?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Phytase is the most widely used feed enzyme in the world. This enzyme inactivates under pelleting conditions such as temperatures above 80 °C. The present study reported covalent attachment of phytase onto functionalized multi-walled carbon nanotubes (F-MWNT). The optimum enzyme loading and immobilization efficiency were 110 µg/mg of functionalized multi-walled carbon nanotubes and 62%, respectively. Characterization, structural changes and immobilization of phytase were studied by scanning electron microscopy (SEM), circular dichroism (CD), zeta potential measurement and Fourier transform infrared (FTIR) spectroscopy. Immobilized phytase exhibited improved stability towards temperature than the free phytase. The free phytase retain 3% and 27% of relative activity at 90 and 80 °C, respectively after 2 min of incubations. While immobilized phytase retained about 33% and 51% at the same condition. Also the results showed that the presence of small quantities of NaCl (<1 M) in the reaction media increased enzyme activity of immobilized phytase up to 78% but free phytase activity was not significantly changed until 1 M NaCl which increased by 30%. Higher concentrations of 1 M NaCl drastically reduced both free and immobilized phytase activities.
Graphical Abstract
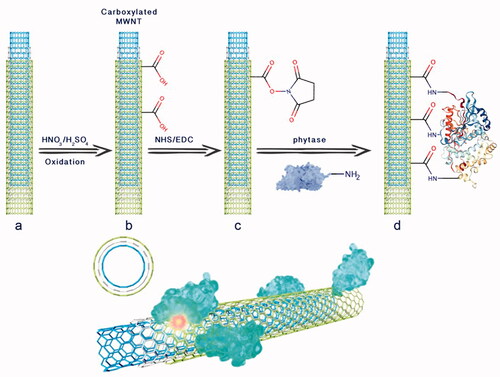
Introduction
Enzymes are natural nano-scaled biocatalysts that catalyze chemical reactions within living cells [Citation1]. Because of high catalytic and specific activity, ease of production, green chemistry, and also their ability to function under mild conditions [Citation2], enzymes are of considerable interest for use in diverse industries and applications which have been well reviewed [Citation3,Citation4]. Despite these advantageous characteristics of enzymes, their use in industrial applications is limited by numerous factors such as their short lifetime also known as instability and low reusability leading to high costs for large scale commercialization [Citation2]. In order to overcome these limitations, immobilization of enzymes has been proposed which has been led to promising results [Citation4]. Enzyme immobilization refers to confining or localizing enzyme molecules within an insoluble phase (matrix/support) while retaining their catalytic activities [Citation2,Citation5]. Apart from being affordable, an ideal carrier matrix should also encompass the following properties: stability, physical strength, inertness, regenerability, ability to reduce product inhibition and resistance to microbial attack [Citation6]. A major benefit of enzyme immobilization is increased stability and resistance of an immobilized enzyme to harsh environmental conditions such as wide ranges of pH and temperature [Citation7]. In the last decades, several methods have been used for immobilization of enzymes on diverse groups of natural and synthetic supports. These techniques are generally classified into four broad categories; that is, covalent binding, adsorption, entrapment and cross-linking [Citation8]. Overall, the chemical and physical properties of the supports used in combination with the biochemical properties of the enzyme play a key role in selecting the most appropriate immobilization technique [Citation9,Citation10].
In the last decades, using nanotechnology in a broad range of areas has become increasingly attractive especially in the case of biocatalysts [Citation11]. Various support materials have been investigated. Nevertheless, due to the recent developments in the field of nanotechnology and the extraordinary features of nanomaterials (such as high surface area/volume ratio, mass transfer resistance and effective enzyme loading), a growing attention has been paid to use these materials as support for enzyme immobilization [Citation12,Citation13]. Ease of separation, low product cost and enzyme reuse are the advantages reportedly attributed to enzymes nano-immobilization. Moreover, it has been claimed that the application of nanosupports would further increase the thermal and pH stability of the immobilized enzyme in comparison with their counterparts immobilized on macrosupports [Citation3,Citation14].
Various nanomaterials, such as nanoparticles, nanoporous, nanofibers and nanotubes have been used as support materials for enzyme immobilization [Citation15]. Among these, carbon nanotubes (CNTs) have been devoted much attention since they exhibit extraordinary mechanical, electrical and thermal properties as well as biocompatibility [Citation16]. Among two main types of CNTs which have been used for enzyme immobilization, multi-walled carbon nanotubes (MWNTs) seem closer to industrial use than single-walled carbon nanotubes (SWNTs) due to lower production costs [Citation17]. The main method used for covalent immobilization of enzymes on CNTs is based on the reaction between carboxylic acid groups located on the sidewalls of CNTs with free amine groups located on the surface of an enzyme (graphical abstract) [Citation16,Citation18].
Phytases (myo-inositol hexakisphosphate phosphohydrolases) are enzymes hydrolyzing phytic acid to myo-inositol and inorganic phosphates leading to the elimination of its anti-nutritional effects. Since there is a lack of intrinsic phytase activity in the digestive tracts of monogastric animals, phytase addition to their diet results in increased availability of calcium, magnesium, phosphorus and proteins; which would be otherwise unavailable as bound to phytate [Citation19]. In the present study, phytase were immobilized on CNTs by the covalent binding method and the thermal stability of the immobilized enzyme was compared with that of their free counterparts. Moreover, optimum enzyme loading rate was investigated and the effect of pH, temperature and NaCl on enzymatic activity in both free and immobilized modes were examined. The results of this study could provide valuable information on covalent-based enzyme immobilization via diimide-activated amidation method on MWNTs.
Materials and methods
Chemicals
Carboxyled MWNTs were purchased from Neutrino Co. (Tehran, Iran). Based on the manufacturer’s data, the MWNTs were 30 µm in length with a purity of 95 wt.% and 1.23 wt.% carboxyl group content. N-hydroxysuccinimide (NHS), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide (EDC) and phytic acid sodium salt hydrate were purchased from Sigma-Aldrich (Munich, Germany). Commercial phytase was obtained from Mianyang Habio Bioengineering Co. (Mianyang, China) and MES buffer was purchased from Bio Basic Co (New York, USA).
Phytase preparation
Phytase was separated from inert materials with three steps of water solubilization, centrifugation and acetone precipitation [Citation20]. At first, 10 g of commercial phytase powder was solubilized in 100 ml deionized water and was centrifuged twice at 18,000g at 4 °C. Subsequently, 300 ml acetone at −20 °C was added to the resultant supernatant. Free phytase was separated as precipitate and was dissolved in 6 ml MES buffer 50 mM (pH 6.1). The protein content of the free and immobilized phytase solutions were determined using the Bradford method including protein assay reagent (using Coomassie Brilliant Blue G-250 dye) and BSA (Bovine Serum Albumin) as standard protein. A range of 5–100 µg/ml of BSA was prepared and incubated with 3 ml dye reagent for 5 min. Samples absorbance were read at 595 nm and the amount of free and bounded protein was calculated by placing the absorbance in the obtained formula from the standard chart [Citation21].
Phytase activity assay
Phytase activity was measured based on the liberated inorganic phosphate using the ammonium molybdate method as described by Heinohen and Lathi with some modifications [Citation22]. Briefly, 50 µl of phytase enzyme either in free or immobilized form was incubated with 0.5 ml of 5 mM sodium phytate for 30 min at 37 °C, pH 5.5 (sodium acetate–acetic acid buffer). The reaction was stopped by the addition of a freshly-prepared solution of acetone (4 ml):5 N H2SO4:10 mM ammonium molybdate (2:1:1 v/v/v). Absorbance was measured at 400 nm. The results were compared with a standard curve prepared with potassium phosphate (KH2PO4), and the phytase activities of the test samples were determined. One phytase unit (FTU) is defined as the amount of enzyme that liberates 1 µmol of inorganic phosphorus per min from 5 mM sodium phytate under standard assay conditions (i.e. 37 °C for 30 min).
Functionalization of carboxylated MWNTs and phytase immobilization
Phytase was immobilized onto carboxylated MWNTs using a two-step carbodiimide reaction [Citation23]. First, 2.0 mg of the carboxylated MWNTs was suspended in 5.0 ml of deionized water by sonicating for 1 min. Then, a solution of 2.3 ml of a 50 mg/ml NHS aqueous solution and 1.0 ml of a 500 mM MES buffer (pH 6.1) were added to the above suspension and were mixed by vortex and the solution was then sonicated for 10 min. Under fast stirring, 1.2 ml of 10 mg/ml fresh EDC aqueous solution was subsequently added to initiate the coupling of NHS to the carboxylic groups on the nanotubes and the mixture was continually stirred (400 rpm) at room temperature for 30 min. The activated nanotube solution was then filtered through a 0.45 µm hydrophilized PTFE membrane and washed twice with a 50 mM MES buffer solution (pH 6.1) to remove excess EDC and NHS. The esterified CNTs were re-dispersed in 9.0 ml of a 50 mM MES buffer solution (pH 6.1). Under continuous shaking (1 h at room temperature), 1.0 ml of different concentrations of phytase (i.e. 57–1800 mg/l) in MES buffer solution was added quickly to CNTs suspension to achieve the optimal loading rate. After shaking the mixtures, they were centrifuged and rinsed with 50 mM MES buffer solution (pH 6.1) three times to remove unbound enzyme molecules. The enzyme–nanotube conjugates were dispersed in 1 ml of a 50 mM MES buffer solution (pH 6.1) for analytical measurements. Moreover, certain amounts of the immobilized enzyme were dried for the Fourier transform infrared (FTIR) and SEM measurements [Citation24].
Characterization techniques
The chemical composition of functionalized MWNTs and immobilized phytase were analyzed by FTIR spectroscopy. FTIR spectra were recorded at room temperature on a Bruker, vector 22 (Ettlingen, Germany) with a wavenumber resolution of 1 cm−1 using KBr pellets in the frequency range of 4000–400 cm−1 [Citation24].
Surface morphologies and the physical structures of the immobilized phytase and functionalized MWNTs were acquired using SEM. The samples were immersed in 2% glutaraldehyde buffer with 0.1 M phosphate buffer at room temperature and were then washed with phosphate buffer. Multi-step dehydration was performed by various concentrations of alcohol (25%, 50%, 75%, 90% and 100%, 2 h for each step). At the last step, a range of samples were prepared and exposed to air until the alcohol evaporated. The samples were then coated with a thin layer of gold and imaged using Zeiss – ultra plus EVO 18 scanning electron microscope (Oberkochen, Germany) [Citation24].
Zeta-potentials were used as a diagnostic tool to confirm enzyme immobilization [Citation25]. Samples were measured in MES buffer (pH 6.5) using Zetasizer Nano ZS Malvern instrument (Malvern, UK). All measurements were performed in triplicate.
Circular dichroism (CD) spectroscopy was performed to determine the structural changes and monitoring the secondary structure of free and immobilized phytase [Citation26]. The UV CD spectra (190–260 nm) were recorded on a CD spectrometer model-215 Aviv (New Jersey, USA) with a bandwidth of 1 nm. Cell length was 10 mm. Test solution containing 0.2 mg/ml of either free or immobilized phytase in MES buffer (50 mM, pH 6.1) was prepared from which 300 μl was used for CD analyses. A MES buffer and solution of functionalized MWNTs in MES buffer were used as control solutions. CDNN software (Magdeburg, Germany) was used for data analysis.
Optimum pH and temperature of free and immobilized enzymes
The optimum pH for free and immobilized phytases was determined by incubating the enzymes with sodium phytate in the different buffers: glycine–HCl (pH 2.0–3.5), sodium acetate–acetic acid (pH 4.0–6.0), Tris–HCl (pH 6.5–8.0) and glycine–NaOH (pH 8.6–10.6). Relative enzyme activities at selected range of pH were measured under standard conditions (37 °C for 30 min). For determining the temperature profile of the free and immobilized phytase, phytic acid was incubated at different temperatures from 30 to 70 °C. Then, 50 µl of phytase was added to each test tube. After 30-min incubation, colour reagent solution were added and absorbance was measured at 400 nm. All measurements were performed a minimum of three times and error bars were plotted by SD values.
Thermal stability of free and immobilized enzymes
To measure the variations in enzyme activity at high temperatures, the free and immobilized phytases were treated at 60, 70, 80 and 90 °C for 1–5 min [Citation27]. The samples were then cooled on ice for 10 min before further analyses. The activity of non-treated enzymes was considered as control for measuring relative activity of both free and immobilized phytases. Residual enzyme activity after each minute was measured under standard conditions (37 °C and pH 5.5 for 30 min). All measurements were performed in triplicate and error bars were plotted by using the SD values.
NaCl effect on free and immobilized enzymes
The effect of NaCl salt on free and immobilized phytase was determined by incubation in different concentrations of NaCl (from 0.05 to 3 M) under standard conditions (37 °C and pH 5.5 for 30 min). Phytase activity was determined and stated as a percentage of the activity of measured in the lack of NaCl. All measurements were performed in triplicate and error bars were plotted by using the SD values.
Results and discussions
Enzyme loading efficiency
The covalent method is preferred over the non-covalent method in order to immobilize phytase on MWNTs, because of stable and strong nature of the bonds formed [Citation16]. To obtain a higher phytase loading efficiency and activity, different concentrations of free phytase (57–1800 µg/ml) vs. 2 mg functionalized MWNT were taken into account and then the protein content for each experiment was measured using the Bradford assay method to obtain loading efficiency (%) (EquationEquation (1))(1)
(1) [Citation21]. For determining the of immobilization yield (%), specific activities of both free and immobilized enzymes were calculated for each initial phytase concentration (57–1800 µg/ml) (EquationEquation (2))
(2)
(2) [Citation26,Citation28]
(1)
(1)
(2)
(2)
with shows that increasing initial phytase concentration from 110 to 187 µg per mg of functionalized MWNTs led to a reduction in loading efficiency from 98.34% to 20.83%, the amount of enzyme immobilized onto functionalized MWNTs increased from 11% to 18.75% though [Citation26]. Therefore, the optimum initial phytase concentration was 110 µg per mg of functionalized MWNTs leading to the highest loading efficiency recorded (, ). The loading efficiency achieved for phytase over MWNTs using covalent immobilization in the present study was similar to those reported for soybean peroxidase (SBP) and Subtilisin Carlsberg (SC), that is, approximately 100–200 µg per mg of MWNTs [Citation29]. Cang-Rong and Pastorin also reported a loading efficiency of 274 µg amyloglucosidase per mg functionalized MWNTs [Citation30]. On the contrary, some studies have reported significantly higher loading efficiencies. For instance, Asuri et al. claimed a loading efficiency of >1000 µg enzyme per mg of CNTs (MWNTs/SWNTs) for Horseradish Peroxidase (HRP) and SC. They also stated that higher enzyme loading rates were achieved for SWNTs compared with MWNTs [Citation31]. As shown in , there is a positive correlation between increasing the enzyme:MWNT ratio and the amount of enzyme successfully immobilized on the nanotubes, however, after a threshold and in spite of the constant rise in the amount of protein content over the nanotubes, immobilization yield dropped. This could be ascribed to the wrong orientation of the immobilized enzyme molecules when high concentrations of the enzyme were involved in the reaction. Accordingly, the results obtained showed that the best immobilization yield stood at 62% at enzyme:MWNT ratio of 0.11 to 0.45 µg per mg of functionalized MWNTs. Various immobilization yields have also reported for different enzymes/proteins using MWNTs as nanosupport. For instance, Cang-Rong and Pastorin reported that covalent immobilization of amyloglucosidase on MWNTs retained 38% of the initial activity [Citation30]. While Das et al. claimed that after the immobilization of protocatechuate 3,4-dioxygenase (3,4-POD) on MWNTs, the retained activity ranged between 66% and 95% under different experimental circumstances [Citation26]. These findings reveal the critical role of the enzyme to nanosupport ratio in achieving the highest immobilization yield possible.
Figure 1. Loading efficiency of immobilized phytase on F-MWNT (a) and immobilization activity yield (b).
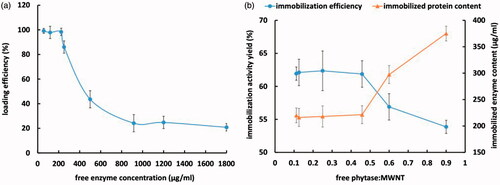
Table 1. Amount of initial and immobilized phytase (µg) on 1 mg of MWNTs and loading efficiency.
It should also be mentioned that immobilization reaction conditions and the characteristics of functionalized MWNT as well as those of the free enzyme molecules may also affect the immobilization yield of a given enzyme. For instance, it has been reported that the pH of the buffer used affected the immobilization yield and that it should be about 0.5 to 1 unit below the pI of the enzyme molecule in order to ensure best attachment [Citation32]. Zhang et al. argued that functionalized MWNTs might site-specifically bind to or near the catalytic site leading to reduced activity of the immobilized enzyme [Citation33]. Accordingly, it could be deduced that the reduction in phytase activity observed herein in response to immobilization could also be attributed to inhibition of the enzyme active site by unfavourable binding of some of the functionalized MWNTs (Graphical abstract).
Characterization of immobilized phytase
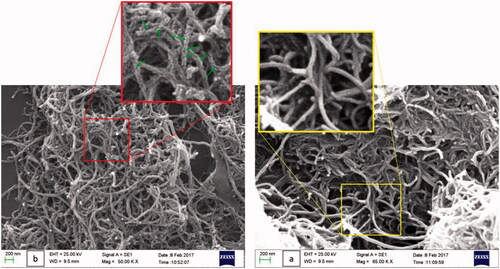
presents the scanning electron microscope (SEM) images of the functionalized MWNTs (a) and immobilized phytase (b). The immobilization of phytase molecule on MWNTs could clearly be seen as bright spots on the surface of CNTs. Since several washing stages were performed after the immobilization, it could be concluded that the enzymes retained over the MWNTs were covalently-bound. In addition, since the morphology of MWNTs before and after the immobilization reaction were not significantly different, it could be hypothesized that the immobilization procedure did not alter the structure of the MWNTs [Citation34].
Figure 2. Scanning electron microscopy of functionalized MWNTs (a) and phytase-MWNTs composites (b).
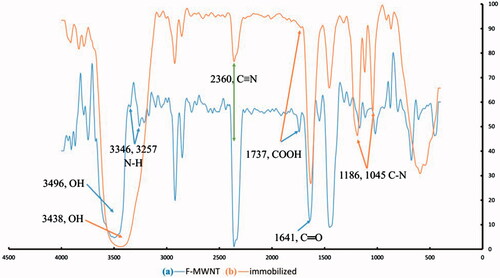
In order to confirm the successful immobilization of phytase on MWNTs, FTIR analysis was carried out to endorse the presence of amino groups. Both functionalized MWNTs and immobilized phytase were examined by FTIR for probing the vibrational changes during the covalent attachment (). As shown in , the peaks at 3438, 1641 and 1737 nm confirmed the presence of (–OH), (–C=O) and (–COOH), respectively [Citation24], indicating complete oxidation of MWNTs. The stretching vibration of (–COOH) shown in indicates the attachment of the enzyme molecules on the nanotubes [Citation26]. As presented in , unique peaks observed at 3346 and 3257 nm belonging to (N–H) bonds in phytase molecule confirmed the presence of phytase on the surface of functionalized MWNTs [Citation34]. Moreover, the aliphatic amide bond (C–N) between the functionalized MWNTs and enzyme molecules led to unique peaks at 1045 and 1186 nm (). These peaks indicates the amidination reaction, which confirms successfully immobilization of phytase enzyme onto the MWNTs. This statement is supported by previous studies [Citation34].
In 2008, Schultz et al. stated that there is a clear correlation between zeta potential and loading efficiency of the enzyme through the immobilization process. They also argued that zeta potential could be used as a diagnostic tool in enzyme immobilization [Citation25]. Their reported zeta potential values are −8.2, −11.4 and −9.5 for free Candida antarctica A-type lipase (CALA), non-porous magnetic microparticles with epoxy (M-PVA E02) and nano-immobilized CALA, respectively. The zeta potential values measured in the present study were −13.6, −7.6 and −12.2 for free phytase, functionalized MWNTs, and immobilized phytase, respectively. Accordingly, the proximity of the zeta potential values of free and immobilized phytase could be an indication of successful immobilization of the phytase enzyme over MWNTs. Furthermore, due to the fact that both the enzyme and the nanosupport were negatively charged, physical adsorption would not be an appropriate method for phytase immobilization over MWNTs and that the choice of covalent immobilization was accurate. Nevertheless, this repulsive force could have still impacted on the orientation of the immobilized enzyme on MWNTs and subsequently, could have played a role in decreasing the enzyme activity.
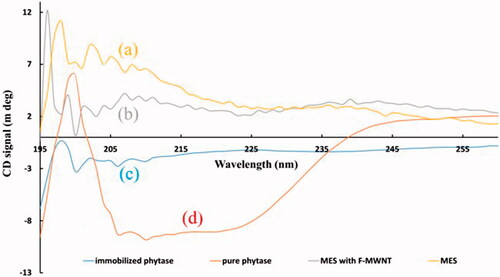
CD spectroscopy was used to investigate the changes in the secondary structures of phytase by covalent attachments on functionalized MWNTs. shows the differences in the secondary structures of the free and immobilized phytase. MES buffer (a) and functionalized MWNT (b) were considered as control solutions and were used for smoothing the images of the immobilized (c) and free (d) phytase. Compared to the free enzyme, the α-helix content decreased from 35.9% to 20.9% in the immobilized enzyme, whereas β-sheet antiparallel and parallel increased from 7.3% to 11.7% and from 8.3% to 14.1%, respectively. Significant changes in β-turn and random coil were not observed. These structural changes may explain the 38% reduction observed in the activity of the phytase enzyme after immobilization.
Temperature and pH profiles of free and immobilized phytase
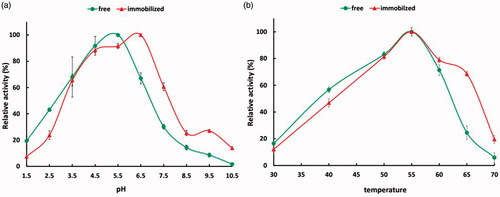
shows the pH (a) and temperature (b) profile relative activities of free and immobilized phytase. As expected, optimum pH of Escherichia coli phytase was in the range of 4.5–5.5. It was observed that the optimum activity of our phytase were in pH 5.5. Results shown that phytase activity decreased at both sides of the optimum pH 5.5 but there was a dramatically decline of enzyme activity at pH condition above 5.5. Retained about 66% and 30% relative activity at pH 6.5 and 7.5, while at pH 4.5 and 3.5 almost 91% and 68% of relative activity were retained, respectively, compared with that of the optimum condition. This reduction is because of a strong electrostatic repulsion which produced by the ionic groups within the phytase molecule in a strong alkaline pH media, leading to enzyme active centre change and the loss of enzyme activity, which is similar to studies [Citation35–37]. The immobilized phytase optimum activity were shifted from pH 5.5 to 6.5 (towards more alkaline) which has similarity to immobilized 3,4-POD on f-MWNT compared to free one [Citation26]. Interestingly, free phytase relative activity in pH 8–10 was lower than immobilized enzyme which is an indication of tendency of the enzyme to the alkaline environment. These results were similar to Dutta et al. which immobilized the phytase on magnesium nanoparticle. They showed that in addition to the shift of the pH optimum from 7.5 to 9, more resistant to the alkaline environment were obtained [Citation38]. The effects might be due to MWNT properties and the environmental conditions which could affect the substrate to product conversion process.
The optimum temperature for enzyme activity of both free and immobilized phytases was 55 °C and similar to some studies [Citation26], fewer relative activity detected for immobilized enzyme at temperatures lower than 50 °C. However, the most differences in temperature effect were observed at upper than 60 °C where free enzyme showed a steep decline in relative activity and on the contrary, immobilized enzyme still resists, and represent about 68% relative activity. In a similar result, lipase was immobilized on MWNT and showed a same optimum temperature with free lipase [Citation39]. Furthermore, immobilized 3,4-POD on MWNT showed a shifted optimum temperature from 55 to 60 °C with more stability at higher temperatures. This results might indicate the higher stability of the immobilized phytase than its free counterpart and also justify the results of thermal stability were explain below.
Salt stability
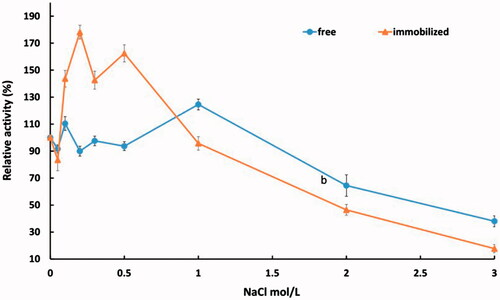
In our study, the effect of NaCl on free and immobilized enzyme activity was determined. indicates that effect of less than 1 M concentration of NaCl on the free phytase activity was not significant (<10% variation except 10% increasing at 0.2 M NaCl). Also by increasing NaCl concentration over 0.2 M changes in activity levels was maintained around 10% lower than the initial activity which indicates the acceptable stability against NaCl concentrations. However, NaCl 1 M increased the relative activity of phytase by 24% compared to 100% relative activity of enzyme in the lack of NaCl. Finally by increasing NaCl concentration over 1 M, relative activity was dramatically declined. On the other hand, the behaviour of immobilized enzyme is very significant and different. As shown in , in like manner with free phytase, NaCl 0.05 M causes reduction of relative activity by 17% but increases 43% in the presence of NaCl 0.1 M. The most important result was obtained when the salt concentration reached 0.2 M and phytase activity increased as much as 80% of the initial value. Stability of immobilized phytase in present of NaCl 0.3 M was slightly reduced but, immobilized enzyme in the present of salt was still 40% more active than in the lack of NaCl. There was a dramatically decline in relative activity of immobilized phytase at higher concentrations of 0.5 M NaCl. Moreover at NaCl 1 M, the activity of enzyme almost was not changed compared to initial activity and just about 17% activity was retained at NaCl 3 M.
Figure 6. Effect of NaCl concentrations on enzymatic activity of free and immobilized enzyme shown at two different scales.
In general, NaCl as a salting-in compound has a positive effect on the activity of some enzymes like dihydrofolate reductases, HIV-1 protease and menadione reductase at specific concentrations by inhibition of incorrect refolding [Citation40,Citation41]. This concentration is different for each enzyme. The activity of a halophilic esterase LipC was studied by Lang et al. under 1–6 M salt concentrations when enzyme was incubated for more than 1 h. Those results showed greatly increased enzyme activity with the rise in salt concentrations up to NaCl 3.4 M and reducing in the presence of over NaCl 3.4 M [Citation42]. Therefore, our results indicate that stability of immobilized phytase is similar to a halophilic enzyme. In another study, the effect of NaCl was investigated in the reaction medium in which increasing salt concentration from 1 to 100 mM causes rising up of 11βHSD2 activity from 30% to 40% then the activity fall down from 100 to 1000 mM NaCl concentration [Citation43]. Also, the effect of NaCl (0–3 M) on Solanum dubium enzyme was studied by Isam et.al. Increasing NaCl concentration from 0 to 2 M in the reaction media causes rising up the relative activity of S. dubium enzyme above 200% compared to 100% activity of initial activity in the absence of salt, then reduce to 180% in the presence of 3 M NaCl. They noted that the NaCl has different effects on proteins involved in the cheese making such as protein solubility and conformation [Citation44]. Furthermore, the presence of NaCl in combination with enzyme prevents microbial contamination. Also, NaCl is one of the appropriate fillers or stabilizers in the downstream production of enzymes [Citation45].
Temperature stability
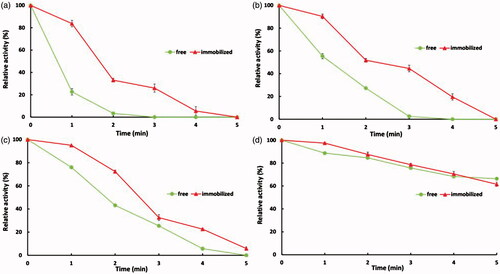
Pelleting is a common feed treatment for poultry diets to enhance broiler performance but phytase becomes deactivated under this mechanical and thermal process since it cannot tolerate a temperature between 80 and 90 °C for 3–5 min [Citation46]. indicates the thermostability of free and immobilized phytase at 90 °C (a), 80 °C (b), 70 °C (c) and 60 °C (d). It is evident that the immobilized phytase was significantly more stable than the free phytase at high temperatures and more prominent when temperature was rose up. All activity of free phytase was lost at 90 and 80 °C and retained just 5% at 70 °C in 4 min while immobilized phytase retained 5%, 19% and 22% at 90, 80 and 70 °C, respectively, at the same time. However, at 60 °C free and immobilized enzyme showed similar stability and showed no significant difference. Compared to our results, immobilized phytase on Bacillus polyfermenticus spores showed similar thermal stability. Results indicates that in 5 min of thermal treatment, free phytase activity was retained 40% at 60 °C while almost zero at 70, 80 and 90 °C, however immobilized phytase showed about 60%, 15%, 10% and 5% retained activity at 60, 70, 80 and 90 °C, respectively [Citation27]. Furthermore, immobilized phytase on allophanic synthetic compounds and montmorillonite nanoclays showed a significant thermal stability [Citation20]. The increased thermal stability observed might be attributed to the nature of support, reduction in molecular structure mobility of protein and changes in support mediated conformational of phytase. In order to confirm our results, most of the find reported in the literature strongly confirm the positive thermal effect of covalent immobilization of various enzymes on carbon nanotubes [Citation26,Citation39,Citation47]. Our results indicate the suitability of MWNTs to maintain the stability of phytase against pelleting temperatures.
Conclusions
Phytase has been successfully immobilized covalently on the functionalized MWNT. The optimum enzyme loading was 96.14% by using optimum amount of 110 µg phytase per 1 mg of F-MWNT which has 62% enzymatic activity. In fact, this study was conducted to find an appropriate support for phytase immobilization to increase resistance against pelleting temperatures. Improving the physicochemical properties of phytase by this method on MWNT, could be the reason to use it more in poultry feed. Now, the most important issue is increasing enzyme loading efficiency through optimization of reaction conditions, going along with the retaining enzyme activity.
Acknowledgements
The authors are grateful to Meisam Tabatabaei for native English editing of this article. The authors are also thankful to Ralf Greiner and Daniel Menezes-Blackburn for their intellectual assistance.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Adrio JL, Demain AL. Microbial enzymes: tools for biotechnological processes. Biomolecules 2014;4:117–139.
- Datta S, Christena LR, Rajaram YRS. Enzyme immobilization: an overview on techniques and support materials. 3 Biotech. 2013;3:1–9.
- Cipolatti EP, Valério A, Henriques RO, et al. Nanomaterials for biocatalyst immobilization–state of the art and future trends. RSC Adv. 2016;6:104675–104692.
- Sirisha V, Jain A, Jain A. Chapter nine-enzyme immobilization: an overview on methods, support material, and applications of immobilized enzymes. Adv Food Nutr Res. 2016;79:179–211.
- Brena B, González-Pombo P, Batista-Viera F. Immobilization of enzymes: a literature survey. Immobilization of enzymes and cells. 3rd ed. Totowa (NJ): Humana Press; 2013. p. 15–31.
- Santos JCSd, Barbosa O, Ortiz C, et al. Importance of the support properties for immobilization or purification of enzymes. ChemCatChem. 2015;7:2413–2432.
- Cipolatti EP, et al. Current status and trends in enzymatic nanoimmobilization. J Mol Catal B: Enzym. 2014;99:56–67.
- Dwevedi A. Basics of enzyme immobilization. In: Enzyme immobilization. Switzerland: Springer: 2016, p. 21–44.
- Cao L. Immobilised enzymes: science or art? Curr Opin Chem Biol. 2005;9:217–226.
- Mateo C, Palomo JM, Fernandez-Lorente G, et al. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb Technol. 2007;40:1451–1463.
- Zhao X, Jia H, Kim J, et al. Kinetic limitations of a bioelectrochemical electrode using carbon nanotube‐attached glucose oxidase for biofuel cells. Biotechnol Bioeng. 2009;104:1068–1074.
- Liu W, Wang L, Jiang R. Specific enzyme immobilization approaches and their application with nanomaterials. Top Catal. 2012;55:1146–1156.
- Wang L, Wei L, Chen Y, et al. Specific and reversible immobilization of NADH oxidase on functionalized carbon nanotubes. J Biotechnol. 2010;150:57–63.
- Ansari SA, Husain Q. Potential applications of enzymes immobilized on/in nano materials: a review. Biotechnol Adv. 2012;30:512–523.
- Verma ML, Puri M, Barrow CJ. Recent trends in nanomaterials immobilised enzymes for biofuel production. Crit Rev Biotechnol. 2016;36:108–119.
- Feng W, Ji P. Enzymes immobilized on carbon nanotubes. Biotechnol Adv. 2011;29:889–895.
- Baughman RH, Zakhidov AA, De Heer WA. Carbon nanotubes – the route toward applications. Science. 2002;297:787–792.
- Huang W, Taylor S, Fu K, et al. Attaching proteins to carbon nanotubes via diimide-activated amidation. Nano Lett. 2002;2:311–314.
- Dersjant-L, Y, et al. Phytase in non‐ruminant animal nutrition: a critical review on phytase activities in the gastrointestinal tract and influencing factors. J Sci Food Agric. 2015;95:878–896.
- Menezes-Blackburn D, Jorquera M, Gianfreda L, et al. Activity stabilization of Aspergillus niger and Escherichia coli phytases immobilized on allophanic synthetic compounds and montmorillonite nanoclays. Bioresour Technol. 2011;102:9360–9367.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem. 1976;72:248–254.
- Heinonen JK, Lahti RJ. A new and convenient colorimetric determination of inorganic orthophosphate and its application to the assay of inorganic pyrophosphatase. Anal Biochem. 1981;113:313–317.
- Jiang K, Schadler LS, Siegel RW, et al. Protein immobilization on carbon nanotubes via a two-step process of diimide-activated amidation. J Mater Chem. 2004;14:37–39.
- Pedrosa VA, Paliwal S, Balasubramanian S, et al. Enhanced stability of enzyme organophosphate hydrolase interfaced on the carbon nanotubes. Colloids Surf B. 2010;77:69–74.
- Schultz N, Metreveli G, Franzreb M, et al. Zeta potential measurement as a diagnostic tool in enzyme immobilisation. Colloids Surf B Biointerfaces. 2008;66:39–44.
- Das R, Hamid SBA, Annuar MSM. Highly efficient and stable novel nanobiohybrid catalyst to avert 3,4-dihydroxybenzoic acid pollutant in water. Sci Rep. 2016;6:33572.
- Cho E-A, Kim E-J, Pan J-G. Adsorption immobilization of Escherichia coli phytase on probiotic Bacillus polyfermenticus spores. Enzyme Microb Technol. 2011;49:66–71.
- Hermanová S, Zarevúcká M, Bouša D, et al. Graphene oxide immobilized enzymes show high thermal and solvent stability. Nanoscale. 2015;7:5852–5858.
- Asuri P, Karajanagi SS, Sellitto E, et al. Water-soluble carbon nanotube–enzyme conjugates as functional biocatalytic formulations. Biotechnol Bioeng. 2006;95:804–811.
- Cang-Rong JT, Pastorin G. The influence of carbon nanotubes on enzyme activity and structure: investigation of different immobilization procedures through enzyme kinetics and circular dichroism studies. Nanotechnology. 2009;20:255102.
- Asuri P, Bale SS, Pangule RC, et al. Structure, function, and stability of enzymes covalently attached to single-walled carbon nanotubes. Langmuir. 2007;23:12318–12321.
- Taheri RA, Rezayan AH, Rahimi F, et al. Evaluating the potential of an antibody against recombinant OmpW antigen in detection of Vibrio cholerae by surface plasmon resonance (SPR) biosensor. Plasmonics. 2017;12:1493.
- Zhang B, Xing Y, Li Z, et al. Functionalized carbon nanotubes specifically bind to α-chymotrypsin’s catalytic site and regulate its enzymatic function. Nano Lett. 2009;9:2280–2284.
- Mubarak NM, Wong JR, Tan KW, et al. Immobilization of cellulase enzyme on functionalized multiwall carbon nanotubes. J Mol Catal B: Enzym. 2014;107:124–131.
- Fei B, Xu H, Zhang F, et al. Relationship between Escherichia coli AppA phytase's thermostability and salt bridges. J Biosci Bioeng. 2013;115:623–627.
- Mandviwala T, Khire J. Production of high activity thermostable phytase from thermotolerant Aspergillus niger in solid state fermentation. J Ind Microbiol Biotechnol. 2000;24:237–243.
- Simon O, Igbasan F. In vitro properties of phytases from various microbial origins. Int J Food Sci Tech. 2002;37:813–822.
- Dutta N, Raj D, Biswas N, et al. Nanoparticle assisted activity optimization and characterization of a bacterial phytase immobilized on single layer graphene oxide. Biocatal Agric Biotechnol. 2017;9:240–247.
- Verma ML, Naebe M, Barrow CJ, et al. Enzyme immobilisation on amino-functionalised multi-walled carbon nanotubes: structural and biocatalytic characterisation. PloS One. 2013;8:e73642.
- Mozhaev VV. Mechanism-based strategies for protein thermostabilization. Trends Biotechnol. 1993;11:88–95.
- Zhao H. Effect of ions and other compatible solutes on enzyme activity, and its implication for biocatalysis using ionic liquids. J Mol Catal B: Enzym. 2005;37:16–25.
- Rao L, Zhao X, Pan F, et al. Solution behavior and activity of a halophilic esterase under high salt concentration. PloS One. 2009;4:e6980.
- Obeyesekere VR, Li KXZ, Ferrari P, et al. Truncation of the N- and C-terminal regions of the human 11β-hydroxysteroid dehydrogenase type 2 enzyme and effects on solubility and bidirectional enzyme activity. Mol Cell Endocrinol. 1997;131:173–182.
- Ahmed IAM, Babiker EE, Mori N. pH stability and influence of salts on activity of a milk-clotting enzyme from Solanum dubium seeds and its enzymatic action on bovine caseins. LWT-Food Sci Technol. 2010;43:759–764.
- Markussen EK, Schmidt AW. Enzyme granulate composition and process for forming enzyme granulates. US4106991. 1978 Aug 15.
- Timmons JR, Angel R, Harter-Dennis JM, et al. Evaluation of heat-stable phytases in pelleted diets fed to broilers from day zero to thirty-five during the summer months. J Appl Poultry Res. 2008;17:482–489.
- Othman AM, González-Domínguez Elena, Sanromán Ángeles, et al. Immobilization of laccase on functionalized multiwalled carbon nanotube membranes and application for dye decolorization. RSC Adv. 2016;6:114690–114697.