Abstract
Tumor invasion is considered a major promoter in the initiation of tumor metastasis, which is supposed to cause most cancer-related deaths. In the present study, octreotide (OCT)-modified daunorubicin plus dihydroartemisinin liposomes were developed and characterized. Evaluations were undertaken on breast cancer MDA-MB-435S cells and MDA-MB-435S xenografts nude mice. The liposomes were ∼100 nm in size with a narrow polydispersity index. In vitro results showed that the OCT-modified daunorubicin plus dihydroartemisinin liposomes could enhance cytotoxicity and cellular uptake by OCT-SSTRs (somatostatin receptors)-mediated active targeting, block on tumor cell wound healing and migration by incorporating dihydroartemisinin. The action mechanism might be related to regulations on E-cadherin, α5β1-integrin, TGF-β1, VEGF and MMP2/9 in breast cancer cells. In vivo, the liposomes displayed a prolonged circulating time, more accumulation in tumor location, and a robust overall antitumor efficacy with no obvious toxicity at the test dose in MDA-MB-435S xenograft mice. In conclusion, the OCT-modified daunorubicin plus dihydroartemisinin liposomes could prevent breast cancer invasion, hence providing a possible strategy for treatment of metastatic breast cancer.
Introduction
Breast cancer is one of the most commonly diagnosed cancers, and it remains the leading cancer-related death of women worldwide [Citation1,Citation2]. Currently, comprehensive treatments for breast cancer include surgery, radiotherapy, immunotherapy and chemotherapy. Although antitumor treatment of early-stage breast cancer shows some positive outcomes, most breast cancer deaths are due to tumor metastasis [Citation3]. Metastasis is the main biological characteristic of malignant tumors, which transforms a local primary tumor into a systemic, metastatic, and life-threatening malignant disease [Citation4]. Once become metastatic, breast cancers turn incurable with a 5-year survival rate reduced to ∼26% [Citation5]. Molecular mechanisms involved in tumor metastasis are complicated and remained to be fully investigated. So far, increasing studies have implicated epithelial-mesenchymal transition (EMT) as a major promoter in process of tumor metastasis [Citation6,Citation7].
EMT is a specific physiological process in which tumor cells lose epithelial characteristics and acquire mesenchymal phenotypes. EMT is often activated during tumor cell migration, invasion, metastatic dissemination and chemotherapy resistance [Citation8–10]. EMT could increase tumor cell invasion which would further render the cells escape from initial sites to other organs. Morphological and behavioral changes during the EMT process are usually accompanied by substantial gene expression changes [Citation11,Citation12]. A series of protein factors have been identified as associated proteins in the initiation and execution of EMT, and also in the tumor invasion and metastatic process, such as transforming growth factor-β1 (TGF-β1), epithelial markers of E-cadherin, matrix metalloproteinases (MMPs) and other factors that regulate tumor cell migration and invasion [Citation13–16]. Hence, if the protein factors regulating the EMT were regulated, the antitumor effects of chemotherapeutic agents could be enhanced.
Daunorubicin, known as a systemic chemotherapy agent, is one of the most effective cytotoxic drugs in treating solid cancers including breast cancer [Citation17]. Daunorubicin action effects are mainly attributed to DNA replication suppression, cell apoptosis induction and generating free radicals [Citation18]. However, the major obstacles of daunorubicin in clinical cancer therapy are the poor tumor selectivity and severe side effects [Citation19]. Originally isolated from the traditional Chinese medicinal plant Artemisia annua, dihydroartemisinin is recommended as a first-line antimalarial drug. Recent studies have revealed that the dihydroartemisinin shows antitumor efficacy and selective cytotoxicity to various human tumors including breast cancer [Citation20,Citation21]. The antitumor effects of dihydroartemisinin are related to inducing apoptosis, modulating tumor-related genes, blocking angiogenesis and inhibiting metastasis [Citation22]. Moreover, the dihydroartemisinin also exhibits remarkable inhibiting effect on the EMT [Citation23]. Octreotide (OCT) is an octapeptide analog of somatostatin (SST) that has a higher affinity with somatostatin receptors (SSTRs), which are widely expressed on cell membranes in various tumors including breast cancer [Citation24]. Therefore, OCT may be used as a specific-targeting ligand to enhance the delivery of antitumor drugs into tumor cells through SSTRs-mediated endocytosis.
In the present study, a kind of PEGylated liposomes containing daunorubicin and dihydroartemisinin with OCT modification was prepared for treating breast cancer. In the targeting liposomes, OCT was modified on the liposomal surface for enhancing tumor cell targeting, dihydroartemisinin was inserted into the lipid bilayer for inhibiting tumor invasion, and daunorubicin was encapsulated into the liposomes by ammonium sulfate gradient method as a cytotoxic agent. Antitumor efficacy of the liposomes was evaluated both in vitro and in vivo, and the involved mechanism of anti-metastasis was also investigated.
Materials and methods
Reagents, cell lines and animals
Daunorubicin hydrochloride and dihydroartemisinin were obtained from Meilun Biotechnology Co., Ltd. (Dalian, China). OCT was synthesized by Shanghai Apeptide Co., Ltd. (Shanghai, China). Egg yolk phosphatidylcholine (EPC) and cholesterol were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL). Polyethylene glycol-distearoyl phosphatidylethanolamine (DSPE-PEG2000) and DSPE-PEG2000-NHS were obtained from NOF Corporation (Tokyo, Japan). Hoechst 33258 and 1,1-dioctadecyl-3,3,3,3-tetramethylindotricarbocyanine iodide (DiR) were purchased from Nanjing Kaiji Biological Technology Development Co., Ltd. (Nanjing, China). Other chemicals were analytical or of chromatographic grade.
Human breast cancer cell line MCF-7 and MDA-MB-435S were obtained from Chinese Academy of Medical Sciences & Peking Union Medical College. Fetal bovine serum (FBS), cell culture medium RPMI-1640, Dulbecco’s Modified Eagle Medium (DMEM) and antibiotics were purchased from GIBCO (Billings, MT). MCF-7 and MDA-MB-435S cells were maintained in RPMI-1640 medium and DMEM medium supplemented with 10% FBS, respectively, with 100 U/mL penicillin and 100 μg/mL streptomycin under an atmosphere of 5% CO2 at 37 °C.
BALB/c nude mice (16–20 g) were obtained from Peking University Experimental Animal Center (Beijing, China). All procedures were performed according to guidelines of the Institutional Authority for Laboratory Animal Care of Peking University.
Synthesis of targeting molecules
OCT was conjugated with terminal NHS-activated DSPE-PEG2000 through a nucleophilic substitution reaction [Citation25]. Briefly, DSPE-PEG2000-NHS was incubated in dimethyl formamide (DMF) with OCT at 1:1 molar ratio, followed by adjusting pH to 10.0 with triethylamine. Reaction lasted 24 h at room temperature under consecutively moderate stirring. Finally, the mixture was dialyzed (molecular weight cut-off 3500 Da) against deionized water for 48 h to remove the unconjugated peptide, followed by lyophilization, and stored at −20 °C until used. The product was characterized by a matrix-assisted laser desorption/ionization time-of-flight mass spectrum (MALDI-TOF-MS, Auto flex speed, Bruker, Germany).
Preparation of liposomes
OCT-modified daunorubicin plus dihydroartemisinin liposomes were prepared with EPC/cholesterol/DSPE-PEG2000/DSPE-PEG2000-OCT/dihydroartemisinin at a molar ratio of 60/40/2/3/20 according to our previous report [Citation26]. Firstly, lipids and drugs were dissolved in chloroform in a pear-shaped flask and evaporated at 40 °C on a rotary evaporator under reduced pressure. The dried lipid film was then hydrated with 250 mM ammonium sulfate and sonicated. External buffer was exchanged by dialyzing through a dialysis tubing (MW cut-off 12,000–14,000 Da) equilibrated with phosphate-buffered saline (137 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4 and 2 mM KH2PO4, PBS pH 7.4). Then, daunorubicin was encapsulated into above liposomes by an ammonium sulfate gradient. Briefly, daunorubicin was added to liposomes at a lipids–drug ratio of 20:1 (w/w) and incubated for 20 min at 40 °C with gentle shaking.
The preparation processes of daunorubicin plus dihydroartemisinin liposomes, daunorubicin liposomes and blank liposomes were the same as described above, except for without DSPE-PEG2000-OCT, dihydroartemisinin and DSPE-PEG2000-OCT and all drugs, respectively. The DiR loaded liposomes were prepared in accordance with the procedure but replacing the drug with fluorescent probe (lipids:DiR, 200:1, w/w).
Characterization of liposomes
Transmission electronic microscopy (TEM, Tecnai G2 20ST, FEI Co., Tokyo, Japan) and atomic force microscopy (AFM, SPI3800N serie SPA-400, NSK Ltd., Tokyo, Japan) were used to characterize morphology of the liposomes. The particle size distribution, polydispersity and zeta potential of the liposomes were determined by a Nano Series Zen 4003 Zetasizer (Malvern Instruments Ltd., Malvern, UK).
For encapsulation efficiency measurement, 0.5 ml liposome suspension was passed through a Sephadex G-50 gel-filtration column, and the contents of daunorubicin and dihydroartemisinin were measured by a high-performance liquid chromatography (HPLC, Agilent Technologies Inc. Cotati, CA) at 233 and 210 nm, respectively. Mobile phase was acetonitrile to 0.02 mol/l sodium dihydrogen phosphate (32:68, volume ratio) for daunorubicin, and acetonitrile to water (60:40 volume ratio) for dihydroartemisinin, respectively. The encapsulation efficiency was calculated as follow: encapsulation efficiency % = (Ae/Atotal) × 100%, where Ae represents the amount of encapsulated drug after eluting, and Atotal is the total amount of drug before eluting.
Cytotoxic effects
Cytotoxicity was performed with both free drugs and drug liposomes using a sulforhodamine B (SRB) staining method [Citation27]. Briefly, MCF-7 and MDA-MB-435S cells were seeded in 96-well plates at a density of 6 × 103 cells/well. After incubation for 24 h, fresh medium containing serial concentrations of various drug formulations was added into the plate well, including free daunorubicin (0–2.5 μM), free dihydroartemisinin (0–25 μM), various concentrations of free daunorubicin (0–2.5 μM) plus a fixed concentration of free dihydroartemisinin (2.5, 5 or 25 μM). In liposome treatment groups, the cells were treated with daunorubicin liposomes, daunorubicin plus dihydroartemisinin liposomes, OCT-modified daunorubicin plus dihydroartemisinin liposomes, respectively. The final concentration of daunorubicin was in the range of 0–10 μM, and blank liposomes was used as a control group. After 48 h treatment, culture medium was removed, 200 μL ice-cold 10% trichloroacetic acid was added to each well for 1 h at 4 °C, followed by stained with 0.4% SRB for 20 min. Then, the unbound dye was removed by 1% acetic acid water solution, and the dye formed by living cells were dissolved in 100 μL Tris base solution. The results were measured using a microplate reader (HBS-1096A, DeTie, Nanjing, China) at 540 nm wavelength. The relative cell survival in comparison with that of the control cells was calculated according to the following formula: cell survival%=AT/AC×100%. Where, AT is the absorbance of treated cells, and AC is the absorbance of control (untreated) cells. The 50% inhibitory concentration (IC50) was calculated from semilogarithmic dose-response plots. Experiments were repeated in triplicate, and data were presented as mean ± standard deviation (SD).
In vitro cellular uptake studies
Flow cytometry analysis
Flow cytometry analysis was performed on the two cell lines (MCF-7 and MDA-MB-435S cells) to observe cell binding and uptake of the liposomes. For each cell line, 2 × 105 cells were cultured in six-well plates and incubated at 37 °C for 24 h to allow cell attachment. Then, the liposomes with a final concentration of 5 μM daunorubicin were incubated with the cells at 37 °C for 4 h. After removal of the medium, cells were washed three times with PBS, then harvested by trypsinization and centrifuged at 1000 rpm for 5 min, and re-suspended in 300 μL PBS and examined using a FACScan flow cytometry (BD Biosciences, Franklin Lakes, NJ).
Fluorescent microscopy analysis
A fluorescent microscope was carried out to further confirm the cellular uptake of SSTRs high expression cell line MDA-MB-435S towards the liposomes. Briefly, MDA-MB-435S cells were cultured on coverslips in 24-well plates for 24 h until total adhesion was achieved. Then, free daunorubicin, daunorubicin liposomes and OCT-modified daunorubicin plus dihydroartemisinin liposomes (final drug concentration 5 μM daunorubicin) were added to the culture medium and incubated at 37 °C for another 4 h. The cells were then washed with cold PBS, fixed with 4% paraformaldehyde for 20 min, followed by cell nuclei staining with Hoechst 33258 for 20 min. Finally, the cells were rinsed another three times with PBS and imaged using a fluorescence microscope (Ti-Eclipse, Nikon, Tokyo, Japan).
Blocking effects on tumor cell wound healing and migration in vitro
Wound-healing assay
MDA-MB-435S cells were seeded in 10% FBS medium on six-well plates at a density of 5 × 105 cells/well and grown overnight to confluence. Three parallel lines were drawn on the monolayer cells by scratching with 10 μL sterile pipette tips vertically. The cells were washed thrice with PBS to remove floating cells and changed with fresh medium. Then, various formulations were added for 24 h. The wounded areas were photographed at the beginning and 24 h following scraping using an inverted microscope (XDS-1B, Chongqing photoelectric Co., Ltd., Chongqing, China). Wound healing was measured as the distance migrated by the leading edge of the wound at each time point. The experiments were performed in triplicate.
Cell migration assay
The migration assay was performed by a transwell assay kit to test the migratory ability of MDA-MB-435S cells. Cells at a density of 1 × 105 cells/well were placed in 200 μL serum-free medium in the presence or absence of various liposomes in the upper chamber of insert at a concentration of 10 μM daunorubicin, and 600 μL complete medium was added into the lower wells and incubated at 37 °C for 12 h. After migration, non-migrating cells on the upper side of the filter were removed with cotton swabs, the migrated cells in the lower surface of the membrane were fixed in 10% paraformaldehyde for 10 min, followed by stained with 1% crystal violet and washed with PBS. Migrated cells were counted using an inverted microscope. Five random fields were counted for each sample.
Regulating effects on the associated proteins
E-cadherin, α5β1-integrin, TGF-β1, VEGF, MMP2 and MMP9 were included in the evaluation on the breast cancer EMT. Briefly, MDA-MB-435S cells were treated with daunorubicin liposomes, daunorubicin plus dihydroartemisinin liposomes and OCT-modified daunorubicin plus dihydroartemisinin liposomes at a concentration of 10 µM daunorubicin, respectively. Culture medium was used as blank control. After incubation for 12 h, the cells were harvested and lysed. Cell lysates were centrifuged at 10,000 rpm at 4 °C for 10 min. The total protein concentrations were measured at 540 nm by a bicinchoninic acid kit. The resulting protein samples were operated according to manufacturer instructions of the kits, and were subsequently analyzed using a microplate reader at 450 nm. The protein expression ratio was calculated using the following formula: protein expression ratio = (A450 nm for treated cells/A540 nm for treated cells)/(A450 nm for control cells/A540 nm for control cells), where A450 nm and A540 nm represent the absorbance values. Each assay was repeated in triplicate.
In vivo imaging in tumor-bearing mice
To monitor the biodistribution and tumor-targeting ability of OCT-modified liposomes in tumor-bearing mice, a noninvasive near-infrared optical-imaging approach was adopted. The in vivo imaging experiment was performed using female BALB/c nude mice and an armpit tumor model. The tumor model was established by subcutaneously injecting MDA-MB-435S cells (5 × 106 cells in 200 μL PBS) into the right shoulder blade. Mice were randomly divided into four groups (n = 3). When the tumors grew to ∼400 mm3, the mice were injected with normal saline, free DiR, DiR liposomes, OCT-modified DiR plus dihydroartemisinin liposomes via tail vein (1 μg DiR each), respectively. Fluorescence and X-ray scans were performed at predetermined time points using an in vivo image system (Carestream, Fx Pro, Rochester, NY).
Antitumor efficacy in tumor-bearing mice
The subcutaneous tumor xenograft models were established as indicated in In vivo imaging in tumor-bearing mice section and used for in vivo antitumor efficacy experiment. Treatments initiated when the tumor volume reached a size of 100 mm3. Mice were randomly divided into five groups (n = 6), namely, normal saline group, free daunorubicin group, daunorubicin liposome group, daunorubicin plus dihydroartemisinin liposome group and OCT-modified daunorubicin plus dihydroartemisinin liposome group. The drug were injected via the tail vein at a dose of 5 mg daunorubicin/kg on Day 17, 19, 21, 23 and 25 post-incubation [Citation28]. Tumor sizes were monitored daily by measuring with a digital caliper, and the tumor volume (V) was calculated using the formula: V = L×W2×0.5, where L and W are the maximum and minor axes of the tumor, respectively. Tumor volume ratio = Vn/V, where Vn represents the tumor volume calculated at day n and V represents the tumor volume measured at Day 17. Throughout the treatment, mice were weighed every other day to assess the toxicity of the daunorubicin formulations.
Histological analysis
At the end of treatment, mice sacrificed by cervical dislocation operation, and major organs including hearts, livers, spleens, lungs and kidneys in the mice were harvested, fixed in 10% paraformaldehyde, embedded in paraffin, and then cut into slices measuring 5 μm in thickness, followed by stained with H&E and observed under a digital microscope.
Statistics
Data analysis were carried out using a SPSS 17.0 software (SPSS Inc., Chicago, IL). Analysis of variance (ANOVA) was used to determine the significances among groups, and a value of p < .05 was considered to be significant difference.
Results
Synthesis and characterization of DSPE-PEG2000-OCT
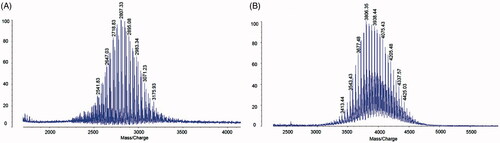
DSPE-PEG2000-OCT was synthesized by the conjugation of DSPE-PEG2000-NHS to OCT. The MALDI-TOF-MS assay showed that the peak was right-shifted after the conjugation. The average masses of DSPE-PEG2000-NHS and DSPE-PEG2000-OCT were m/z 2807.33 () and m/z 3806.36 (). The difference in the mass of DSPE-PEG2000-NHS and DSPE-PEG2000-OCT was consistent with the mass of polypeptide chain OCT.
Characterization of liposomes
Daunorubicin-loaded liposomes were detected to be round in shape with a smooth surface under AFM observation, and were identified as spherical vesicles under TEM observation (). The mean particle sizes of the daunorubicin-loaded liposomes analyzed by dynamic light scattering were ∼100 nm with a polydispersity index <0.2. These results were in according with those observed by AFM and TEM, indicating that the liposomes owned small particle sizes with a narrow size distribution ( and ). The encapsulation efficiencies of daunorubicin and dihydroartemisinin in all the liposomes were about 90% (). Zeta potentials were 0.64 ± 0.11, 2.34 ± 0.55 and 1.84 ± 0.54 mV for daunorubicin liposomes, daunorubicin plus dihydroartemisinin liposomes, OCT-modified daunorubicin plus dihydroartemisinin liposomes, respectively. The zeta potentials changed slightly after the modification of OCT.
Figure 2. Characterization of OCT-modified daunorubicin plus dihydroartemisinin liposomes. (A) A schematic representation of OCT-modified daunorubicin plus dihydroartemisinin liposomes, (B) AFM image of OCT-modified daunorubicin plus dihydroartemisinin liposomes, (C) size distribution of OCT-modified daunorubicin plus dihydroartemisinin liposomes, (D) TEM of OCT-modified daunorubicin plus dihydroartemisinin liposomes.
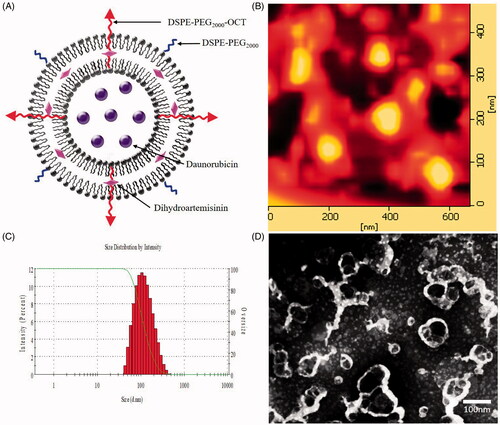
Table 1. Characterization of liposomes.
Cytotoxic effects
The cytotoxic effects of free drug and liposomal formulations on MCF-7 cells and MDA-MB-435S cells are graphically represented in . The IC50 values in daunorubicin-loaded formulations are also showed in . Daunorubicin as a chemotherapeutic agent showed an obvious inhibitory effect and free dihydroartemisinin alone owned a minimal cytotoxic effect on these two cell lines. Free daunorubicin combined with certain concentrations of free dihydroartemisinin (2.5, 5 or 25 μM), exhibited an enhanced cytotoxic effect showing a dihydroartemisinin dose-dependent manner (). The OCT-modified daunorubicin plus dihydroartemisinin liposomes showed the strongest inhibitory effects on MCF-7 cells and MDA-MB-435S cells among all liposomal formulations (). Blank-targeting liposomes exhibited minimal inhibitory effects. The IC50 values of OCT-modified daunorubicin plus dihydroartemisinin liposomes were 0.64 ± 0.03 μM against MCF-7 cells and 0.44 ± 0.03 μM against MDA-MB-435S cells, respectively ().
Figure 3. Inhibitory effects to breast cancer cells after treatment with OCT-modified daunorubicin plus dihydroartemisinin liposomes. p < .05; 1, versus free dihydroartemisinin; 2, versus free daunorubicin; 3, versus free daunorubicin plus 2.5 μM dihydroartemisinin liposomes; 4, versus free daunorubicin plus 5 μM dihydroartemisinin liposomes; a, versus blank liposomes; b, versus daunorubicin liposomes; c, versus daunorubicin plus dihydroartemisinin liposomes. Data are presented as mean ± SD (n = 6).
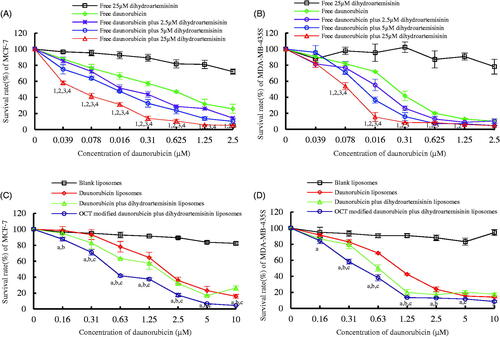
Table 2. IC50 values of daunorubicin (μM) against breast cancer cells.
Cellular uptake and targeting effects
Flow cytometry was used to quantify the total daunorubicin uptake by MCF-7 cells and MDA-MB-435S cells for different daunorubicin formulations. As shown in and , the intracellular daunorubicin in the OCT-modified daunorubicin plus dihydroartemisinin liposomes was higher than that of the daunorubicin liposomes and daunorubicin plus dihydroartemisinin liposomes. In addition, the highest uptake was found with free daunorubicin group due to a direct diffusion into the cells.
Figure 4. Cellular uptake and targeting effects after incubation with varying formulations. (A) Cellular uptake of MCF-7 cells, (B) cellular uptake of MDA-MB-435S cells, (C) fluorescence microscopy images of MDA-MB-435S cells incubated with varying formulations. (a) Blank control; (b) daunorubicin liposomes; (c) OCT-modified daunorubicin plus dihydroartemisinin liposomes; (d) free daunorubicin.
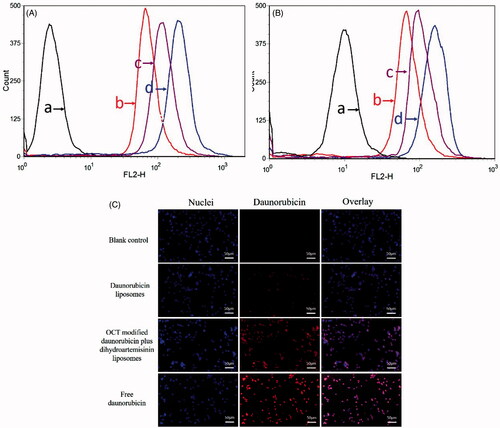
The images acquired by fluorescent microscopy confirmed the flow cytometry data (). Free daunorubicin exhibited the highest fluorescence intensities in all of the daunorubicin formulations, and the daunorubicin fluorescence was found to be almost overlapped with the Hoechst 33258 stained cell nuclei. For OCT-modified daunorubicin plus dihydroartemisinin liposomes, the images showed a higher intense fluorescence of daunorubicin in the cells than those of general liposome groups in MDA-MB-435S cells.
Blocking effects on tumor cell wound healing and migration in vitro
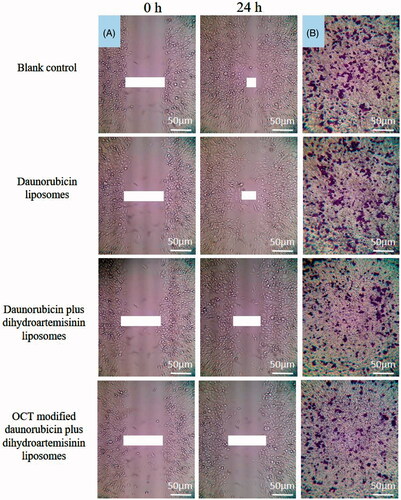
Dihydroartemisinin has been reported to play an important role on the metastasis of tumor cells. Therefore, the effect of dihydroartemisinin on MDA-MB-435S cells metastasis in vitro was investigated through the invasion and migration assays. As determined by the wound-healing assay, OCT-modified daunorubicin plus dihydroartemisinin liposomes showed the strongest inhibition on MDA-MB-435S cell invasion and the lowest cell fusion capacity among the three liposome groups (). The migration assay also revealed the similar tendency, the cell migration was strongly inhibited by the OCT-modified daunorubicin plus dihydroartemisinin liposomes ().
Regulating effects on the associated proteins
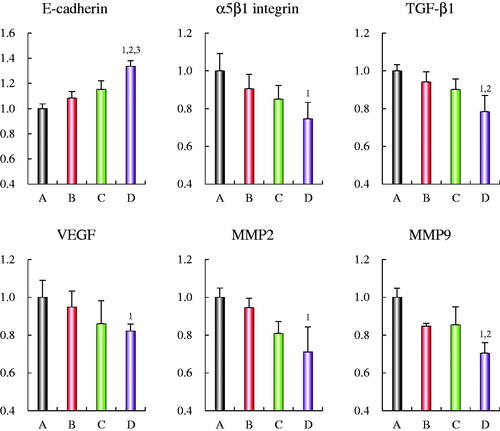
depicts the regulatory effects on associated proteins of the tumor invasion in MDA-MB-435S cells after treatment with varying formulations. After addition of OCT-modified daunorubicin plus dihydroartemisinin liposomes, the expression ratios of α5β1-integrin, TGF-β1, VEGF, MMP2 and MMP9 were evidently lower than other groups. An opposite trend was seen on E-cadherin expression ratio as compared with the control formulations. As for OCT-modified daunorubicin plus dihydroartemisinin liposomes, the expression ratios of E-cadherin, α5β1-integrin, TGF-β1, VEGF, MMP2 and MMP9 were 1.33 ± 0.04, 0.75 ± 0.09, 0.78 ± 0.09, 0.82 ± 0.04, 0.71 ± 0.13, and 0.71 ± 0.06, respectively.
Figure 6. Regulating effects on the protein indicators of the EMT in MDA-MB-435S cells after treatments with OCT-modified daunorubicin plus dihydroartemisinin liposomes. (A) Blank control; (B) daunorubicin liposomes; (C) daunorubicin plus dihydroartemisinin liposomes; (D) OCT-modified daunorubicin plus dihydroartemisinin liposomes. p < .05; 1, versus A; 2, versus B; 3, versus C. Data are presented as mean ± SD (n = 4).
In vivo imaging in tumor-bearing mice
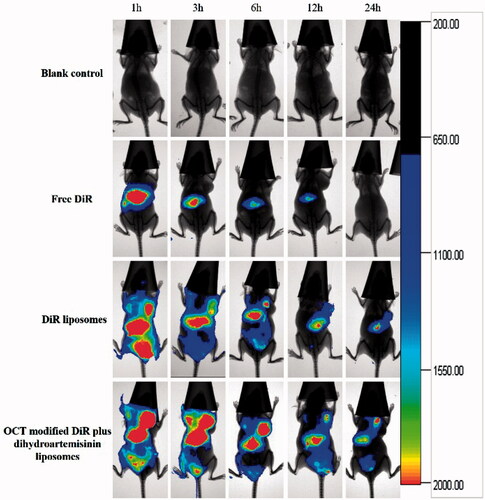
In vivo fluorescent imaging was performed to further investigate the biodistribution of OCT-modified liposomes in tumor-bearing mice. shows the representative images of tumor-bearing nude mice after administered free DiR, DiR liposomes and OCT-modified DiR plus dihydroartemisinin liposomes, respectively. Results showed that free DiR mainly distributed in liver during the whole test, and little signal was detected in tumor tissues. For DiR liposome groups, the fluorescence accumulation was found in tumors at 1 h. With the extension of time, OCT-modified DiR plus dihydroartemisinin liposomes has a stronger fluorescence signal in tumor tissues than those of DiR liposome group at all observed time-points. In addition, the modification of OCT on liposomes increased drug accumulation at tumor sites as compared with liposomes without OCT.
Antitumor efficacy in tumor-bearing mice
To determine the antitumor activity of the developed liposomes in vivo, the effects of these liposomes on tumor-bearing mice were investigated. displays the tumor volumes of each group during the 10-day treatment process. All the administered groups showed certain inhibitions on tumor growth compared with the control group. The most significant antitumor efficacy was observed in the OCT-modified daunorubicin plus dihydroartemisinin liposome group.
Figure 8. Anticancer efficacy on invasive breast cancer MDA-MB-435S cell xenografts nude mice after treatment with varying formulations. (A) Antitumor efficacy after treatment with varying formulations; (B) body weight changes during the treatment process. p < .05; 1, versus physiological saline. Data are presented as mean ± SD (n = 6).
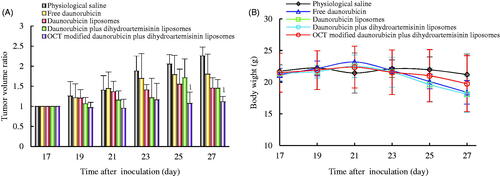
Changes in body weights of those tumor-bearing mice were also examined. As presented in , no noticeable weight loss was observed for all groups of mice during the treatment process. H&E staining was applied to examine the histopathologic changes in major organs (). Similar to the physiological saline group, no significant organ damage was observed after treatment with varying daunorubicin formulations at the current therapeutic dosage.
Figure 9. Histopathological analysis of main organs with H&E staining. (1) Physiological saline; (2) free daunorubicin; (3) daunorubicin liposomes; (4) daunorubicin plus dihydroartemisinin liposomes; (5) OCT-modified daunorubicin plus dihydroartemisinin liposomes. Images were obtained under 20× objectives.
Discussions
Breast cancer has become the most common cause of cancer-related deaths among women in recent years [Citation29,Citation30]. Most deaths of breast cancer patients are not caused directly by in situ breast cancer, but metastases, which are bestowed upon the primary tumor cells via the EMT [Citation31]. EMT is a biological process that enables transforming cells to lose their epithelial membrane adhesions, invading through the epithelium basement membrane, and elevating tumor cell migratory ability to move to distant organs via vascular routes. Final changes in cell phenotype result from altered expression of a series of associated cytokines [Citation32]. Increasing indicators are known that can regulate tumor invasion and metastasis including E-cadherin, TGF-β1, VEGF, integrin on tumor cells and MMPs [Citation14,Citation15,Citation33]. Thus, defining these factors may provide new therapeutic targets on inhibiting tumor metastasis [Citation34].
In the present study, daunorubicin and dihydroartemisinin were incorporated into the liposomes with OCT modification. The MALDI-TOF-MS spectrum analysis confirmed that DSPE-PEG2000-OCT conjugate was successfully synthesized (). Physicochemical characterizations of the liposomes are important for their further evaluations both in vitro and in vivo. As shown in and , modification of OCT on liposomes showed no significant variance in particle size, particle size distribution, zeta potential and encapsulation efficiency, and therefore might not influence the comparison of these liposomal systems in the following biological studies.
As demonstrated in cytotoxicity assays, free daunorubicin and daunorubicin-loaded liposomes exhibited obvious cytotoxic effects on both MCF-7 and MDA-MB-435S cells (). The IC50 values in revealed that the cytotoxicity of free daunorubicin were significantly higher than those of daunorubicin-loaded liposomes on both cells. Intensive inhibitory effects of free daunorubicin might be attributed to its direct diffusion into the cells. As compared with other liposomal formulations, OCT-modified daunorubicin plus dihydroartemisinin liposomes displayed higher cytotoxicity, indicating that the OCT could enhance cellular uptake of daunorubicin, which is subsequently confirmed by the flow cytometry ( and Supplementary Figure S1). Possible mechanism of increased cellular uptake for the liposomes is attribute to an OCT-SSTRs-mediated phagocytosis, which facilitates the transportation across tumor cell membranes to take action intracellularly. The results from fluorescent microscopy images further verify the flow cytometry data (). In detail, fluorescence of the OCT-modified daunorubicin plus dihydroartemisinin liposomes in the cytoplasm was higher than that of control liposomes.
MDA-MB-435S cells are more aggressive and metastatic than MCF-7 cells, therefore we evaluated the potential of dihydroartemisinin to inhibit migration and invasion of MDA-MB-435S cells. Results showed that the dihydroartemisinin loaded liposomes displayed more inhibition effect on cell invasion and transwell migration as compare with those of daunorubicin liposomes and control group ( and Supplementary Figure S2). These indicate that the dihydroartemisinin potently hampers migration, invasion, and colony formation of MDA-MB-435S cells. Hence, dihydroartemisinin may be a good preventive or therapeutic agent against metastatic breast cancer.
Metastasis is responsible for more than 90% of cancer-associated mortality. EMT is considered a fundamental step in the initiation of the metastatic cascade, in which process tumor cells lose epithelial polarity and acquire invasive properties [Citation35]. Many cytokine factors have been associated with tumor invasion and metastasis. TGF-β1, a cytokine with multiple biological functions, is first described as an inducer of EMT in normal mammary epithelial cells, and subsequent studies report important roles of TGF-β-induced EMT in tumor metastasis [Citation36]. Elevated level of TGF-β1 is associated with enhanced malignant behaviors such as invasion and metastasis [Citation37]. VEGF is a potent growth and angiogenic cytokine that stimulate angiogenesis by stimulating proliferation and survival of endothelial cells, thus promoting vascular permeability in tumor regions [Citation38,Citation39]. Substantial evidences implicate that cancer overexpressed VEGF is prone to invade and metastasize [Citation40,Citation41]. TGF-β1 can stimulate the production of VEGF and other angiogenic factors permitting the final metastatic colonization at distant sites [Citation42]. E-cadherin is a crucial type of cell–cell adhesion to hold the cells tight together. E-cadherin down-regulation decreases the strength of cellular adhesion, resulting in increased cell motility which allows tumor cells to cross the basement membrane and invade the surroundings [Citation43,Citation44]. The α5β1-integrin binds to matrix macromolecules and proteinases to stimulate angiogenesis. The activated integrins may participate in EMT process, mostly by stimulating various intracellular signaling pathways [Citation45]. Adhesion signaling mediated by integrins were shown to induce EMT in association with TGF-β receptor signaling. MMP-2/9 play critical roles in tumor cell invasion and metastasis by hydrolyzing extracellular matrix [Citation46]. In the present study, inhibition of metastasis is achieved as the OCT-modified daunorubicin plus dihydroartemisinin liposomes increase cell–cell adhesion by enhancing E-cadherin activity and markedly downregulate TGF-β1, VEGF, α5β1-integrin, and MMP-2/9 in breast cancer cells ().
A real-time living imaging was conducted to investigate the biodistribution of OCT-modified liposomes in vivo (). The fluorescence intensity in tumor locations for OCT-modified DiR plus dihydroartemisinin liposomes was stronger and lasted longer than those of normal DiR liposomes. Increased drug accumulation in tumor region leads stronger therapeutic effect. Higher distribution of OCT-modified DiR plus dihydroartemisinin liposomes in tumor region might be attributed to the enhanced permeability and retention (EPR) effect of liposomes and OCT modification, which increase the accumulation and cellular internalization in tumor via an OCT-SSTRs-mediated active targeting.
As for the results from the in vivo experiments, OCT-modified daunorubicin plus dihydroartemisinin liposomes showed remarkably therapeutic effects compared to other control formulations (). The in vivo antitumor effects were not only in good accordance with the cellular uptake and cytotoxicity results in vitro, but also with the data of living imaging study in vivo. The enhanced antitumor activity can account for the EPR effect, the receptor-meditated endocytosis by OCT, and incorporating dihydroartemisinin. A systemic drug delivery with low toxicity in vivo is a crucial criterion for clinical application. Therefore, we also performed the body weight and histological analyses on these formulations ( and ). No significant body weight loss and toxic pathological changes in the main organs were observed in all treatment groups. Due to passive targeting by the EPR effect and a specific SSTRs-mediated endocytosis by OCT, OCT-modified daunorubicin plus dihydroartemisinin liposomes could selectively accumulate in the tumor locations and avoid systemic toxicity to the normal tissues.
Conclusions
In the present study, daunorubicin and dihydroartemisinin were encapsulated in PEGylated liposomes, and a specific agent OCT was modified on surface of the liposomes to achieve the targeting effect by SSTRs-mediated endocytosis. The liposomes were ∼100 nm in size, and demonstrated a strong antitumor efficacy on breast cancer. Evaluations were carried out on breast cancer cells and xenograft nude mice. The action mechanisms involve the following aspects: (i) pegylated liposomes with suitable particle sizes render a prolonged circulation in blood by escaping the rapid uptake of reticuloendothelial system and thus enhancing drug concentrations via leaky vasculatures in the tumor location; (ii) OCT peptide facilitated cellular uptake and enhanced the intracellular concentrations of therapeutic agents; (iii) dihydroartemisinin incorporation increased the suppression on breast cancer invasion by regulating associated proteins (E-cadherin, α5β1-integrin, TGF-β1, VEGF and MMP2/9) in breast cancer cells. In conclusion, the established liposomal delivery system containing daunorubicin and dihydroartemisinin might provide a potential strategy for treatment of breast cancer along with preventing the metastasis.
Rui-Jun_et_al._Supplemented_Material.zip
Download Zip (124.6 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Otten JD, Broeders MJ, Fracheboud J, et al. Impressive time-related influence of the Dutch screening programme on breast cancer incidence and mortality, 1975–2006. Int J Cancer. 2008;123:1929–1934.
- Walker G, Xenophontos M, Chen L, et al. Long-term efficacy and safety of exemestane in the treatment of breast cancer. Patient Prefer Adherence. 2013;7:245–258.
- Shi Y, Zhao Y, Shao N, et al. Overexpression of microRNA-96-5p inhibits autophagy and apoptosis and enhances the proliferation, migration and invasiveness of human breast cancer cells. Oncol Lett. 2017;13:4402–4412.
- Yang X, Shi L, Yi C, et al. MiR-210-3p inhibits the tumor growth and metastasis of bladder cancer via targeting fibroblast growth factor receptor-like 1. Am J Cancer Res. 2017;7:1738–1753.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA-Cancer J Clin. 2016;66:7–30.
- Fei F, Zhang D, Yang Z, et al. The number of polyploid giant cancer cells and epithelial-mesenchymal transition-related proteins are associated with invasion and metastasis in human breast cancer. J Exp Clin Cancer Res. 2015;34:158.
- Shapiro IM, Cheng AW, Flytzanis NC, et al. An EMT-driven alternative splicing program occurs in human breast cancer and modulates cellular phenotype. PLoS Genet. 2011;7:e1002218.
- Kim RK, Kaushik N, Suh Y, et al. Radiation driven epithelial-mesenchymal transition is mediated by Notch signaling in breast cancer. Oncotarget. 2016;7:53430–53442.
- Nieto MA. The ins and outs of the epithelial to mesenchymal transition in health and disease. Annu Rev Cell Dev Biol. 2011;27:347–376.
- Eivazy P, Atyabi F, Jadidi-Niaragh F, et al. The impact of the codelivery of drug-siRNA by trimethyl chitosan nanoparticles on the efficacy of chemotherapy for metastatic breast cancer cell line (MDA-MB-231). Artif Cells Nanomed Biotechnol. 2017;45:889–896.
- Bhowmik SK, Ramirez-Pena E, Arnold JM, et al. EMT-induced metabolite signature identifies poor clinical outcome. Oncotarget. 2015;6:42651–42660.
- Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–4751.
- Huang RY, Guilford P, Thiery JP. Early events in cell adhesion and polarity during epithelial-mesenchymal transition. J Cell Sci. 2012;125:4417–4422.
- Lin CY, Tsai PH, Kandaswami CC, et al. Matrix metalloproteinase-9 cooperates with transcription factor Snail to induce epithelial-mesenchymal transition. Cancer Sci. 2011;102:815–827.
- Lo HW, Hsu SC, Xia W, et al. Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer Res. 2007;67:9066–9076.
- Sigurdsson V, Hilmarsdottir B, Sigmundsdottir H, et al. Endothelial induced EMT in breast epithelial cells with stem cell properties. PloS One. 2011;6:e23833.
- Simeonova M, Ivanova G, Enchev V, et al. Physicochemical characterization and in vitro behavior of daunorubicin-loaded poly(butylcyanoacrylate) nanoparticles. Acta Biomater. 2009;5:2109–2121.
- Lotfi K, Zackrisson AL, Peterson C. Comparison of idarubicin and daunorubicin regarding intracellular uptake, induction of apoptosis, and resistance. Cancer Lett. 2002;178:141–149.
- Minotti G, Menna P, Salvatorelli E, et al. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56:185–229.
- Singh NP, Lai H. Selective toxicity of dihydroartemisinin and holotransferrin toward human breast cancer cells. Life Sci. 2001;70:49–56.
- Zhang S, Ma Y, Jiang J, et al. Inhibition of urokinase-type plasminogen activator expression by dihydroartemisinin in breast cancer cells. Oncol Lett. 2014;7:1375–1380.
- Hwang YP, Yun HJ, Kim HG, et al. Suppression of PMA-induced tumor cell invasion by dihydroartemisinin via inhibition of PKCalpha/Raf/MAPKs and NF-kappaB/AP-1-dependent mechanisms. Biochem Pharmacol. 2010;79:1714–1726.
- Li X, Zhou Y, Liu Y, et al. Preclinical efficacy and safety assessment of artemisinin-chemotherapeutic agent conjugates for ovarian cancer. EBioMedicine. 2016;14:44–54.
- Zhang HY, Xu WQ, Wang YW, et al. Tumor targeted delivery of octreotide-periplogenin conjugate: synthesis, in vitro and in vivo evaluation. Int J Pharm. 2016;502:98–106.
- Zhang J, Jin W, Wang X, et al. A novel octreotide modified lipid vesicle improved the anticancer efficacy of doxorubicin in somatostatin receptor 2 positive tumor models. Mol Pharm. 2010;7:1159–1168.
- Li XT, Ju RJ, Li XY, et al. Multifunctional targeting daunorubicin plus quinacrine liposomes, modified by wheat germ agglutinin and tamoxifen, for treating brain glioma and glioma stem cells. Oncotarget. 2014;5:6497–6511.
- Ju RJ, Zeng F, Liu L, et al. Destruction of vasculogenic mimicry channels by targeting epirubicin plus celecoxib liposomes in treatment of brain glioma. Int J Nanomed. 2016;11:1131–1146.
- Ying X, Wen H, Lu WL, et al. Dual-targeting daunorubicin liposomes improve the therapeutic efficacy of brain glioma in animals. J Control Release. 2010;141:183–192.
- Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. JCO. 2006;24:2137–2150.
- Tran TH, Nguyen AN, Kim JO, et al. Enhancing activity of artesunate against breast cancer cells via induced-apoptosis pathway by loading into lipid carriers. Artif Cells Nanomed Biotechnol. 2016;44:1979–1987.
- Choi HJ, Park JH, Park M, et al. UTX inhibits EMT-induced breast CSC properties by epigenetic repression of EMT genes in cooperation with LSD1 and HDAC1. EMBO Rep. 2015;16:1288–1298.
- Martin-Belmonte F, Perez-Moreno M. Epithelial cell polarity, stem cells and cancer. Nat Rev Cancer. 2011;12:23–38.
- Renner G, Noulet F, Mercier MC, et al. Expression/activation of alpha5beta1 integrin is linked to the beta-catenin signaling pathway to drive migration in glioma cells. Oncotarget. 2016;7:62194–62207.
- Maschler S, Gebeshuber CA, Wiedemann EM, et al. Annexin A1 attenuates EMT and metastatic potential in breast cancer. EMBO Mol Med. 2010;2:401–414.
- Zheng S, Jia Q, Shen H, et al. Treatment with the herbal formula Songyou Yin inhibits epithelial-mesenchymal transition in hepatocellular carcinoma through downregulation of TGF-beta1 expression and inhibition of the SMAD2/3 signaling pathway. Oncol Lett. 2017;13:2309–2315.
- Mahmood MQ, Reid D, Ward C, et al. Transforming growth factor (TGF) beta1 and Smad signalling pathways: a likely key to EMT-associated COPD pathogenesis. Respirology. 2017;22:133–140.
- Lakhani HA, de Mel A, Seifalian AM. The effect of TGF-beta1 and BMP-4 on bone marrow-derived stem cell morphology on a novel bioabsorbable nanocomposite material. Artif Cells Nanomed Biotechnol. 2015;43:230–234.
- Roskoski R Jr. Vascular endothelial growth factor (VEGF) and VEGF receptor inhibitors in the treatment of renal cell carcinomas. Pharmacol Res. 2017;120:116–132.
- Sharifpanah F, Behr S, Wartenberg M, et al. Mechanical strain stimulates vasculogenesis and expression of angiogenesis guidance molecules of embryonic stem cells through elevation of intracellular calcium, reactive oxygen species and nitric oxide generation. BBA-Mol Cell Res. 2016;1863:3096–3105.
- Baker GJ, Yadav VN, Motsch S, et al. Mechanisms of glioma formation: iterative perivascular glioma growth and invasion leads to tumor progression, VEGF-independent vascularization, and resistance to antiangiogenic therapy. Neoplasia. 2014;16:543–561.
- Jun-Jiang C, Huan-Jiu X. Vascular endothelial growth factor 165-transfected adipose-derived mesenchymal stem cells promote vascularization-assisted fat transplantation. Artif Cells Nanomed Biotechnol. 2016;44:1141–1149.
- Cesi V, Casciati A, Sesti F, et al. TGFbeta-induced c-Myb affects the expression of EMT-associated genes and promotes invasion of ER + breast cancer cells. Cell Cycle. 2011;10:4149–4161.
- Canel M, Serrels A, Frame MC, et al. E-cadherin-integrin crosstalk in cancer invasion and metastasis. J Cell Sci. 2013;126:393–401.
- Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–558.
- Ihara Y, Inai Y, Ikezaki M. Alteration of integrin-dependent adhesion and signaling in EMT-like MDCK cells established through overexpression of calreticulin. J Cell Biochem. 2011;112:2518–2528.
- Musial K, Bargenda A, Zwolinska D. Urine survivin, E-cadherin and matrix metalloproteinases as novel biomarkers in children with chronic kidney disease. Biomarkers. 2015;20:177–182.