Abstract
Cardiovascular diseases (CVDs) are the leading cause of morbidity and mortality worldwide. Due to the significant impact of CVD on humans, there is a need to develop novel treatment modalities tailored to major classes of cardiac diseases including hypertension, coronary artery disease, cardiomyopathies, arrhythmias, valvular disease and inflammatory diseases. In this article, we discuss recent advancements regarding development of therapeutic strategies based on stem cells, aptamers, exosomes, drug-eluting and dissolvable stents, immunotherapy and nanomedicine for the treatment of CVD. We summarize current research and clinical advances in cardiovascular therapeutics, with a focus on therapies that move beyond current oral- or sublingual-based regimens. This review article provides insight into current research and future treatment strategies that hold a great relevance for future clinical practice in pursuit of improving quality of life of patients suffering from CVD.
Introduction
Cardiovascular disease (CVD) is the leading cause of mortality, worldwide and is an ever-increasing problem especially with the geriatric populations [Citation1,Citation2]. When examining heart disease, we not only have to consider its effects related to mortality, but also the morbidity and the disabling effects it has on individuals. CVD is a broad term that encompasses disorders of the cardiac muscle, vessels, or interstitial tissues. CVD can be classified based on: underlying cause such as hypertension, ischemia, inflammation or rheumatic by location of disease such as coronary artery disease, valvular disease, or pericarditis or by the type of disease such as cardiomyopathy, heart rhythm disturbance or infections. The objective for the treatment of CVD is to reduce the amount of hospitalizations that occur each year due to these diseases, to reduce mortality from heart disease, and to reduce heart disease-related disability.
Although current treatments in ischemic events are continually improving, the main factor that determines the level of disability sustained by a cardiac event, and even whether the patient survives, is usually time. The faster an individual receives percutaneous coronary intervention (PCI), the better his or her outcome upon discharge [Citation3,Citation4]. Current strategies are designed to speed up the time from cardiac event onset to receiving the appropriate medical intervention. In addition, there are also many clinical trials currently underway that aim to reduce or reverse the disease state and pre-emptively mitigate the level of disability encountered by the patient following an ischemic event [Citation5,Citation6]. These procedures are also being evaluated for their ability to alter the pathophysiology of hypertensive, inflammatory and myopathic diseases.
In this article, we will summarize the recent advancements regarding new treatment modalities based on stem cells, aptamers, exosomes and nanomedicine including emerging therapeutics that are under clinical trials in pursuit of improving quality of life of patients suffering from CVD.
Stem cells
Stem cells not only have the capability of self-renewal and proliferation, but also have the ability to develop into multiple cell types that can ultimately constitute viable tissue. Early on, proposed mechanisms of stem-cell therapy for CVD focused on angiogenesis and myogenesis [Citation7]. Since then, excitement for stem cell therapy has resulted in an abundance of promising preclinical data and a rise in adult stem cell clinical trials. Stem cells for CVD applications can be obtained from bone marrow-derived cells, inducible pluripotent stem cells, resident cardiac stem cells, mesenchymal or adipose tissue-derived stem cells, skeletal myoblasts, and circulating stem cells (). These stem cells can be then directly injected into CVD site. A number of studies have demonstrated the efficacy of stem cells for CVDs including myocardial infarct and ischemic heart disease () [Citation8–14]. However, due to the lack of understanding of basic principles, pathways and transcription factor networks, as well as the complexity of the heart as an organ, the use of stem cell therapy is not yet standard clinical practice [Citation15]. Major challenges include optimal stem cell type, the optimum timing of stem cell delivery, and the ideal application route. Thus far, results of clinical trials are heterogeneous in terms of efficacy and outcomes [Citation15]. Nonetheless, the field of regenerative cardiac medicine is still considered a promising therapy for the future.
Figure 1. Stem cell therapy in cardiovascular disease. Potential sources of stem cell therapy for cardiovascular disease are bone marrow-derived cells, inducible pluripotent stem cells, resident cardiac stem cells, mesenchymal or adipose tissue-derived stem cells, skeletal myoblasts, and circulating stem cells. Once differentiated into cardiospheres, they can be directly injected into an infarct zone or indirectly delivered to an ischemic zone via intracoronary or transcoronory cell injection.
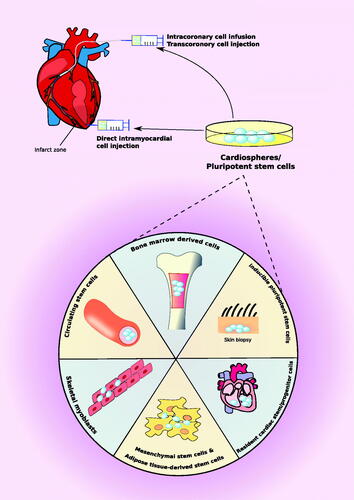
Table 1. Application of stem cell therapy for the treatment of cardiovascular diseases.
Mesenchymal stem cells (MSCs), a specific stem cell type, have shown regenerative properties at disease sites including the secretion of trophic and immunologic factors. However, MSCs have been difficult to control when grafting into the target site, as their cytokine production is sporadic and can promote inflammatory conditions. If researchers can develop methods to control the factors that enhance tissue repair and reduce tissue damage, MSCs could prove to be therapeutically beneficial.
Some researchers are studying exosomes, small bi-lipid membrane secreted vesicles released by MSCs, as potential cell signalling methods to shift MSCs into resolution, rather than an inflammatory state. In ischemic and coronary artery disease, these exosomes have been shown to reduce ischemic and reperfusion injuries at the site of infarction in cardiac tissue [Citation16]. MSCs can also induce endothelial, cardiovascular and neovascular differentiation in vitro and ex vivo [Citation17–20].
The current therapies for CVDs predominantly act to prolong the onset or progression of symptoms, and newer treatments are being designed to reverse the disease process in individuals. Cell-based therapies for patients suffering acute myocardial infarction (AMI) or living with congestive heart failure have captured the attention of the research community because current interventional strategies cannot compensate for the irreversible loss of functional cardiomyocytes. At the forefront of therapy are mesenchymal and resident cardiac stem cells, which are activated to differentiate into several different cell types [Citation19]. These new developments refute previous notions that the heart is fully differentiated and has limited restorative capabilities [Citation21]. Although these cells have been shown to have a high degree of cell turnover in vitro, their capabilities ex vivo or in vivo are less understood, though the potential underlying mechanisms may be useful in several different cardiac ailments [Citation22].
Hypertensive disease
Although there are no current strategies involving stem cell therapy in vascular hypertensive disease, there have been some promising results using stem cells to treat pulmonary hypertension (PH) in rodent models [Citation23]. MSCs can reduce hypoxia-induced damage and fibrosis, reverse injury to the alveoli, and normalize lung function, and ultimately reverse moderate signs of PH [Citation24]. PH commonly leads to right ventricle hypertrophy (RVH), which can ultimately result in right-sided heart failure and systemic symptoms. In another rodent model, the PH and RVH decreased in response to autologous MSC therapy and as a result normalized right ventricle ejection fraction and lung weight [Citation25].
Inflammatory disease
MSCs have also been studied for their potential to treat rheumatic disease. MSCs promote angiogenesis and secrete cytokines and growth factors in response to cellular damage. This promotes paracrine function between cells and leads to the reversal of immune responses, promoting the healing of damaged myocardium [Citation26]. Genetic modification of some stem cell lineages have been shown to lead to better engraftment and chemotaxis at inflammation sites and ultimately achieve a better healing response [Citation27].
Coronary artery disease
Perhaps the largest target of stem-cell therapy is coronary artery disease treatment post myocardial infarct. In randomized, double-blinded studies, it was found that MSCs have the ability to improve left ventricular ejection fraction and reverse cardiac remodelling [Citation28]. These benefits are believed to be due to the successful replication and differentiation of endogenous cardiac stem cells. In animal studies, injected MSCs induced differentiation and proliferation in resident cardiac stem cells [Citation28]. Other animal studies have shown that MSCs have the ability to engraft and differentiate into cardiomyocytes and myocardial vasculature, which leads to the secretion of paracrine and exosomal factors to promote healing [Citation28]. Current clinical trials aim to better elucidate the mechanism by which transplanted MSCs elicit this action. For example, the functional integrated myocytes derived from MSCs are too small in size to know if the healing effects are due to these cells, their paracrine functions proteins or from their effect on other neighbouring cells. Identification of these paracrine factors and administering them as therapeutics will promote a reversal of remodelling [Citation29].
Stem cells have not yet shown therapeutic potential in many of the other classifications of CVD such as cardiomyopathies and arrhythmias. However, there are a growing number of cell models of these conditions, which may aid in future understanding. For example, dysregulation of calcium cycling is seen in hypertrophic cardiomyocytes in hypertrophic myopathy [Citation30]. In addition, MSCs and pluripotent stem cells have been used for many years as systems for studying dysrhythmias in a cardiac environment [Citation31,Citation32].
Other recent developments include induced pluripotent stem cells (iPSs) and embryonic stem cells (ESCs), which have opened many exciting and controversial opportunities for cardiac tissue regeneration and engineering. The potential of reprogramming cells creates the opportunity for personalized stem cell therapies and the ability to model cardiac disease using patient-specific iPS cells. Though promising, more research is needed to precisely understand and harness the potential healing power of stem cells.
Aptamers
Aptamers are small RNA or DNA oligonucleotides that function as “chemical antibodies” and exhibit a range of important diagnostic and therapeutic applications [Citation33,Citation34]. Aptamers are developed completely in vitro through a system referred to as “Systematic Evolution of Ligands by Exponential Enrichment” (SELEX), which uses a series of target binding, washing and amplification steps to select DNA or RNA oligonucleotides based on affinity for the target molecule [Citation33]. Through this process, aptamers can be created that can bind with high specificity and affinity to a wide array of biomedical targets including proteins and small molecules [Citation33,Citation35]. Although the development of aptamer drugs is still in its infancy, aptamers possess several potential advantages over monoclonal antibodies. Specifically, aptamers can be developed purely in vitro, there is low batch-to-batch variability, and production and scale up is economical [Citation33,Citation34]. Aptamers are also thermally stable, do not require refrigeration, and can be easily transported [Citation34]. Lastly, aptamers lack the possible toxicities and immunogenicity of antibodies [Citation33,Citation34]. However, aptamers do harbour several pertinent disadvantages. For one, due to their smaller size, they are more quickly than antibodies, and are also vulnerable to endo- and exo-nucleases in vivo [Citation34,Citation35]. To combat these challenges, aptamers must be modified in vitro via chemical additions, which slows excretion and protects them against nucleases, however, in the process may also precipitate harmful side effects [Citation34,Citation35]. Additionally, aptamers have hard-to-predict pharmacokinetics, a narrow efficacy-safety profile, can exhibit non-target selectivity, and lack accurate means of titration or reversibility [Citation34].
Despite these challenges, aptamers have shown promise in a variety of cardiovascular therapeutic applications, most prominently as antithrombotics and anticoagulants [Citation33–35]. Currently, clinical trials are underway to assess aptamers against Von-Willebrand Factor (vWF), thrombin and Factor IX [Citation33,Citation34]. vWF-induced platelet activation plays a key role in multiple CVDs, including CAD, acute coronary syndrome (ACS), AMI and peripheral vascular disease [Citation33,Citation34]. As such, it is speculated that anti-vWF aptamers may prove useful in the treatment of these conditions. Similarly, thrombin is a key regulator of intrinsic coagulation pathway and can also activate platelets and inflammatory cytokines involved in plaque formation [Citation33]. Anti-thrombin aptamers are therefore being examined as possible therapeutic tools. Aptamers that inhibit Factor IX, another key element of the intrinsic coagulation cascade, are also undergoing clinical evaluation as possible antithrombotics [Citation34]. Beyond vWF, thrombin and Factor IX, research is also underway to investigate aptamer therapeutics in many other realms of cardiovascular pathology, including tissue factor inhibition [Citation35], factor XII inhibition [Citation33], inhibition of auto-antibodies present in cardiomyopathies [Citation36,Citation37], targeting of MSCs, vasopressin binding, and intra-coronary stent coating to improve endotheliazation [Citation34]. Aptamers are also being developed to select for protein profiles that can help predict future cardiovascular events [Citation38]. In summary, while the field of aptamer therapeutics is still in its infancy, the wide-ranging therapeutic possibilities of aptamers provide hope that they will be of increasing importance in future clinical practice.
Exosomes
Many cells in the cardiovascular system secrete nano-sized, lipid-bilayer vesicles (40–100 nm) called exosomes which carry signalling molecules such as protein, mRNA and miRNA are key players in intercellular communication that mediate survival and homeostasis. Exosomes utilize a cell-cell communication method based upon release and uptake of membrane-bound extracellular vesicles (EVs). Exosomes first bind to target surface ligands inducing receptor-mediated signal transduction, transfer surface receptors of EVs to target cell, and finally deliver functional protein, lipids and RNAs within the EVs. The following are reasons why EVs provide a unique advance over other cell-cell communication such as direct contact, gap junction, and signalling molecules – (1) a bilayer membrane allows for increased stability and prevents enzymatic degradation of proteins and molecules within the EV, (2) exosomes can be selectively enriched in certain components and can display high concentrations of signalling molecule, ensuring affinity and specificity, (3) allow for transfer of hydrophobic molecules across the target cell’s membrane and (4) a multi-molecular system allows for complex messages to be sent [Citation39–41]. Exosomes serve as a promising tool for diagnostic and prognostic biomarkers as well as efficient delivery of targeted therapy for CVD.
In particular, exosomes play an important part in the pathophysiology of myocardial infarction due to the increased need of orchestrating important signalling molecules such as chemokines, growth factors and miRNAs involved with hypoxic stress of cardiomyocytes. Secretion of exosomes has been shown to be involved with cardioprotection and remodelling of the heart after ischemic in the compensatory and repair process, acting as paracrine signalling mediators. They may also play a role in stimulating angiogenesis and promoting cardiomyocyte survival in stem cell therapy [Citation42]. A number of experiments have demonstrated exosome secretion from cardiomyocytes, especially under hypoxic stress. One study showed that injection of exosomes from cardiac progenitor cells into mice with myocardial infarction resulted in decreased cardiomyocyte apoptosis, increased angiogenesis and enhanced LV ejection fraction [Citation43]. Another study that derived exosomes from human and rat plasma showed that exosomal HSP70 activating MAPK/ERK1/2 signalling via TLR4 result in molecular cardioprotection against ischemia and reperfusion injury [Citation44]. It is also suggested the basis for stem cell therapy for myocardial repair and regeneration is highly dependent on paracrine signalling effects mediated in large part by exosomes [Citation45]. While there have been many promising animal studies showing the cardioprotective nature of exosomes against ischemia and infarction, exosome efficacy as well as pharmacokinetics and pharmacodynamics requires further elucidation [Citation46].
Novel drug eluting and dissolvable stent
Stent implantation has become a standard practice in interventional cardiology. In the past decades, major efforts have been made to improve implantation technique and stent design, and significant advances have been made from traditional balloon angioplasty and bare-metal stents. One major revolution includes the development of drug eluting and dissolvable stents, also called bio-resorbable scaffolds (BRSs). Drug-eluting stents are a novel approach to allow local drug delivery to prevent in-stent restenosis by inhibiting intimal thickening, inflammation and proliferative developments of the extracellular matrix in that particular region () [Citation47]. Furthermore, dissolvable stents are a novel development, which allow stents to act as a temporary scaffold, permitting increased natural healing and decreasing risk of thrombosis [Citation47]. Nonetheless, although with its advantages, certain risks still remain including late stent thrombosis, continuous neo-atherosclerosis, potential stent fracture and incomplete re-endothelialization. Different materials have been used to make BRS such as poly-l-lactic acid (PLLA) and magnesium being the most common. Each material has different strengths, resorption time, thrombotic properties and corrosion, and degradation rate.
Figure 2. Drug-eluting and biodegradable stent in coronary artery. Drug-eluting and biodegradable stents maximize the effectiveness of stent in atherosclerosis while minimizing side effects. These stents allow specific drug delivery into endothelial and smooth muscle cells in the affected area, and decrease chances of inflammatory process and restenosis. Stents are covered by polymer coating, which can be degraded gradually as drug delivery is completed.
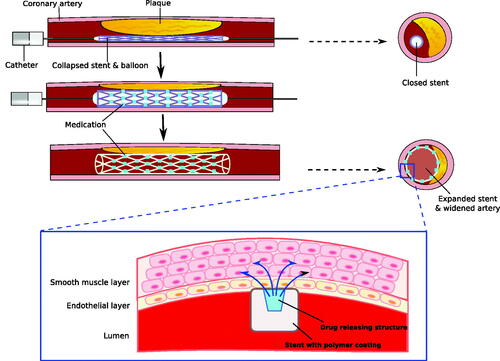
The first BRS to be used was the Igaki-Tamai stent, which is a PLLA-based BRS that is self-expandable upon heating and continues to expand until equilibrium between dilation and resistance of vessel wall is reached [Citation48]. While there are currently BRSs in design and production, none has been approved by the U.S. Food and Drug Administration. Two BRSs have the Conformite´ Europe´enne (CE) mark for use in coronary artery disease, which includes the Absorb bio-resorbable vascular scaffold (BVS) (Abbott Vascular) and the DESolve scaffold (Elixir Medical Corporation, Milpitas, CA). More clinical trials and outcome data is needed to prove that the BRS’s theoretical advantages outweigh its limitations. Nonetheless, BRS is a promising interventional treatment for coronary artery disease [Citation48].
Immunotherapy
Chronic inflammation and abnormal immune responses have been shown to be an integral part in the development of CVD. Autoimmune diseases such as rheumatoid arthritis (RA), systemic lupus erythematosus, scleroderma are well-defined chronic inflammatory diseases that also greatly affected by the cardiovascular system. Interestingly, there is growing understanding of the interaction between metabolism and inflammation through the interaction of lipids and leukocytes. Circulating monocytes, neutrophils, and platelets also have played an important role in CVD disease initiation and progression [Citation49]. Understanding the immune origins may delineate novel mechanisms to identify targeted immunotherapy for prevention and management of heart disease. For example, RA is an autoimmune disease in which cytokines such as tumor necrosis factor (TNF) and interferon attack normal, healthy cardiomyocytes. This results in dysfunction of endothelial lining of coronary blood vessels, leading to lipid deposition, plaque accumulation, atherosclerosis and an increased risk of thrombosis. RA and CVD have many similar innate and adaptive immune response mechanisms such as chronic elevation of acute phase reactant proteins, cytokines, and a common cell-mediated immune response [Citation50]. Many anti-inflammatory agents have been shown to be promising in the management of CVD. For example, the use of methotrexate, a disease-modifying anti-rheumatic drug used to treat many chronic inflammatory disorders, has been shown to be associated with lower risk for CVD in autoimmune disease states [Citation51]. Anti-TNF-α therapy (infliximab), another staple drug used to treat RA, has been shown to reduce risk of all cardiovascular events, myocardial infarction, and cerebrovascular accidents [Citation52]. Other anti-inflammatory therapeutics such as IL-1, IL-6, and T- and B- cell inhibitors (Rituximab) are also of interest due to their potential in reducing inflammation in CVD [Citation50]. However, anti-inflammatory agents have also been shown to increase other health risks such as increase total cholesterol and triglycerides as well as alter haematopoiesis and stem cell proliferation [Citation53,Citation54]. Current trials are on-going in order to better understand how anti-inflammatory drugs and immunotherapy should be used in the prevention and management of CVD.
Nanomedicine
Recently, nanomedicine has been extensively investigated for the diagnosis and therapy of CVDs (). Its unique design allows for precise structure control and targeted drug delivery, making it an effective method to treat a range of CVDs () [Citation55,Citation56]. Several types of drug delivery systems have been utilized depending on the therapeutic agent of choice and disease state. Therapeutic agents include a variety of small molecules and more recently DNA, siRNA, peptides and proteins. Drug delivery systems, including nanoparticles and liposomes, are highly dependent on drug-inherent properties such as solubility, molecular weight and the therapeutic goal of the drug. This section of the review will outline current nanomedical drug delivery systems that have been investigated for various specific CVDs such as atherothrombotic disease and injured myocardium.
Figure 3. Nanoparticle drug delivery to ischemic cardiac myocyte. A nanoparticle specific for ischemic cardiac myocyte is prepared by following: addition of organic spacers, attachment of functional groups (e.g. NH2) to organic spacers, and binding of Annexin V on the surface of a nanoparticle. Then, this newly assembled nanoparticle is intravenously injected, and delivered to the infarct area. Here, Annexin V on the nanoparticle recognizes phopshatidylserine expressed on the surface of a cardiac myocyte and successfully delivers drug content into the cell.
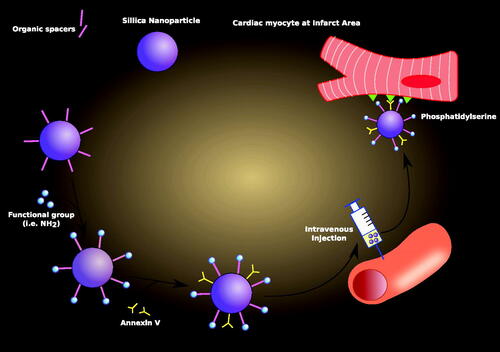
Table 2. Targeted drug delivery for cardiovascular diseases using nanoparticles.
Atherothrombotic disease
A polysaccharide-based nanosystem is a popular avenue for targeting certain cardiovascular pathologies such as atherothrombotic disease. Polysaccharides are long-chain carbohydrate molecules that are made up of identical monosaccharide units. These individual units are held together by glycosidic bonds. Polysaccharides not only have great structural diversity but also play a role in a variety of cellular functions including cell signalling and adhesion [Citation57]. Atherothrombotic pathology is highly associated with polysaccharide recognition, which allows for both targeted and inhibitory therapy [Citation58]. The well-known polysaccharide-based nanosystems used to combat atherosclerotic-related pathological disease include chitosan and dextran-coated nanoparticles. Chitosan is a linear polysaccharide that is protonated in acidic to neutral solutions rendering it as a hydrophilic cationic polyelectrolyte [Citation59]. Due to electrostatic interactions, chitosan has been noted to strongly interact with fibrin, a negatively charged fibrous protein [Citation60]. Chung et al. conducted an in vitro study to examine whether chitosan-coated nanoparticles could enhance clot penetration by reducing time needed for thrombolysis using tissue-plasminogen activator (t-PA) [Citation61]. Results showed that t-PA-loaded nanoparticles had a significantly lesser t-PA-related thrombolysis time than t-PA administered alone in solution. Furthermore, results showed that t-PA-loaded nanoparticles also greatly helped with clot permeation.
Another targeted platform for drug delivery consists of a superparamagnetic iron-oxide nanoparticle that has a cross-linked dextran coating (CLIO) [Citation62]. Dextran, a glucose polysaccharide, prevents nanoparticle aggregation and enables target ligand interactions through chemical modifications [Citation58]. McCarthy et al. conducted a study to look at use of CLIO platform for thrombus-targeted fibrinolytic therapy. Peptide-affinity ligands on the dextran coating targeted two different components of a thrombus: fibrin and activated factor XIII (FXIIIa). FXIIIa is a transglutaminase that functions to stabilize the thrombus by crosslinking fibrin molecules. While the applicability of the CLIO-platform thrombus treatment was demonstrated both in vitro and in vivo, more studies need to be conducted to optimize this fibrinolytic therapy [Citation63]. In another study, dextran-coated iron-oxide magnetic nanoparticles (MNPs) were synthesized and conjugated with urokinase, a plasminogen activator [Citation64]. It was observed that the magnetic field helped in focusing MNPs to the site of the thrombus and enhanced the thrombolytic efficacy of the conjugate particle.
The CLIO-platform has also been studied as a novel treatment modality for atheroma using light-activating inflammatory cell ablation. A study investigated about the light-activated nanoagents that are internalized by macrophages and are localized at the atherosclerotic lesions [Citation65]. Macrophage cellular uptake was detected by tagging the active drug with fluorescence and then using intravital fluorescence microscopy to observe localization. The cells were subsequently irradiated with a therapeutic dose of light, and researchers noticed that extensive apoptosis was induced through the production of cytotoxic oxygen radicals. This resulted in eradication of the inflammatory macrophages and ablation of inflammatory macrophages.
Another polymer-based nanoparticle is polylactic-co-glycolic acid (PLGA), a polymer of polylactic acid (PLA) and polyglycolic acid (PGA). A study examined whether pitavastatin-loaded PLGA nanoparticles inhibited atherosclerotic plaque destabilization in a mouse model by regulating the recruitment of inflammatory monocytes [Citation66]. The results showed that intravenous treatment with pitavastatin-incorporated nanoparticles did inhibit plaque destabilization and rupture by regulating MCP-1/CCR2-dependent monocyte recruitment in their model. PLGA nanoparticles have also been successfully used for the delivery of pioglitazone, an agonist of peroxisome proliferator-activated receptor-γ (PPARγ) [Citation67]. In another study, PLGA nanoparticles were loaded with thiazolidinedione (TZD) pioglitazone in hyperlipidaemic mice to determine if the mice could inhibit the activation of macrophages. It was observed that PLGA nanoparticles that were injected intramuscularly led to therapeutic arteriogenesis in targeted ischemic tissue.
Cardiovascular applications for polysaccharide-based nanoparticles not only include therapy for thrombosis and atheroma, but also magnetic imaging to help with diagnosis and treatment of atherosclerotic disease. Ultra-small superparamagnetic particles of iron oxide (USPIOs) have been experimentally shown to accumulate in macrophages in ruptured and rupture-prone plaques in humans. A study determined whether USPIO induced magnetic resonance (MR) signal changes in human atherosclerotic plaques. The accumulation of USPIOs in macrophages was a time-dependent process and caused a decrease in MR signalling [Citation68]. A subsequent study showed that uptake of USPIO-enhanced MRI contrast took up to 24–48 h after administration, suggesting that there was an optimal time window for detection [Citation69].
In another recent study, fibrin-targeted imaging and antithrombotic nanomedicine, termed FTIAN was constructed. FITIAN is developed from a near-infrared (NIR) fluorescent dye that is conjugated to boronate antioxidant polymers (fBAP) and fibrin targeting lipopeptides. The purpose of this study was to specifically image thrombus vessels through photoacoustic imaging and inhibit thrombus formation. Photoacoustic imaging has been shown to highlight atherosclerotic plaque and composition. At a cellular level, platelet recruitment during the formation of a thrombus is associated with a burst of hydrogen peroxide, H2O2, which in turn facilities inflammation at the site of endothelial injury. The administration of FTIAN inhibits the generation of H2O2, serving as an anti-inflammatory and anti-platelet modality. FTIAN targeted the obstructive thrombus and significantly enhance the fluorescence/photoacoustic sign [Citation70].
Injured myocardium
Injured myocardium is another potential therapy target for nanoparticle treatment. Infarcted myocardium is replaced by poorly conducting, fibrotic scar tissue, making it a good target for repair therapy. A study determined the interaction of thymosin beta 4 (Tβ4)-loaded nanoparticles at a site of cardiac repair. Recently, Tβ4, a small peptide, has been clinically suggested to be a therapeutic agent for myocardial infarction. It functions to promote the survival of cardiomyocytes and activates epicardial progenitor cells. However, the therapeutic benefit is limited by its local concentration found at the site of the infarct. The researchers developed a fibrin-targeting delivery system with the intention to reduce the dosing of Tβ4 systemically and promote efficiency. They used CREKA, a clot-binding peptide containing a cysteine–arginine–glutamate–lysine–alanine chain, as the targeting moiety and Tβ4 as the choice of drug administration. CREKA has been shown to bind to fibrin in microthrombi and the stroma of tumour. Results showed that CREKA-Tβ4 nanoparticles favoured the retention of Tβ4 at the zone of the infarction and helped promote repair and healing [Citation71]. The pitavastatin-PLGA nanoparticle delivery system has also been studied in patient post-myocardial infarction to see if this drug delivery approach could prevent further ischemic tissue damage [Citation72]. The results of the study showed that the nanoparticles did indeed reduce the ischemic-reperfusion (I/R) injury in the heart and reduced overall inflammation.
Stent repair in coronary artery disease
Nanoparticles have been indicated to act as drug carriers to treat coronary restenosis and support the healing process post-PCI in patients with coronary artery disease. There are two current therapeutic strategies for nanoparticle drug-eluting stents: an anti-restenosis strategy to prevent smooth cell proliferation and a pro-healing strategy to restore functional endothelium [Citation73].
Conclusion and future directions
As the leading cause of mortality and morbidity worldwide, CVD is being heavily studied with advances rapidly evolving in just the last decade. Conventional therapy which targets physiological properties such as arterial constriction, blood volume, coagulation cascade and lipid metabolism pathways encounter clinical limitations due to complications such as systemic toxicity, side effects, aberrant immune activation, unsustainable long-term patient compliance and thrombosis in stents. As our understanding of cellular processes underlying cardiovascular events increases, we are better able to develop treatments that are more targeted in the prevention and management of CVD on a molecular level.
Improving current stent repair methodology through the use of drug-eluting and dissolvable stents as well as nanoparticles in combination can prevent thrombotic complications and promote healing in cardiac endothelium. Nanomedicine, which allows us to create drug delivery and target systems on a cellular level, offers a wide array of opportunity in the areas of diagnosis, imaging, and treatment that can be targeted to biological structures at a nanometre scale. In addition to creating molecular-based therapy, treatment modalities using delivery systems such as aptamers and exosomes can regulate release, specificity and efficacy of therapeutic agents by utilizing antibodies/antigen and biomarkers that are present in pathological states. Gene therapy using miRNA-directed regulation can regulate gene expression and protein production that contribute to certain cardiovascular pathology as well as dyslipidaemia regulation. Beyond targeted, molecular therapy, advances in stem cell therapy has the potential for regeneration and reversal of disease process that can aid in the healing process after cardiovascular events. Finally, with the growing understanding of the underlying inflammatory and immune-mediated reactions that occur in CVD, we can explore the use of immunotherapy to decrease overall inflammation and decrease immune-mediated damage, thereby slowing the progress of CVD states. Increasing our understanding of molecular and pathophysiological pathways that underline CVD is important and will allow us to develop therapeutic modalities and drug-delivery platforms that are timely, targeted, specific and efficacious.
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- de Mestral C, Stringhini S. Socioeconomic status and cardiovascular disease: an update. Curr Cardiol Rep. 2017;19:115.
- Kwan GF, Mayosi BM, Mocumbi AO, et al. Endemic cardiovascular diseases of the poorest billion. Circulation. 2016;133:2561–2575.
- Karjalainen PP, Nammas W. Percutaneous revascularization of coronary chronic total occlusion: toward a reappraisal of the available evidence. J Cardiol. 2017;69:799–807.
- Nammas W, Pietila M, Romppanen H, et al. Outcome of poor initial TIMI flow in patients presenting with acute coronary syndrome. Scand Cardiovasc J. 2017;51:248–254.
- Quyyumi AA, Vasquez A, Kereiakes DJ, et al. PreSERVE-AMI: a randomized, double-blind, placebo-controlled clinical trial of intracoronary administration of autologous CD34+ cells in patients with left ventricular dysfunction post STEMI. Circ Res. 2017;120:324–331.
- Traverse JH, Henry TD, Pepine CJ, et al. The TIME trial - effect of timing of stem cell delivery following st-elevation myocardial infarction on the recovery of global and regional left ventricular function: final 2-year analysis. Circ Res. 2018;122:479–488.
- Perin EC. Stem cell therapy for cardiovascular disease. Tex Heart Inst J. 2006;33:204.
- Pelacho B, Nakamura Y, Zhang J, et al. Multipotent adult progenitor cell transplantation increases vascularity and improves left ventricular function after myocardial infarction. J Tissue Eng Regen Med. 2007;1:51–59.
- Ghostine S, Carrion C, Souza LC, et al. Long-term efficacy of myoblast transplantation on regional structure and function after myocardial infarction. Circulation. 2002;106:I131–I136.
- Abdel-Latif A, Bolli R, Tleyjeh IM, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989–997.
- Strauer BE, Yousef M, Schannwell CM. The acute and long-term effects of intracoronary Stem cell Transplantation in 191 patients with chronic heARt failure: the STAR-heart study. Eur J Heart Fail. 2010;12:721–729.
- Schachinger V, Erbs S, Elsasser A, et al. Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: final 1-year results of the REPAIR-AMI trial. Eur Heart J. 2006;27:2775–2783.
- Meluzin J, Janousek S, Mayer J, et al. Three-, 6-, and 12-month results of autologous transplantation of mononuclear bone marrow cells in patients with acute myocardial infarction. Int J Cardiol. 2008;128:185–192.
- Bartunek J, Behfar A, Dolatabadi D, et al. Cardiopoietic stem cell therapy in heart failure: the C-CURE (cardiopoietic stem cell therapy in heart failURE) multicenter randomized trial with lineage-specified biologics. J Am Coll Cardiol. 2013;61:2329–2338.
- Hare JM, Chaparro SV. Cardiac regeneration and stem cell therapy. Curr Opin Organ Transplant. 2008;13:536.
- Lai RC, Arslan F, Lee MM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222.
- Tomita S, Li RK, Weisel RD, et al. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation. 1999;100:II247–II256.
- El Sayed Shafei A, Ali MA, Ghanem HG, et al. Mesenchymal stem cells therapy: a promising cell based therapy for treatment of myocardial infarction. J Gene Med. 2017;19:e2995.
- Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776.
- Ranganath SH, Levy O, Inamdar MS, et al. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012;10:244–258.
- Laugwitz KL, Moretti A, Lam J, et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653.
- Oh H, Bradfute SB, Gallardo TD, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci USA. 2003;100:12313–12318.
- Takemiya K, Kai H, Yasukawa H, et al. Mesenchymal stem cell-based prostacyclin synthase gene therapy for pulmonary hypertension rats. Basic Res Cardiol. 2010;105:409–417.
- Hansmann G, Fernandez-Gonzalez A, Aslam M, et al. Mesenchymal stem cell-mediated reversal of bronchopulmonary dysplasia and associated pulmonary hypertension. Pulm Circ. 2012;2:170–181.
- Umar S, de Visser YP, Steendijk P, et al. Allogenic stem cell therapy improves right ventricular function by improving lung pathology in rats with pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2009;297:H1606–H1616.
- Mirotsou M, Jayawardena TM, Schmeckpeper J, et al. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J Mol Cell Cardiol. 2011;50:280–289.
- Karp JM, Teo GSL. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206–216.
- Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109:923–940.
- Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937.
- Lan F, Lee AS, Liang P, et al. Abnormal calcium handling properties underlie familial hypertrophic cardiomyopathy pathology in patient-specific induced pluripotent stem cells. Cell Stem Cell. 2013;12:101–113.
- Zhang YM, Hartzell C, Narlow M, et al. Stem cell-derived cardiomyocytes demonstrate arrhythmic potential. Circulation. 2002;106:1294–1299.
- Navarrete EG, Liang P, Lan F, et al. Screening drug-induced arrhythmia using human induced pluripotent stem cell-derived cardiomyocytes and low-impedance microelectrode arrays. Circulation. 2013;128:S3–S13.
- Li W, Wang K, Zhao M, et al. Development of aptamer oligonucleotides as anticoagulants and antithrombotics for cardiovascular diseases: current status. Thromb Res. 2014;134: 769–773.
- Wang P, Yang Y, Hong H, et al. Aptamers as therapeutics in cardiovascular diseases. Curr Med Chem. 2011;18:4169–4174.
- Hu PP, Zhang KH. The modulation of coagulation by aptamers: an up-to-date review. Blood Coagul Fibriolysis. 2015;26:1–6.
- Port JD, Bristow MR. Aptamer therapy for heart failure? Circ Res. 2011;109:982–983.
- Haberland A, Wallukat G, Dahmen C, et al. Aptamer neutralization of beta1-adrenoceptor autoantibodies isolated from patients with cardiomyopathies. Circ Res. 2011;109:986–992.
- Sabatine MS. Using aptamer-based technology to probe the plasma proteome for cardiovascular disease prediction. JAMA. 2016;315:2525–2526.
- Zhao W, Zheng XL, Zhao SP. Exosome and its roles in cardiovascular diseases. Heart Fail Rev. 2015;20:337–348.
- Emanueli C, Shearn AI, Angelini GD, et al. Exosomes and exosomal miRNAs in cardiovascular protection and repair. Vascul Pharmacol. 2015;71:24–30.
- Xu JY, Chen GH, Yang YJ. Exosomes: a rising star in falling hearts. Front Physiol. 2017;8:494.
- Ratajczak J, Wysoczynski M, Hayek F, et al. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20:1487–1495.
- Barile L, Lionetti V, Cervio E, et al. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc Res. 2014;103:530–541.
- Vicencio JM, Yellon DM, Sivaraman V, et al. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J Am Coll Cardiol. 2015;65:1525–1536.
- Madonna R, Van Laake LW, Davidson SM, et al. Position paper of the European society of cardiology working group cellular biology of the heart: cell-based therapies for myocardial repair and regeneration in ischemic heart disease and heart failure. Eur Heart J. 2016;37:1789–1798.
- Davidson SM, Takov K, Yellon DM. Exosomes and cardiovascular protection. Cardiovasc Drugs Ther. 2017;31:77–86.
- Garcia-Garcia HM, Vaina S, Tsuchida K, et al. Drug-eluting stents. Arch Cardiol Mex. 2006;76:297–319.
- Tamai H, Igaki K, Kyo E, et al. Initial and 6-month results of biodegradable poly-l-lactic acid coronary stents in humans. Circulation. 2000;102:399–404.
- Soehnlein O, Swirski FK. Hypercholesterolemia links hematopoiesis with atherosclerosis. Trends Endocrinol Metab. 2013;24:129–136.
- Crowson CS, Liao KP, Davis JM, et al. Rheumatoid arthritis and cardiovascular disease. Am Heart J. 2013;166:622–628 e1.
- Westlake SL, Colebatch AN, Baird J, et al. The effect of methotrexate on cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology (Oxford). 2010;49:295–307.
- Barnabe C, Martin BJ, Ghali WA. Systematic review and meta-analysis: anti-tumor necrosis factor α therapy and cardiovascular events in rheumatoid arthritis. Arthritis Care Res (Hoboken). 2011;63:522–529.
- Kraakman MJ, Dragoljevic D, Kammoun HL, et al. Is the risk of cardiovascular disease altered with anti-inflammatory therapies? Insights from rheumatoid arthritis. Clin Trans Immunol. 2016;5:e84.
- Choy E, Ganeshalingam K, Semb AG, et al. Cardiovascular risk in rheumatoid arthritis: recent advances in the understanding of the pivotal role of inflammation, risk predictors and the impact of treatment. Rheumatology (Oxford). 2014;53:2143–2154.
- Chappell JC, Song J, Burke CW, et al. Targeted delivery of nanoparticles bearing fibroblast growth factor-2 by ultrasonic microbubble destruction for therapeutic arteriogenesis. Small. 2008;4:1769–1777.
- Kim J, Cao L, Shvartsman D, et al. Targeted delivery of nanoparticles to ischemic muscle for imaging and therapeutic angiogenesis. Nano Lett. 2011;11:694–700.
- Raman R, Sasisekharan V, Sasisekharan R. Structural insights into biological roles of protein-glycosaminoglycan interactions. Chem Biol. 2005;12:267–277.
- Silva AK, Letourneur D, Chauvierre C. Polysaccharide nanosystems for future progress in cardiovascular pathologies. Theranostics. 2014;4:579–591.
- Szymanska E, Winnicka K. Stability of chitosan-a challenge for pharmaceutical and biomedical applications. Mar Drugs. 2015;13:1819–1846.
- Chung TW, Yang MC, Tsai WJ. A fibrin encapsulated liposomes-in-chitosan matrix (FLCM) for delivering water-soluble drugs. Influences of the surface properties of liposomes and the crosslinked fibrin network. Int J Pharm. 2006;311:122–129.
- Chung TW, Wang SS, Tsai WJ. Accelerating thrombolysis with chitosan-coated plasminogen activators encapsulated in poly-(lactide-co-glycolide) (PLGA) nanoparticles. Biomaterials. 2008;29:228–237.
- Tassa C, Shaw SY, Weissleder R. Dextran-coated iron oxide nanoparticles: a versatile platform for targeted molecular imaging, molecular diagnostics, and therapy. Acc Chem Res. 2011;44:842–852.
- McCarthy JR, Sazonova IY, Erdem SS, et al. Multifunctional nanoagent for thrombus-targeted fibrinolytic therapy. Nanomedicine. 2012;7:1017–1028.
- Bi F, Zhang J, Su Y, et al. Chemical conjugation of urokinase to magnetic nanoparticles for targeted thrombolysis. Biomaterials. 2009;30:5125–5130.
- McCarthy JR, Korngold E, Weissleder R, et al. A light-activated theranostic nanoagent for targeted macrophage ablation in inflammatory atherosclerosis. Small. 2010;6:2041–2049.
- Katsuki S, Matoba T, Nakashiro S, et al. Nanoparticle-mediated delivery of pitavastatin inhibits atherosclerotic plaque destabilization/rupture in mice by regulating the recruitment of inflammatory monocytes. Circulation. 2014;129:896–906.
- Koga J, Matoba T, Egashira K. Anti-inflammatory nanoparticle for prevention of atherosclerotic vascular diseases. J Atheroscler Thromb. 2016;23:757–765.
- Kooi ME, Cappendijk VC, Cleutjens KB, et al. Accumulation of ultrasmall superparamagnetic particles of iron oxide in human atherosclerotic plaques can be detected by in vivo magnetic resonance imaging. Circulation. 2003;107:2453–2458.
- Trivedi RA, U-King-Im JM, Graves MJ, et al. In vivo detection of macrophages in human carotid atheroma: temporal dependence of ultrasmall superparamagnetic particles of iron oxide-enhanced MRI. Stroke. 2004;35:1631–1635.
- Kang C, Gwon S, Song C, et al. Fibrin-targeted and H2O2-responsive nanoparticles as a theranostics for thrombosed vessels. ACS Nano. 2017;11:6194–6203.
- Huang Z, Song Y, Pang Z, et al. Targeted delivery of thymosin beta 4 to the injured myocardium using CREKA-conjugated nanoparticles. Int J Nanomedicine. 2017;12:3023–3036.
- Nagaoka K, Matoba T, Mao Y, et al. A new therapeutic modality for acute myocardial infarction: nanoparticle-mediated delivery of pitavastatin induces cardioprotection from ischemia-reperfusion injury via activation of PI3K/Akt pathway and anti-inflammation in a rat model. PLoS One. 2015;10:e0132451.
- Yin RX, Yang DZ, Wu JZ. Nanoparticle drug- and gene-eluting stents for the prevention and treatment of coronary restenosis. Theranostics. 2014;4:175–200.
- Lian L, Tang F, Yang J, et al. Therapeutic angiogenesis of PLGA-Heparin Nanoparticle in mouse ischemic limb. J Nanomaterials. 2012;2012:193704.
- Xie J, Wang H, Wang Y, et al. Induction of angiogenesis by controlled delivery of vascular endothelial growth factor using nanoparticles. Cardiovasc Ther. 2013;31:e12–e18.
- Chang MY, Yang YJ, Chang CH, et al. Functionalized nanoparticles provide early cardioprotection after acute myocardial infarction. J Control Release. 2013;170:287–294.
- Gray WD, Che P, Brown M, et al. N-acetylglucosamine conjugated to nanoparticles enhances myocyte uptake and improves delivery of a small molecule p38 inhibitor for post-infarct healing. J Cardiovasc Transl Res. 2011;4:631–643.
- Somasuntharam I, Boopathy AV, Khan RS, et al. Delivery of Nox2-NADPH oxidase siRNA with polyketal nanoparticles for improving cardiac function following myocardial infarction. Biomaterials. 2013;34:7790–7798.
- Smalling RW, Feld S, Ramanna N, et al. Infarct salvage with liposomal prostaglandin E1 administered by intravenous bolus immediately before reperfusion in a canine infarction-reperfusion model. Circulation. 1995;92:935–943.
- Verma DD, Hartner WC, Levchenko TS, et al. ATP-loaded liposomes effectively protect the myocardium in rabbits with an acute experimental myocardial infarction. Pharm Res. 2005;22:2115–2120.
- Scott RC, Rosano JM, Ivanov Z, et al. Targeting VEGF-encapsulated immunoliposomes to MI heart improves vascularity and cardiac function. FASEB J. 2009;23:3361–3367.
