora [Citation37] and Punica granatum [Citation38] have been successfully reported for the biosynthesis of AgNPs. The demand for AgNPs has increased due to their vast applications in biomedicine, pharmacy, agriculture and food industry which leads to the development of clean, nontoxic and biocompatible AgNPs. Plant synthesized AgNPs are being successfully employed not only as anti-bacterial, antifungal [Citation26–28], anti-viral [Citation39], anti-inflammatory [Citation40], wound healing [Citation41], anti-angiogenic treatment [Citation42] cancer diagnosis and drug delivery but also in bio imaging [Citation9–11,Citation43]. Further, plant synthesized AgNPs are biocompatible and non-hemolytic [Citation10,Citation13].
Nanotechnology translated to nanomedicine, has made an important contribution to oncology. Indeed, the application of NPs for targeted drug delivery and diagnostics is at the forefront of cancer nanomedicine projects. Nanomedicine employing AgNPs, has emerged as an alternative approach to the conventional therapies of cancer for delivery of drugs into their targets and cancer bioimaging [Citation9–11]. A number of plants belonging to Rhynchosia genus have been reported for their anti-cancer, anti-oxidant and anti-fungal properties. Rhynchosia suaveolens belongs to family Fabaceae, is a viscous hairy shrub found widely in South India, whose leaves are rich in flavones and phenolic compounds [Citation44]. The presence of these compounds reduce the silver cations (Ag+) present in the silver nitrate solution during interaction and trigger the formation of RS-AgNPs [Citation45]. In the present study, we have evaluated the anti-carcinogenic potential of the RS-AgNPs against various cell lines including DU145 and PC-3 (human prostate carcinoma cell lines), SKOV3 (human ovarian carcinoma) and A549 (human lung adenocarcinoma). Furthermore, we have conducted the experiments to provide the direct evidence of the cytotoxic strength of RS-AgNPs and molecular mechanism of action on SKOV3 cells.
Materials and methods
Chemicals and antibodies
Dulbecco’s Modified Eagle’s medium (DMEM), Rosewell park memorial institute (RPMI)-1640 medium, antibodies to caspases 3,8 and 9, p53, Bax and Bcl-2 were obtained from Sigma Aldrich (St. Louis, MO). Antibody to actin was purchased from Calbiochem (Darmstadt, Germany). Alkaline phosphatase conjugated secondary antibodies and BCIP/NBT substrates were obtained from Genei Pvt. Ltd. (Bangalore, India). Fetal bovine serum (FBS) was purchased from JRH Biosciences (Lenexa, KS). Polyvinylidene difluoride (PVDF) membranes were purchased from Millipore, Billerica, MA. Antibiotics were obtained from Gibco (Thermo Fisher Scientific, Grand Island, NY). Silver nitrate (AgNO3) and all other chemicals were purchased from Sigma Aldrich (St. Louis, MO) wherever not specified.
Collection of plant material and preparation of extract
The leaves of R. suaveolens were collected from Talakona forest ranges of Seshachalam hills, Eastern Ghats, Tirupati (north latitudes 18° 10° to 19° 44° and east longitudes of 82° 53° to 84° 05°), Andhra Pradesh, South India. The plant was identified by the renowned plant taxonomist and the voucher specimens (SVUTYRS-01-05) were deposited in the herbarium, Department of Botany, Sri Venkateswara University, Tirupati.
Initially, 100 g of leaves were thoroughly cleansed with tap water to remove the dust particles adhered. Then the leaves were surface-sterilized by sterile Milli-Q water. The cleaned leaves were crushed using mortar and pestle and transferred to a beaker filled with 500 ml of Milli-Q water. The mixture was boiled 2–3 times for three minutes each time with a 30 min interval (800 w, LG) using a microwave oven, kept under vigorous stirring and centrifuged at 12,000 rpm for 10 min. The supernatant was stored at 4 °C which was used as the reducing and capping agent and sterile condition was maintained throughout the experiment.
Biosynthesis of RS-AgNPs
Bio synthesis of RS-AgNPs was carried out according to the method of Mukherjee et al. [Citation10]. Briefly, about 95 ml of 1 mM AgNO3 was taken into amber colored bottle and 5 ml of aqueous leaf extract of R. suaveolens (Pale Brown) was added and incubated for 3–24 h in dark with constant stirring at 45 °C temperature.
Characterization of RS-AgNPs
Initially, the bioreduction of silver (Ag+) into RS-AgNPs was confirmed by visual analysis. UV-Vis spectrum of the colloidal solution of RS-AgNPs was recorded between 200–700 nm by Spectra MAX Plus; Molecular Devices, Sunnyvale, CA; supported by SOFTmax PRO-5.4. Colloidal solution of RS-AgNPs was centrifuged for 40 min at 15,000 g at 15 °C using KUBOTA centrifuge (Japan). After centrifugation, the supernatant was discarded and only the concentrated loose black coloured RS-AgNPs pellet was collected. The pellet was air dried to get pure powder of RS-AgNPs which is used for further characterizations and in vitro experiments. Fourier transform infrared (FTIR) analysis was carried out to reveal the functional groups that plausibly participated in the bioreduction and stabilization of RS-AgNPs. X-ray diffraction (XRD) analysis was carried out to determine the crystalline nature of RS-AgNPs (PANalytical Empyrean). The size, shape and morphology was determined by Scanning electron microscopy (SEM) followed by Transmission electron microscopy (TEM) using JEM 2000EXII apparatus (M/s. JEOL, Switzerland). Energy dispersive X-ray (EDX) spectrum of RS-AgNPs was also recorded using SEM to determine the elemental composition. Thermal stability of the biogenic RS-AgNPs was examined by thermo gravimetric analysis (TGA) where temperature aided decomposition/desorption behavior of the RS-AgNPs was studied [Citation46]. Dynamic light scattering (DLS) was used to determine the zeta potential value of RS-AgNPs.
Cell lines and cell culture
The human prostate cancer (DU145 and PC-3), ovarian carcinoma (SKOV3) and lung carcinoma (A549) cell lines were obtained from the National Centre for Cellular Sciences, Pune, India. Cells were grown in either RPMI-1640 (PC3), or DMEM (DU145, SKOV3 and A549). The media were supplemented with 10% (v/v) heat-inactivated FBS, 100 units/mL penicillin and 100 µg/mL streptomycin. All cell lines were maintained in culture at 37 °C in a humidified atmosphere of 5% CO2 and 95% air.
In vitro cytotoxicity assay
Cytotoxic activity of RS-AgNPs was carried out by 3-(4,5-dimethylthiazo-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay [Citation47]. Briefly, the exponentially growing cells (2 × 104 to 3 × 104 cells/100 μL) were seeded in 96-well plates and incubated for 12 h. Cells were then treated continuously by the addition of test compound (RS-AgNPs in 100 µL of media) to obtain final concentrations of 1–10 µg/mL. After 24 h, cell survival was evaluated by adding 10 μL of MTT at a concentration of 5 mg/mL in phosphate-buffered saline (PBS; pH 7.4). After 3–4 h of incubation at 37 °C, the medium and the MTT were removed and 100 μL of dimethyl sulfoxide (DMSO) was added to dissolve the precipitate of reduced MTT. The formazan crystals were then dissolved and the absorbance was recorded at 570 nm with a microplate reader (Spectra MAX Plus; Molecular Devices; supported by SOFTmax PRO-5.4). The MTT assay distinguishes between viable and nonviable cells on the basis of the requirement of physiologically active mitochondria to metabolize the MTT only in viable cells. The IC50 was calculated as the concentration of RS-AgNPs causing a 50% inhibition compared to that of cells treated with media alone.
Morphological changes of cells
The altered morphology of cells (1 × 105) at different concentrations of RS-AgNPs (IC25, IC50 and IC75) was studied after 24 h using an inverted phase contrast microscope (Optika, Italy). Simultaneously, other batch of cells were washed with PBS (0.01 M, pH 7.4) and the cells were fixed in 70% ethanol for two hours at 4 °C. Later, cells were stained individually with 10 μL of 100 µg/mL Hoechst 33,342 solution and 5 μL of 100 µg/mL AO-EB in a dark chamber for 30 min at room temperature. Finally, the stained cells were washed three times with PBS and were observed under a fluorescence microscope with standard filters.
DNA extraction and electrophoresis analysis
DNA extracted from RS-AgNPs treated cancer cells was analyzed on agarose gel electrophoresis. Briefly, 24 h exposed cells were centrifuged at 2000 rpm for 10 min at 4 °C. Cells were washed with PBS and recentrifuged at 13,200 rpm for 10 min at 4 °C. DNA from treated cells was extracted using cetyl trimethylammonium bromide (CTAB) method. The extracted DNA was subjected to agarose gel electrophoresis, which was run at 50 V for 120 min. The bands were visualized under gel documentation and photographed (UVDI, Major Science, India).
Caspase 3/7 activity
The caspase 3/7 activity was detected by cellEventTM (caspase-3/7 green detection reagent Life technologies, Eugene, OR, Cat: C10423) according to manufacturer’s instructions.
Measurement of intracellular reactive oxygen species (ROS)
The level of intracellular ROS generation was estimated using 2, 7-dichlorofluorescein diacetate (DCFDA; Sigma, St Louis, MO) dye by the method of Wan et al. [Citation48]. Cells were seeded in a 96-well black bottom plate (104 cells/well). After 24 h, the cells were exposed to RS-AgNPs (IC25, IC50, IC75, 5 mM NAC and NAC with IC50) for 6 and 12 h, respectively. Following exposure, the cells were washed twice with PBS and were incubated with DCFDA dye (20 µM) for 30 min at 37 °C. Later the reaction mixture was replaced by 200 µL of PBS and fluorescence intensity was measured on multimode plate reader (Spectramax M2e, Molecular Devices) using Softmax pro 5.0 software at excitation and emission wavelengths of 485 and 528 nm, respectively. The qualitative analysis of ROS generation was done using a microscope with a fluorescence attachment (Optika, Italy).
Intra cellular reduced glutathione estimation
Reduced glutathione (GSH) content was measured in accordance with method of Jollow et al. [Citation49]. Briefly, 1 × 106 cells/well were seeded in six-well plates and treated with different concentrations of RS-AgNPs (IC25, IC50 and IC75 concentrations) along with controls (without test compound) for 24 h. About 60 μL of the supernatant was added to 50 μL of Trichloroacetic acid (TCA) (5%) and then 25 μL of Ellman’s reagent (1.2 mM DTNB in 1% sodium citrate) and the final assay volume was made to 150 μL with phosphate buffer. The color developed was read at 412 nm to determine percentage of GSH levels in comparison with control.
Lipid peroxidation assay
Malondialdehyde (MDA) a lipid peroxidation end product in treated cultured cells was measured according to the method described by Utley et al. [Citation50]. Briefly, 0.125 ml of homogenate and 0.125 ml of reaction mixture (10 mM ferrous sulfate, 200 mM ascorbate, 0.125 M potassium chloride and 0.02 M Tris-hydrochloride buffer, pH 7.4) was incubated for 30 min at 37 °C, followed by the addition of 0.25 ml of 20% chilled TCA and 0.5 ml of 0.67% thiobarbituric acid to the above mixture and boiled at 100 °C for 10 min. The absorbance of the pink colored extract in n-butanol was measured at 532 nm using a spectrophotometer (Molecular Devices) supported by SOFTmax Pro 5.4. The amount of MDA formed was calculated from standard curve and was expressed in nanomoles of MDA formed/mg protein.
Cell migration inhibition assay
In order to assess the effect RS-AgNPs on the cellular migration as well as growth rate of inhibition, a wound healing assay was performed. The seeded SKOV3 monolayer was scratched with sterile edge of a plastic tip and washed with DPBS. Then the cells were treated with different concentrations (IC25, IC50, and IC75) of RS-AgNPs and incubated up to 24 h. The cells migration was analyzed by phase contrast images.
Immunoblot analysis
SKOV3 cells (1 × 106) exposed to different test concentrations of RS-AgNPs (IC25, IC50 and IC75 concentrations) were collected at 24 h, and re-suspended in SDS-PAGE sample buffer (4% SDS, 10% glycerol, 625 mM Tris-HCl, 200 mM β-mercaptoethanol and protease inhibitory cocktail, pH 6.8). The concentration of total proteins was determined using Bradford protein assay (Bio-Rad Laboratories, Hercules, CA). Twenty-Five micrograms of total protein from each concentration was resolved by using 12% SDS-PAGE followed by electrotransferring onto Immobilon-P transfer membrane (Millipore, Billerica, MA). The blots were incubated in blocking solution containing 5% fat free milk powder in TBST solution (10 mM Tris-HCl (pH 8.0), 0.15 M NaCl and 0.1% Tween 20) at 4 °C for an hour . They were then washed thrice with TBST solution and incubated with antibody (diluted 1/1000) against the specified protein overnight at 4 °C. Rabbit pAb :anti-caspase-3, anti-caspase-8and anti-caspase-9; rabbit antihuman: Bax and Bcl-2 and P53 were used. Blots were washed thrice with TBST solution and incubated for an hour with alkaline phosphatase-conjugated secondary antibodies (1:1500) against the interacting protein. Equal protein loading in each lane was routinely confirmed by incubating with actin antibodies (1:1000). 5-bromo-4-chloro-3-indolyl-phosphate (BCIP)/ nitro blue tetrazolium (NBT) substrates (1 ml) were used to develop the blots.
Statistical analysis for cell viability
The data obtained from MTT assays were subjected to linear regression analysis (using mean values of three independent assays). The regression lines were plotted for the best straight- line fit. The IC50 (50% inhibition of the cell viability) concentrations were calculated using the respective regression equation.
Results and discussion
The genus Rhynchosia is a member of the legume (pea) family Fabaceae which consists of about 80 species, found throughout the tropics and subtropics regions. Rhynchosia genus plants have high content of phenolic and flavonoids which plausibly play a key role in the formation and stabilization of AgNPs. R. suaveolens is an endemic medicinal plant found in Talakona forest range hills of Andhra Pradesh, South India. The aqueous extract of the R. suaveolens was used to synthesize AgNPs (RS-AgNPs). The present study reports the successful synthesis of RS-AgNPs and their anticancer activity and plausible mechanism study.
Biosynthesis of RS-AgNPs
The biological synthesis of RS-AgNPs using aqueous leaf extracts of R. suaveolens was evident by a variation in the color from colorless to brownish-yellow of oxidized silver nitrate solution in dark condition. Color change was an initial indication for the formation of RS-AgNPs [Citation10,Citation26–28]. Further the confirmation of the biosynthesis of RS-AgNPs was done by UV-Vis analysis.
UV-Vis analysis of RS-AgNPs
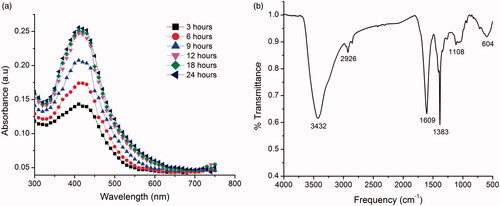
UV-Vis analysis of colloidal solution of RS-AgNPs showed the absorption peak at 426 nm. Furthermore, the bio-reduction of silver ions into RS-AgNPs in colloidal solution was monitored at different time intervals (3, 6, 9, 12, 18 and 24 h, respectively) using UV-Vis spectra (). The formation of the RS-AgNPs was started at 3 h and completed at 24 h. The peak was found to stable at 12, 18 and 24 h at the same wavelength of 426 nm. The absorption peak is due to the excitation of SPR which is characteristic of RS-AgNPs and consistent with earlier reports [Citation26–28]. To the best of our knowledge, this was the first report on the biosynthesis of RS-AgNPs using leaf extract of R. suaveolens. This suggests that the phytochemicals present in R. suaveolens leaves act as a reducing and capping agents which will be confirmed by FTIR analysis.
FTIR analysis of RS-AgNPs
FTIR spectrum showed peaks at 3432, 2926, 1608, 1383, 1106 and 604 cm−1 (), respectively. The peak at 3432 cm−1 can be assigned to O-H stretching vibration of polyphenolic compounds or flavonoids [Citation51]. The peak at the 2926 cm−1 could be due to C-H stretching of proteins [Citation12]. The sharp bands at 1608 and 1383 cm−1 correspond to amide I and II, respectively, arising due to carbonyl stretch in proteins. The peak at 1108 cm−1 developed due to C-N stretching. The peak at 604 cm−1 assigned to CH out of plane bending vibrations are substituted ethylene systems –CH = CH [Citation51]. FTIR spectrum clearly revealed the participation of flavonoids and proteins in the biosynthesis and stabilization of RS-AgNPs. Proteins coated/capped around the RS-AgNPs confer the stability for RS-AgNPs by preventing the agglomeration. The stability of the RS-AgNPs will be further confirmed by zeta potential measurement.
SEM, TEM and EDX studies of RS-AgNPs
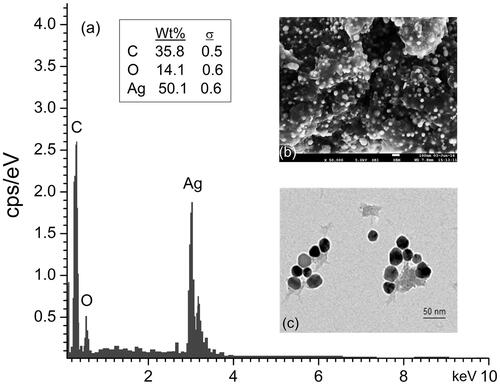
EDX spectrum showed the elemental composition of the RS-AgNPs (). EDX spectrum revealed that silver (50.1%) was the major constituent element compared to carbon (35.8%) and oxygen (14.1%), which confirms the formation of AgNPs. The SEM and TEM revealed the size and morphology of the RS-AgNPs. The SEM analysis revealed that the RS-AgNPs were spherical in shape (). To further confirm the morphological distribution and size, we performed TEM analysis of RS-AgNPs which proved the spherical shape, with the sizes ranging between 10–30 nm ().
XRD analysis of RS-AgNPs
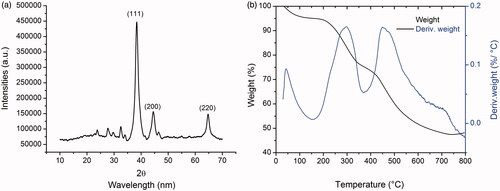
The crystalline nature of biogenic RS-AgNPs was confirmed by XRD analysis (). The XRD pattern of the RS-AgNPs mainly exhibited three characteristic Bragg’s diffraction peaks at 2θ values 38.14, 44.33 and 64.56, respectively corresponding to (111), (200) and (220) planes of the face centered cubic crystal (FCC) lattice of RS-AgNPs. All of these diffraction patterns are consistent with standard data file (JCPDS Card no 04-0783). It indicates that synthesized RS-AgNPs are pure crystalline in nature with FCC structure.
TGA and zeta potential measurement of RS-AgNPs
It has been observed from TGA plot that total dominant weight loss of 52.75% occurred in three steps in the temperature range of 0 to 700 °C while 47.25% of residual weight remained in the sample (). The initial step weight loss was observed at ∼100 to 200 °C and this can be attributed to evaporation of moisture content adsorbed on the surface of RS-AgNPs. Second step weight loss appeared in the temperature range of ∼300–500 °C and this could be due to desorption of biomolecules such as phenols and proteins present in the colloidal solution of RS-AgNPs and then third step is the minor one. Further the stability of the RS-AgNPs was evaluated by DLS analysis. DLS analysis showed that RS-AgNPs possess a zeta potential value of −28.1 mV. This higher negative surface charge indicates that the synthesized RS-AgNPs are prevented from agglomeration in the medium and monodispersed in nature which leads to long-term stability.
Owing to their ultra-small size and high stability, we state that these RS-AgNPs are suitable for efficient and safe transferring of secondary metabolites to the cancerous cells with minimum side effects.
Anticancer activity of RS-AgNPs
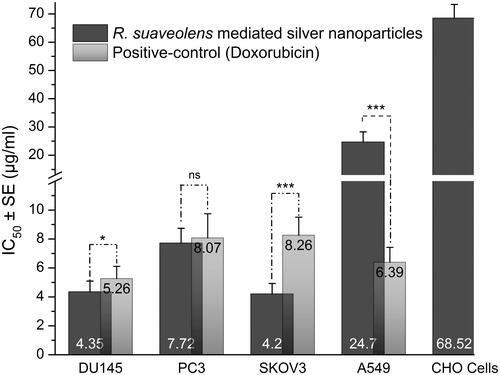
The anticancer activity of RS-AgNPs was determined by the MTT assay by measuring the percentage of cell viability (live cells) and IC50 values were calculated for each cell line (). FDA approved drug molecule doxorubicin was used as a positive control. RS-AgNPs exhibited concentration-dependent potential cytotoxicity against DU145 and PC3, SKOV3 and A549 cells. RS-AgNPs induced 50% cytotoxicity against DU14, PC-3, SKOV3 and A549 at the concentrations of 4.35, 7.72, 4.2 and 24.7 μg/mL, respectively. Lower IC50 value indicates highest inhibitory activity. Hence, RS-AgNPs were more cytotoxic against SKOV3 cells, followed by DU14, PC3 and A549. It is evident from the results that RS-AgNPs exhibit highest inhibition against SKOV3 cells with an IC50 value of 4.2 µg/mL. Nevertheless, these RS-AgNPs are 16-fold less toxic to most common mammalian cells, Chinese hamster ovary (CHO) cells. The results are supported by previous findings. Biogenic AgNPs from the leaf extract of Olax scandens exhibited potent cytotoxicity against A549, B16F10 (mouse melanoma) and MCF-7 (Human breast cancer), but showed negligible cytotoxicity towards normal cell lines including CHO, human umbilical vein endothelial cells (HUVEC) and rat cardiomyoblast (H9C2) cells [Citation10]. Biosynthesized AgNPs from Clerodendrum phlomidis exhibited potent cytotoxicity against HT29 human colorectal adenocarcinoma (HT29) and Ehrlich ascites carcinoma(EAC) cells [Citation52]. Biosynthesized AgNPs from Cibotium barometz and Chaenomeles sinensis showed anticancer activity against MCF-7 cells [Citation27,Citation28]. AgNPs synthesized with leaf extract of Sesbania grandiflora showed potent cytotoxicity against MCF-7 cells [Citation53]. Biogenic AgNPs from rhizome extract of Acorus calamus demonstrated to be cytotoxic against A431 carcinoma cells [Citation54].
Figure 4. MTT assay results confirming the in vitro cytotoxicity effect of Rs-AgNPs against the different cancerous cell lines for 24 h. Data were expressed as mean ± SE of three independent experiments. Significant difference from positive control values, *p < .05, **p < .01 and ***p < .001. ns: non-significant.
Morphological study of cancer cells treated withRS-AgNPs
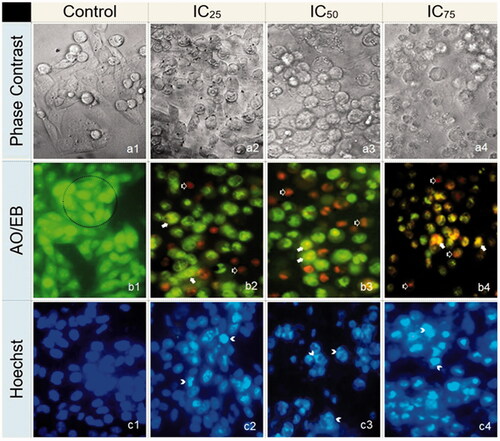
In the present study, several morphological characteristics, including the disruption of membrane integrity, decreased cell growth, cytoplasmic condensation and cell clumping were observed in SKOV3 cells treated with IC25, IC50 and IC75 concentrations of RS-AgNPs, whereas control cells remained active ()). Acridine orange/ethylene dibromide (AO/EB) fluorescent DNA binding dye indicated different DNA states in viable and apoptotic cells. Cells stained green represent viable cells, whereas yellow staining represents early apoptotic cells and reddish or orange staining represents late apoptotic cells. Cells treated with lower concentration (IC25) of RS-AgNPs showed early apoptosis and at higher concentration (IC75) showed late apoptotic features. Hoechst staining clearly indicated as an early apoptosis leading to the margination of chromatin into a horseshoe shaped structure followed by apoptotic blebbing on cell surface treated with IC25 and IC50. However, the cells treated with IC75 concentration exhibited destructive fragmentation of the nucleus with formation of apoptotic bodies.
Figure 5. The effect of IC25, IC50 and IC75 concentrations of RS-AgNPs on SKOV3 cells for 24 h exposure (400×). (a) Phase contrast microscopy, (b) AO/EB staining and (c) Hoechst staining. Intact cells in control (a1, b1 and c1). Cells in the circle of AO/EB staining represents viable cells (b1), white arrows represent early apoptotic (b2, b3 and b4) and black arrow represents late apoptotic cells (b2, b3 and b4). White arrow head in Hoechst staining indicates as an early and late apoptosis (c2, c3 and c4).
RS-AgNPs induce ROS generation and DNA damage
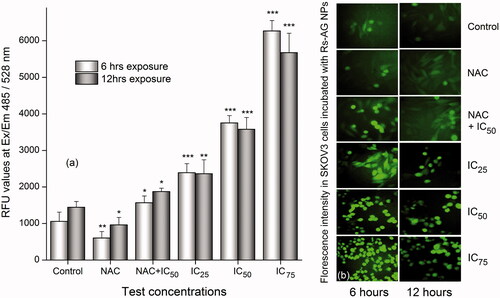
Different anticancer agents induce the production of ROS that could play a major role in the apoptotic signaling pathways. In this study, the generation of ROS in SKOV3 cells were analyzed using DCFH-DA 2, 7-dichlorodihydrofluorescein diacetate(DCFH-DA) a redox sensitive probe/dye which oxidizes DCFH to the 2',7'-dichlorofluorescin(DCF). It is evident from the results that the cells treated with different concentrations of RS-AgNPs (Control, IC25, IC50, and IC75) for six and 12 h has significantly enhanced the levels of ROS in a concentration-dependent manner (). The induction level was much higher at 6 h than the same at 12 h. To re-confirm the influence of RS-AgNPs on ROS levels, additionally cells were pre-treated with ROS scavenger, N-acetyl cysteine [NAC: 5 mM] for 2 h and exposed to IC50 concentration of Rs-AgNPs for 6 and 12 h, respectively. A significant reduction of ROS levels was noticed in the cells treated with NAC alone at six and 12 h. However, the combined effect of NAC + IC50 of RS-AgNPs has nullified the action of NAC and enhanced the ROS production after six and 12 h, which is comparatively less than the individual effect of Rs-AgNPs at similar concentrations (). Furthermore, the fluorescence microscopic studies have additionally demonstrated that the SKOV3 cells treated with RS-AgNPs induced the ROS levels in a concentration-dependent manner (). From the it was clear that SKOV3 cells treated with RS-AgNPs exhibited intense green fluorescence that controlled cells (without treatment). Further, the intensity of green fluorescence was increased with increase in the concentration of RS-AgNPs. The results clearly revealed that the enhanced apoptotic efficacy/anticancer efficiency of RS-AgNPs directly correlate with the elevated levels of ROS. A few recent studies suggested that the cytotoxic activity of the AgNPs is related to the generation of ROS inside the cells [Citation55,Citation56].
Figure 6. (a) Effect of RS-AgNPs on cellular ROS generation by spectrophotometer using H2DCF-DA as a fluorescent Probe for six and 12 h. Each value is the mean ± SE of three individual observations. Significant difference from control values, NS: non-significant. *p < .05; **p < .01 and ***p < .001 (t-test) and (b) The intracellular ROS was assessed by fluorescence microscopy and the representative images showed comparatively higher fluorescence intensity in SKOV3 cells incubated with RS-AgNPs than that of control at six and 12 h.
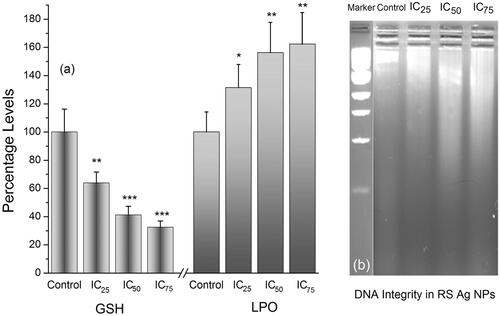
Lipid peroxidation (LPO) plays a significant role in the control of cell division and it is the manifestation of oxidative damage caused by ROS. GSH, a major non-protein free-radical scavenger, plays an important role in maintaining the cellular redox state and protects the cells from oxidative damages due to ROS [Citation47]. It is well known that the presence of ROS induces the oxidation of GSH to glutathione disulfide (GSSG). Increase in the concentration of RS-AgNPs decreases the GSH levels drastically which is due to enhanced ROS levels. Further increase in the concentration of RS-AgNPs elevates the lipid peroxidation levels (). Generation of ROS, decreased levels of GSH and increased levels of LPO might affect the DNA fragmentation in the cancer cells. The agarose gel electrophoresis results revealed that the treated cells at 24 h exhibited a characteristic DNA damage with concentration dependent manner. while untreated controls did not show any signs of DNA damage (). DNA damage is considered as one of the major causes for apoptosis. AgNPs execute well as cancer therapeutics because they can disrupt the mitochondrial respiratory chain, which induces the generation of ROS which in turn can induce DNA damage. Fragmentation of DNA was evident as one of the distinctive features of apoptotic process or cell death [Citation55–57]. Altogether these results showed that ROS elevation is crucial for Rs-AgNPs induced apoptosis.
Figure 7. (a) Effect of IC25, IC50 and IC75 concentrations of RS-AgNPs on intracellular GSH and LPO levels at 24 h. Each value is the mean ± SE of three individual observations. Significant difference from control values *indicates p < .05; **p < .01 and ***p < .001 (t-test) and (b) Analysis of the DNA integrity in RS-AgNPS treated SKOV3 cells by agarose gel electrophoresis. DNA from cells was extracted and electrophoresed through a 1.8% agarose gel and visualized by staining with ethidium bromide.
Molecular mechanism of apoptosis
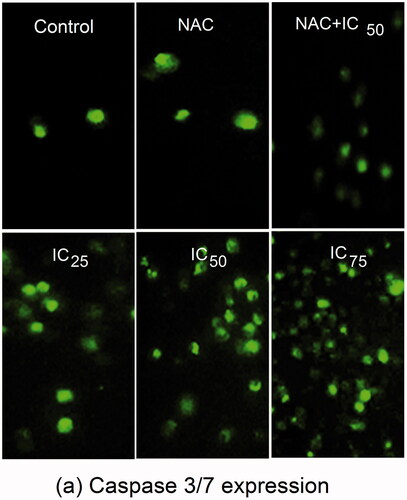
The caspase family members participated in triggering different types of cell death. After treatment with different concentrations (IC25, IC50, and IC75) of RS-AgNPs for 24 h, caspase-3/7 activities were directly monitored by the Cell Event caspase-3/7 green detection reagent, which is a nucleic acid binding dye that harbors the caspase-3/7 cleavage sequence, DEVD (Asp-Glu-Val-Asp) and is fluorescent after being cleaved and bound to DNA. The amount of fluorescence is directly proportional to caspase-3/7 activity. On microscopic observations, activated caspase-3/7 signals significantly increased in a concentration dependent manner after RS-AgNPs treatment (). A significant reduction of caspase-3/7 signals was noticed in the cells treated with NAC alone. However, the combined effect of NAC + IC50 of RS-AgNPs has nullified the action of NAC and enhanced the caspase-3/7 signals.
Figure 8. Fluorescence images showing an increase in expression of caspase 3/7 in SKOV3 cells treated with NAC, IC25, IC50, IC75 and NAC + IC50 concentrations of RS-AgNPs at 24 h, which showed a concentration dependent increase an expression of caspase 3/7.
The wound healing effects of RS-AgNPs on cultured SKOV3 cell monolayers were examined by using an in vitro wound healing assay. The cell-free wound gaps of SKOV3 monolayers healed quickly in control group. However, in the presence of RS-AgNPs, the closure of the wounded area was significantly reduced (). RS-AgNPs showed potent and concentration dependent inhibition on the healing of monolayer cells. At low concentration of the RS-AgNPs (IC25), fewer cells appeared in the wounded gap. Whereas, the number of cells reduced at median lethal concentration (IC50) and clear area (no cells) was observed at higher concentration (IC75). Moreover, some floating cells appeared in the wounded area, which shows that reduced healing of the wounded area is concentration dependent.
Figure 9. (a) Effect of RS-AgNPs in SKOV3 cell migration inhibition assay after 12 h. Cell migration inhibition was evident with the test concentrations that shown inhibition in a concentration dependent manner. (b) Western blot analysis of P53, Bax, Bcl-2, actin, caspases-3, -8, and -9, levels in SKOV3 cells after 24 h treatment with RS-AgNPs.
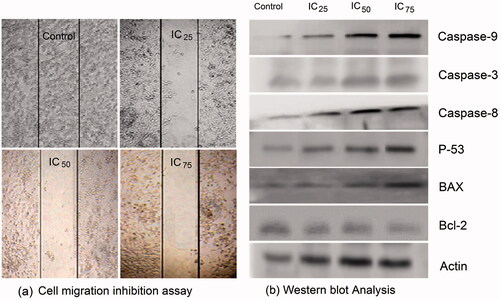
Apoptotic protein expression analysis was performed to examine apoptotic proteins (caspase -3, -8 and -9, p53 and BAX) and anti-apoptotic protein (Bcl-2) using immunoblot (western blot) analysis in SKOV3 cells exposed to IC25, IC50, and IC75 concentrations of RS-AgNPs for 24 h. The Bcl-2 family of proteins play a major role in the regulation of apoptosis. Expression levels of Bcl-2 protein were decreased with increased concentration of RS-AgNPs. The relative expression levels revealed that induction of apoptosis by RS-AgNPs was accompanied by a decrease in anti-apoptotic Bcl-2 with an increase in pro-apoptotic BAX.
The expression levels of caspase -3, -8 and -9, p53 and BAX proteins were increased with increase in concentration of RS-AgNPs. The results indicated up-regulation of p53, active caspase-3 and BAX proteins, down-regulation of Bcl-2 and stimulated caspases -8 and -9 to carry out the execution of apoptosis in RS-AgNPs treated SKOV3 cells compared with untreated cells (). The expression of actin used as loading control was also analyzed, the results suggests that RS-AgNPs induces apoptosis by oxidative stress, in which Bcl-2 playing an important role in mitochondrial outer membrane permeabilization and loss of mitochondrial membrane potential. Based on our findings, the possible mechanism of inhibition of Bcl-2 in SKOV3 cells by RS-AgNPs could be the induction of mitochondrial dysfunction and energy depletion in cancer cells in turn inducing the imbalance between oxidant and antioxidant levels in the cells.
We state that RS-AgNPs causes apoptosis of cancer cells by upregulating the proapoptotic proteins caspase -3, -8 and -9, p53 and BAX and by down regulating the Bcl-2.
Conclusions
The present study concerns with cost effective and environment friendly synthesis of RS-AgNPs using the aqueous leaf extract of Rhynchosia suaveolens. The synthesized RS-AgNPs were highly stable, ultra-small in size (10–30 nm) and crystalline in nature. Biognic RS-AgNPs showed concentration dependent anti-proliferative and pro-apoptotic activity against SKOV3, DU145, PC-3, and A549 cancer cell lines. The synthesized RS-AgNPs were highly stable and low cytotoxicity against the normal mammalian cell line CHO. The anticancer activity of RS-AgNPs was due to apoptosis, ROS generation and switching on apoptotic signaling pathways after DNA fragmentation. The results should support the use of RS-AgNPs for future therapeutic application in cancer therapy and other biomedical applications with which further studies needs to be carried out. RS-AgNPs anti-cancer activity is similar to the doxorubicin activity, so it is an effective anti-cancer agent or drug delivery system in near future.
Acknowledgements
We would like to thank Director, CSIR-Indian Institute of Chemical Technology for his constant encouragement throughout the study. Mr. Murali Satyanarayana Bethu is also thankful to Academy of Scientific and Innovative Research (AcSIR), New Delhi.
Disclosure statement
Authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
Additional information
Funding
References
- Kelly KL, Coronando E, Zhao LL, et al. The optical properties of metal nanoparticles: the influence of size, shape and dielectric environment. J Phys Chem B. 2002;107:668–677.
- El-Sayed MA. Some interesting properties of metals confined in time and nanometer space of different shapes. Acc Chem Res. 2001;34:257–264.
- Feiner LF. Nanoelectronics: crossing boundaries and borders. Nat Nanotechnol. 2006;1:91–92.
- Vilchis NAR, Sanchez MV, Camacho LMA, et al. Solvent less synthesis and optical properties of Au and Ag nanoparticles using Camellia sinensis extract. Mater Lett. 2008;62:3103–3105.
- Wang T, Kaempgen M, Nopphawan P, et al. Silver nanoparticle decorated carbon nanotubes as bifunctional gas-diffusion electrodes for zinc-air batteries. J Pow Sources. 2010;5:4350–4355.
- Campelo JM, Luna D, Luque R, et al. Sustainable preparation of supported metal nanoparticles and their applications in catalysis. Chem Sus Chem. 2009;2:18–45.
- Cobley CM, Skrabalak SE, Campbel DJ, et al. Shape controlled synthesis of silver nanoparticles for plasmonic and sensing applications. Plasmonics. 2009;4:171–179.
- Wei H, Li J, Wang Y, et al. Silver nanoparticles coatedwith adenine: preparation, self-assembly and application in surface-enhanced Raman scattering. Nanotechnology. 2007;18:610–615.
- Ravindran A, Chandran P, Khan S. Biofunctionalized silver nanoparticles: advances and prospects. Colloids Surf B Biointerfaces. 2013;105:342–352.
- Mukherjee S, Chowdhury D, Kotcherlakota R, et al. Potential theranostics application of bio-synthesized silver nanoparticles (4-in-1 system). Theranostics. 2014;4:316–335.
- De Jong WH, Borm PJ. Drug delivery and nanoparticles: applications and hazards. Int J Nanomedicine. 2008;3:133–149.
- Netala VR, Kotakadi VS, Domdi L, et al. Biogenic silver nanoparticles: efficient and effective antifungal agents. Appl Nanosci. 2016;6:475–484.
- Netala VR, Bethu MS, Bobbu PL, et al. Biogenesis of silver nanoparticles using endophytic fungus Pestalotiopsis microspora and evaluation of their antioxidant and anticancer activities. Int J Nanomedicine. 2016;11:5683–5696.
- Li Y, Kim YN, Lee EJ, et al. Synthesis of silver nanoparticles by electron irradiation of silver acetate. Nucl Instruments Methods Phys Res Sect B Beam Interact with Mater Atoms. 2006;251:425–428.
- Madhu KR, Lakshmeesha RB, Asha S, et al. Gamma radiation assisted biosynthesis of silver nanoparticles and their characterization. Adv Mater Lett. 2015;6:1088–1093.
- Pyatenko A, Shimokawa K, Yamaguchi M, et al. Synthesis of silver nanoparticles by laser ablation in pure water. Appl Phys A. 2004;79:803–806.
- Togashi T, Saito K, Matsuda Y, et al. Synthesis of water-dispersible silver nanoparticles by thermal decomposition of water-soluble silver oxalate precursors. J Nanosci Nanotechnol. 2014;14:6022–6027.
- Zhao X, Xia Y, Li Q, et al. Microwave-assisted synthesis of silver nanoparticles using sodium alginate and their antibacterial activity. Colloids Surf A Physicochem Eng Aspects. 2014;444:180–188.
- Byeon JH, Kim YW. A novel polyol method to synthesize colloidal silver nanoparticles by ultrasonic irradiation. Ultrason Sonochem. 2012;19:209–215.
- Hosseini M, Momeni MM. Silver nanoparticles dispersed in polyaniline matrixes coated on titanium substrate as a novel electrode for electro-oxidation of hydrazine. J Mater Sci. 2010;45:3304–3310.
- Frattine A, Pellegri N, Nicastro D, et al. Preparation of amine coated silver nanoparticles using triethylenetetraamine. Mater Chem Phys. 2005;94:148–152.
- Kutsenko AS, Granchak VM. Photochemical synthesis of silver nanoparticles in polyvinyl alcohol matrices. Theor Exp Chem. 2009;45:313–318.
- Sivalingam P, Antony JJ, Siva D, et al. Mangrove Streptomyces sp. BDUKAS10 as nanofactory for fabrication of bactericidal silver nanoparticles. Colloids Surf B Biointerfaces. 2012;98:12–17.
- Netala VR, Kotakadi VS, Bobbu PL, et al. Endophytic fungal isolate mediated biosynthesis of silver nanoparticles and their free radical scavenging activity and antimicrobial studies. 3 Biotech. 2016;6:1–9.
- Sastry M, Ahmad A, Islam Khan M, et al. Biosynthesis of metal nanoparticles using fungi and actinomycete. Curr Sci. 2003;85:162–170.
- Singh H, Du J, Yi TH. Green and rapid synthesis of silver nanoparticles using Borago officinalis leaf extract: anticancer and antibacterial activities. Artif Cells Nanomed Biotech. 2016;45:1310–1316.
- Wang D, Josus M, Wang C, et al. Green synthesis of gold and silver nanoparticles using aqueous extract of Cibotium barometz root. Artif Cells Nanomed Biotech. 2016;45:1548–1555.
- Keun HO, Veronika S, Josus M, et al. Biosynthesized gold and silver nanoparticles by aqueous fruit extract of Chaenomeles sinensis and screening of their biomedical activities. Artif Cells Nanomed Biotech. 2017. DOI:https://doi.org/10.1080/21691401.2017.1332636
- Dehghanizade S, Arasteh J, Mirzaie A. Green synthesis of silver nanoparticles using Anthemis atropatana extract: characterization and in vitro biological activities. Artif Cells Nanomed Biotech. 2017. DOI:https://doi.org/10.1080/21691401.2017.1304402
- Johnson P, Krishnan V, Loganathan C, et al. Rapid biosynthesis of Bauhinia variegata flower extract-mediated silver nanoparticles: an effective antioxidant scavenger and a-amylase inhibitor. Artif Cells Nanomed Biotech 2017. DOI:https://doi.org/10.1080/21691401.2017.1374283
- Pooja M, Sumitra C. Synthesis and characterization of silver nanoparticles using Caesalpinia pulcherrima flower extract and assessment of their in vitro antimicrobial, antioxidant, cytotoxic, and genotoxic activities. Artif Cells Nanomed Biotech. 2016;45:1556–1567.
- Hatem F, Li H, Dawood H, et al. Controlling Aedes albopictus and Culex pipiens pallens using silver nanoparticles synthesized from aqueous extract of Cassia fistula fruit pulp and its mode of action. Artif Cells Nanomed Biotech. 2017. DOI:https://doi.org/10.1080/21691401.2017.1329739
- Hina S, Juan D, Priyanka S, et al. Ecofriendly synthesis of silver and gold nanoparticles by Euphrasia officinalis leaf extract and its biomedical applications. Artif Cells Nanomed Biotech. 2017. DOI:https://doi.org/10.1080/21691401.2017.1362417
- Yue H, Priyanka S, Yeon JK, et al. Biological synthesis of gold and silver chloride nanoparticles by Glycyrrhiza uralensis and in vitro applications. Artif Cells Nanomed Biotech. 2017. DOI:https://doi.org/10.1080/21691401.2017.1307213
- Vijayan R, Joseph S, Mathew B. Indigofera tinctoria leaf extract mediated green synthesis of silver and gold nanoparticles and assessment of their anticancer, antimicrobial, antioxidant and catalytic properties. Artif Cells Nanomed Biotech. 2017. DOI:https://doi.org/10.1080/21691401.2017.1345930
- Pavithra BV, Ragavendran C, Murugan N, et al. Ipomoea batatas (Convolvulaceae)-mediated synthesis of silver nanoparticles for controlling mosquito vectors of Aedes albopictus, Anopheles stephensi, and Culex quinquefasciatus (Diptera:Culicidae). Artif Cells Nanomed Biotech. 2016;45:1568–1580.
- Geeta A, Kumari RM, Gupta N, et al. Green synthesis of silver nanoparticles using Prosopis juliflora bark extract: reaction optimization, antimicrobial and catalytic activities. Artif Cells Nanomed Biotech. 2017. DOI:https://doi.org/10.1080/21691401.2017.1354302
- Rijuta GS, Shin SH, Gopalakrishnan K, et al. Exploiting antidiabetic activity of silver nanoparticles synthesized using Punica granatum leaves and anticancer potential against human liver cancer cells (HepG2). Artif Cells Nanomed Biotech. 2017. DOI:https://doi.org/10.1080/21691401.2017.1337031
- Mori Y, Ono T, Miyahira Y, et al. Antiviral activity of silver nanoparticle/chitosan composites against H1N1 influenza A virus. Nanoscale Res Lett. 2013;8:93.
- Wong KKY, Cheung SOF, Huang L, et al. Further evidence of the anti-inflammatory effects of silver nanoparticles. Chem Med Chem. 2009;4:1129–1135.
- Gunasekaran T, Nigusse T, Dhanaraju MD. Silver nanoparticles as real topical bullets for wound healing. J Am Coll Clin Wound Spec. 2011;3:82–96.
- Baharara J, Namvar F, Mousavi M, et al. Anti-angiogenesis effect of biogenic silver nanoparticles synthesized Using Saliva officinalis on chick chorioalantoic membrane (CAM). Molecules. 2014;19:13498–13508.
- Shi J, Kantoff PW, Wooster R, et al. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2016;17:20–37.
- Aluru R, Duvvuri G, Netala VR, et al. Structure elucidation and antioxidant activity of the phenolic compounds from Rhynchosia suaveolens. Nat Prod Comm. 2015;10:609–611.
- Raveendran P, Fu J, Wallen SL. A simple and “green” method for the synthesis of Au, Ag, and Au–Ag alloy nanoparticles. Green Chem. 2006;8:34.
- Ankireddy K, Iskander M, Vunnam S, et al. Thermal analysis of silver nanoparticles for flexible printed antenna fabrication. J Appl Phys. 2013;114:124303.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63.
- Wan CP, Myung E, Lau BHS. An automated micro-fluorometric assay for monitoring oxidative burst activity of phagocytes. J Immunol Methods.1993;159:131–138.
- Jollow D, Mitchell JR, Zampaglione N, et al. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11:151–169.
- Utley HG, Bernheim F, Hochstein P. Effect of sulfhydryl reagents on peroxidation in microsomes. Arch Biochem Biophys. 1967;118:29–32.
- Daisy P, Saipriya K. Biochemical analysis of Cassia fistula aqueous extract and phytochemically synthesized gold nanoparticles as hypoglycemic treatment for diabetes mellitus. Int J Nanomedicine. 2012;7:1189–1202.
- Sriranjani R, Srinithya B, Vellingiri V, et al. Silver nanoparticle synthesis using Clerodendrum phlomidis leaf extract and preliminary investigation of its antioxidant and anticancer activities. J Mol Liquids. 2016;220:926–930.
- Das J, Paul Das M, Velusamy P. Sesbania grandiflora leaf extract mediated green synthesis of antibacterial silver nanoparticles against selected human pathogens. Spectrochim Acta - Part A Mol Biomol Spectrosc. 2013;104:265–270.
- Nayak D, Pradhan S, Ashe S, et al. Biologically synthesised silver nanoparticles from three diverse family of plant extracts and their anticancer activity against epidermoid A431 carcinoma. J Colloid Interface Sci. 2015;457:329–338.
- Franco-Molina M, Mendoza-Gamboa E, Sierra-Rivera C, et al. Antitumor activity of colloidal silver on MCF-7 human breast cancer cells. J Exp Clin Cancer Res. 2010;29:148.
- Kovács D, Igaz N, Keskeny C, et al. Silver nanoparticles defeat p53-positive and p53-negative osteosarcoma cells by triggering mitochondrial stress and apoptosis. Sci Rep. 2016;6:27902.
- Castilla J, Saá P, Hetz C, et al. In vitro generation of infectious scrapie prions. Cell. 2005;121:195–206.