Abstract
Escherichia coli O157:H7 is a pathogen, which can generate Shiga-like toxins (SLTs) and cause hemolytic-uremic syndrome. Foodborne illness outbreaks caused by E. coli O157:H7 have become a global issue. Since SLTs are quite toxic, effective medicines that can reduce the damage caused by SLTs should be explored. SLTs consist of a single A and five B subunits, which can inhibit ribosome activity for protein synthesis and bind with the cell membrane of host cells, respectively. Pigeon ovalbumin (POA), i.e. a glycoprotein, is abundant in pigeon egg white (PEW) proteins. The structure of POA contains Gal-α(1→4)-Gal-β(1→4)-GlcNAc ligands, which have binding affinity toward the B subunit in SLT type-1 (SLT-1B). POA immobilized gold nanoparticles (POA-Au NPs) can be generated by reacting PEW proteins with aqueous tetrachloroauric acid in one-pot. The generated POA-Au NPs have been demonstrated to have selective trapping-capacity toward SLT-1B previously. Herein, we explore that POA-Au NPs can be used as protective agents to neutralize the toxicity of SLT-1 in SLT-1-infected model cells. The results show that the cells can be completely rescued when a sufficient amount of POA-Au NPs is used to treat the SLT-1-infected cells within 1 h.
Graphical Abstract

Introduction
Food contaminated by microorganisms may cause illness and death [Citation1]. Thus, food safety has got considerable attention. Among foodborne pathogenic bacteria, Escherichia coli O157:H7 is one of the most toxic pathogens because it can cause hemolytic uremic syndrome and kidney failure [Citation2–11]. Shiga like toxins (SLTs) generated from E. coli O157:H7 are the main causes of the pathogenicity [Citation12,Citation13]. SLTs consists of a single A subunit and five B subunits. The A subunit is responsible for the inhibition of protein synthesis, while the B subunit can selectively bind with globotriaosylceramide (Gal-α(1→4)-Gal-β(1→4)-Glc-β-O-ceramide; Gb3) on the cell membrane of the host cells [Citation14–16]. Furthermore, each B subunit contains three binding sites toward Gb3 [Citation17]. Cell apoptosis was observed in the presence of SLTs with a concentration as low as ∼10 ng/mL [Citation18]. In addition, a previous study also showed that a crude cell lysate containing SLTs with a concentration up to ∼14 nM can cause damage on human vascular endothelial cells [Citation19].
Antibiotics, such as cefixime and tetracycline, are commonly used to treat patients suffering with E. coli O157:H7 infections [Citation20,Citation21]. However, the use of antibiotics in inhibiting the growth of SLT-produced bacteria does not directly inhibit the toxicity of SLTs that has been generated. Furthermore, antimicrobial treatment is not suitable for the patients that have developed hemolytic uremic syndrome [Citation21]. Thus, neutralizing SLT toxicity can be considered as one of the options for treating the illnesses caused by SLTs. Brefeldin A [Citation22] and baicalin [Citation23] have been used as an inhibitor for SLTs. Moreover, some studies have shown the possibility of using Gb3 analogues as protective agents to prevent cell damage caused by SLT-1 through the competition of the binding with SLT-1B in human vascular endothelial cells in vitro [Citation24,Citation25]. Nonetheless, the availability of medicines that can be used to directly neutralize the toxicity of SLTs is still limited.
The growth of nanotechnology has opened up a new possibility for the exploration of new medicines. Nanomedicine has become one of the choices for developing new drugs/therapies. Nanomaterials coated with Gb3 analogues have been successfully used to effectively target SLT-1B [Citation26–29]. The beauty of using Gb3 immobilized nanoparticles (Gb3-NPs) instead of Gb3 analogues alone is that multivalent interactions are involved in the binding between Gb3-NPs with SLT-1B, leading to high binding affinity. Nonetheless, Gb3 analogues are usually obtained through tedious synthesis steps. Thus, Gb3-containing molecules directly obtained from inexpensive natural products will be desirable. Pigeon egg white (PEW) proteins contain abundant pigeon ovotransferrin, pigeon ovomucoid and pigeon ovalbumin (POA), in which POA dominates ∼60% [Citation30,Citation31]. POA is a glycoprotein and contains Galα(1→4)-Gal-β(1→4)-GlcNAc termini [Citation32], and it has been used to target SLT-1B [Citation27]. In addition, POA contains three cysteines [Citation33], which can serve as reducing/capping agents [Citation34–39] for generation of Au NPs by reacted with aqueous tetrachloroauric acid through one-pot reactions [Citation40]. Furthermore, the POA immobilized Au NPs (POA-Au NPs) possessed selective targeting capacity toward SLT-1B [Citation40]. Herein, POA-Au NPs are explored to have the capability to neutralize the toxicity of SLT-1 in the SLT-1 infected cells. The feasibility of using POA-Au NPs as protective agents to inhibit the toxicity of SLT-1 in the SLT-1 infected cells was investigated in this study.
Experimental section
Generation of POA-Au NPs
PEW obtained from fresh pigeon eggs was collected and lyophilized. The resultant lyophilized PEW was stored in a freezer (−20 °C). POA-Au NPs were generated based on the method reported previously [Citation40]. When preparing POA-Au NPs, PEW proteins were denatured first. Briefly, PEW was dissolved (∼6.25 mg) in aqueous ammonium bicarbonate solution (100 mM, 0.5 ml, pH 8) followed by addition of urea (16 M, 0.5 ml). The mixture was incubated at 37 °C for 30 min. Then dithiothreitol (0.4 mg) was added to the resultant mixture and the PEW proteins were denatured by heating the mixture at 45 °C for 1 h. Later, the mixture was cooled to room temperature and iodoacetic acid (1 mg) was added. The vial containing the mixture was wrapped with aluminum foil to prevent light illumination. The vial was vortex-mixed for 2.5 h. To remove unreacted species and small proteins, the resultant solution was filtrated with an Amicon Ultra-4 centrifugal filter (cut-off mass: 50 kDa) under centrifugation at 3750 g for 10 min. The remaining species on the membrane were rinsed by deionized water (1 ml × ∼10 under the same centrifugation speed for 10 min). Subsequently, the remaining proteins on the filter membrane were re-dissolved in deionized water (1 ml). Later the resultant protein solution (300 μL), aqueous tetrachloroauric acid (10 mM, 100 μl), and deionized water (594 μl) were mixed together and the resultant mixture was stirred at ∼60 °C for 10 min. Aqueous sodium hydroxide (2 M, 2 μl) was added to the mixture under stirring for 1 min. The same aqueous sodium hydroxide solution (1 μL) was added to the mixture again under stirring. Subsequently, the reaction solution was moved to a water bath, maintained at 60 °C and was stirred at 400 rpm for ∼4 h. The resultant POA-Au NPs were collected by centrifugation at 26,810 g (4 °C) for 30 min. The supernatant was removed and the remaining POA-Au NPs were rinsed by re-suspending in deionized water (1 ml × 3) and centrifuged at 3750 g for 10 min to remove precipitates that contained large Au NPs. The supernatant containing POA-Au NPs was collected was used for further experiments.
Preparation of SLT-1 infected model cells
A human liver cancer cell line, Hep G2 cell (∼4 × 104 cells mL−1, 100 μL) and a mouse cell line L929 (∼4 × 104 cells mL−1, 100 μL) were loaded individually to 96-well plates and incubated (5% CO2, 37 °C) for 16 h. The solution from the plates was discarded and the cell lysate containing SLT-1 derived from E. coli O157:H7 (OD600 = 1) with different dilution factors (2.8, 5.6, 11.2, 22.4 and 44.8) was added to the remaining cells on the plates and incubated for 1 h (5% CO2, 37 °C). Control samples without the addition of the cell lysate were conducted in parallel. The solution from the plates was removed and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) assay was conducted. The steps were the same as described above. The cell viability of these SLT-infected cells was examined accordingly.
Model cells treated by the conjugates of SLT-1 and Au@POA NPs
The model cells were treated with the conjugates of SLT-1B and POA-Au NPs. This treatment was used to examine the toxicity of the conjugates. The conjugates were first prepared. POA-Au NPs (1 ml) with different concentrations (0, 62.5, 125, 250, 500, and 1000 µgmL−1) were centrifuged at 26,810 g (4 °C) for 10 min and the supernatant was discarded. The precipitates obtained were re-dispersed in Tris buffer (10 μL, 25 mM, pH 7.4). The resultant suspensions that contain POA-Au NPs (10 μL) were mixed with the cell lysate sample containing SLT-1 derived from E. coli O157:H7 (40 μL), under microwave heating (power: 450 W) for 90 s. Subsequently, the resultant solution was diluted by medium (1 ml). Model cells (L929 cells and Hep G2 cells; ∼4 × 104 cells mL−1, 100 μL) were loaded to a 96-well plate and incubated at 37 °C (5% CO2) for 16 h. The solution in the plates was discarded and the cell lysate solution (100 μL) that had been mixed with POA-Au NPs was added to the cells. The mixture was incubated in the incubator at 37 °C (5% CO2) for 2 h. Control samples were also conducted in parallel. Subsequently, the solution on the plates was discardedand MTT was conducted to estimate the cell viability.
Examination of the protective roles of POA-Au NPs toward SLT-1-infected cells
Model cells (∼4 × 104 cells mL−1, 100 μL), including Hep G2 cells and L929 cells, were loaded to 96-well plates and were incubated (5% CO2, 37 °C) for 16 h. The solution in the plates was discarded. A 2.8-fold diluted cell lysate (100 μL) that contains SLT-1 derived from E. coli O157:H7 was added and incubated at 37 °C (5% CO2) for 1, 2 and 4 h, respectively. The solution on the plates was then discarded. POA-Au NPs (100 μL) with different concentrations (0, 62.5, 125, 250, 500 and 1000 μgmL−1) were added to the remaining cells. The mixture was incubated at 37 °C (5% CO2) for 2 h and the solution from the plate was eliminated. The remaining samples were examined by the same MTT assay as described aboveand the cell viability was estimated accordingly.
Statistics analysis
When examing cell viability of the cells and the SLT-1 infected cells with and without NP treatment, three replicates were condcuted. The average and standard deviation derived from three replicated data were used to plot bar graphs.
Results and discussion
Characterization of POA-Au NPs
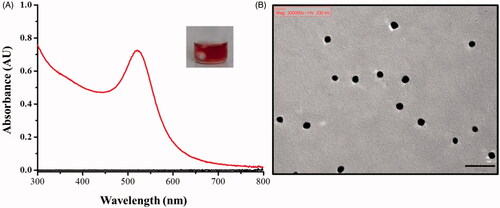
POA-Au NPs were prepared according to the method reported previously [Citation40]. The generated POA-Au NPs were first characterized by ultraviolet-visible (UV-Vis) absorption spectroscopy. The maximum absorption band appeared at the wavelength of ∼520 nm (), and the generated POA-Au NPs had red color (inset photograph in ). The generated POA-Au NPs have similar UV-Vis absorption spectrum and color to that in the previous reported [Citation40]. shows the transmission electron microscope (TEM) image of the generated POA-Au NPs. The particle size was ∼10 nm. It is appealing that a white-layer of materials surrounded the surface of these AuNPs, indicating the success of protein immobilization.
Examination of cell biocompatibility of the generated POA-Au NPs
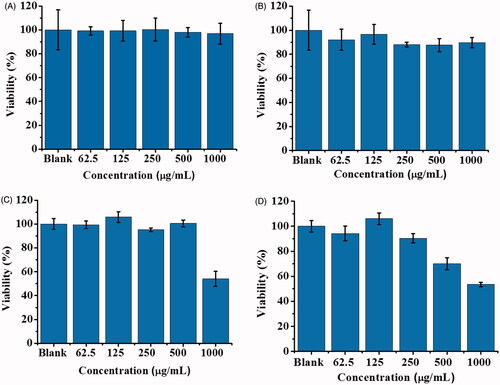
The purpose of this study was to investigate the protective role of the generated POA-Au NPs for SLT-1-infected cells. Thus, the cell biocompatibility of the generated POA-Au NPs was initially investigated. MTT assay was conducted for evaluation. show the cell viability results that were obtained after L929 cells (∼4 × 104 cells mL−1, 100 μL) were treated with POA-Au NPs at different concentrations for 1, 2, 4 and 6 h, respectively. Apparently, the cells were not damaged when the incubation time lasted for 1 () and 2 h (), respectively even when the concentration of POA-Au NPs was increased to 1000 μgmL−1. However, when the incubation time was increased to 4 () and 6 h (), respectively the cell viability was decreased with the treatment of POA-Au NPs at the concentration of 1000 μgmL−1. These results indicated that the generated POA-Au NPs have a desirable cell biocompatibility.
Figure 2. Examination of cell biocompatibility of POA-Au NPs toward L929 cells. L929 cells (∼4 × 104 cells mL−1, 100 μL) prepared in DMEM medium were treated with POA-Au NPs (100 μL) with different concentrations (0, 62.5, 125, 250, 500 and 1000 µgmL−1) that were prepared in DMEM medium, for (A) 1 h (B) 2 h (C) 4 h and (D) 6 h, respectively. Blank stands for the control sample that was not treated with POA-Au NPs. Three replicates were conducted for obtaining the bar graphs.
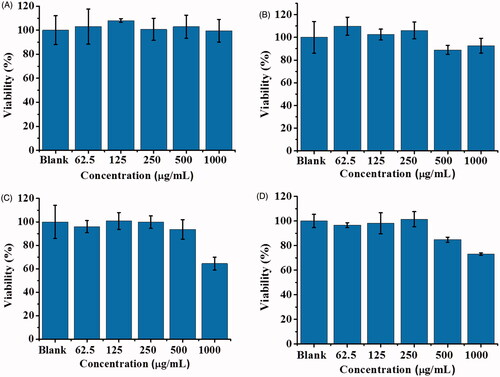
Liver is an important organ that can metabolize many molecules including proteins. Given that SLT-1 and POA are proteins, we selected Hep G2 cells, which are liver epithelial cells, as the model cells to examine the cell biocompatibility of POA-Au NPs. show the cell viability of Hep G2 cells that were treated by POA-Au NPs with different concentrations for 1, 2, 4 and 6 h, respectively. The results were similar to those shown in . That is, the toxicity of POA-Au NPs was not apparent until the incubation time was extended to 6 h and the concentration of POA-Au NPs reached more than 1000 μgmL−1.
Figure 3. Examination of the cell biocompatibility of POA-Au NPs toward Hep G2 cells. Hep G2 cells (∼4 × 104 cells mL−1, 100 μL) prepared in MEM medium were treated with POA-Au NPs (100 μL) with different concentrations (0, 62.5, 125, 250, 500 and 1000 µgmL−1) that were prepared in MEM medium, for (A) 1 h (B) 2 h (C) 4 h and (D) 6 h, respectively. Blank stands for the control sample that was not treated with POA-Au NPs. Three replicates were conducted.
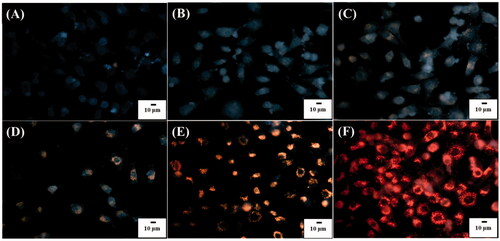
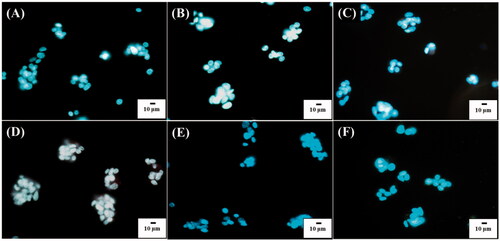
Since it is the nature that cells can internalize NPs when incubated with NPs, the cell uptake of POA-Au NPs by the model cells was also observed under an optical microscope. show the optical images of the L929 cells under dark field that were obtained after the incubation with POA-Au NPs at the concentrations of 0, 62.5, 125, 250, 500 and 1000 µgmL−1, respectively, for 6 h. Apparently, with the increase of the concentration of POA-Au NPs, the intensity of the scattering light with orange color derived from POA-Au NPs became apparent. The NPs were mainly located in the cytoplasm. The results indicated that POA-Au NPs were readily internalized by the cells and the AuNP uptake amount by the cells was increased with the increase of the treated concentration of POA-Au NPs.
Figure 4. Dark field optical images of L929 cells (1 ml, ∼106 cells/well) obtained after the treatment with POA-Au NPs at the concentations of (A) 0, (B) 62.5, (C) 125, (D) 250, (E) 500 and (F) 1000 µgmL−1 for 6 h, respectively. Exposure time was set to 40 ms. The scale bar is 10 µm.
To visualize the location of the nuclei of the cells, the cells that were treated with POA-Au NPs were also stained by Hoechst 33342 with blue fluorescence. show the overlapped images of the L929 cells that were obtained after being treated by POA-Au NPs with the concentrations of 0, 62.5, 125, 250, 500, and 1000 µgmL−1, respectively, for 6 h. Fluorescence and dark field microscopy were used to obtain the overlapped images. The results clearly showed that the blue nuclei was surrounded by a ring of orange light, which was derived from AuNP scattering. The results again confirmed that POA-Au NPs were readily internalized by the cells and the uptake amount was increased as the concentration of POA-Au NPs were increased.
Figure 5. Overlapped images of L929 cell (1 ml, ∼106 cells mL−1) obtained after incubated with POA-Au NPs at the concentrations of (A) 0, (B) 62.5, (C) 125, (D) 250, (E) 500 and (F) 1000 µgmL−1 for 6 h, from dark field and fluorescence microscopy. The cells were stained by Hoechst 33342. The excitation wavelength was set at 330–380 nm and the exposure time was set to 20 ms. The scale bar is 10 µm.
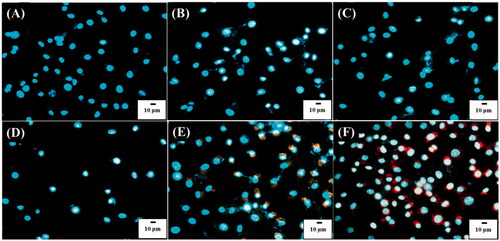
Figure 6. Optical images of Hep G2 cells (1 ml, 106 cells mL−1) under dark field obtained after treated with POA-Au NPs with the concentations of (A) 0, (B) 62.5, (C) 125, (D) 250, (E) 500 and (F) 1000 µgmL−1 for 6 h. Exposure time was set to 40 ms. The scale bar is 10 µm.
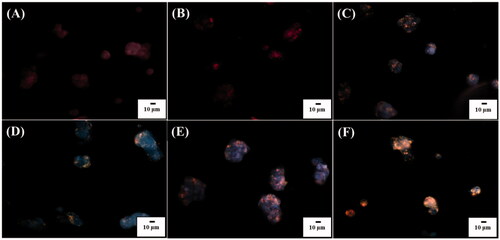
The uptake of POA-Au NPs by Hep G2 cells was also examined by using optical microscopy. show the optical images of Hep G2 cells under dark field that were obtained after being incubated with POA-Au NPs at the concentations of 0, 62.5, 125, 250, 500, and 1000 µgmL−1, respectively, for 6 h. The intensity of the scattering light derived from POA-Au NPs in the cells was apparently increased as the concentration of POA-Au NPs was increased. However, the location of the cell nuclei was hard to be observed because Hep G2 cells were aggregated to a large extent. Thus, blue fluorescent Hoechst 33342 was used to stain the cells and the cell nuclei were located. show the overlapped images of Hep G2 cells that were obtained by being incubated with POA-Au NPs with the concentrations of 0, 62.5, 125, 250, 500, and 1000 µgmL−1, respectively, for 6 h. The overlapped images were obtained by using optical microscopy under dark field and fluorescence microscopy. The blue colorindicated the cell nuclei and orange color indicated the distribution of POA-Au NPs. The blue color was too bright and suppressed the observation of the location of POA-Au NPs. Nevertheless, a red emission was derived from POA-Au NPs that surrounded the blue nuclei, as shown in .
Figure 7. Overappled images of Hep G2 cells (106 cells mL−1, 1 ml) obtained after being incubated with POA-Au NPs at the concentations of (A) 0, (B) 62.5, (C) 125, (D) 250, (E) 500 and (F) 1000 µgmL−1 for 6 h, respectively obtained from dark field and fluorescence microscopy. The cells were stained by Hoechst 33342. The excitation wavelength was set at 330–380 nm and the exposure time was set to 10 ms.
Infection of model cells by SLT-1-containing cell lysate
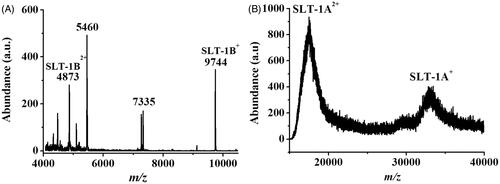
To examine the toxicity caused by the E. coli O157:H7, L929 cells and Hep G2 cells that were incubated with the cell lysate containing SLT-1 were used as the model cells. MTT assay was used to examine the cell viability. The cell lysate was originally obtained from an E. coli 157:H7 suspension (OD600 = ∼1). To confirm the existence of SLT-1 in the prepared cell lysate sample, matrix-assisted laser desorption/ionization (MALDI)-MS, which is a suitable tool for characterization of large biomolecules, was used for confirmation. shows the MALDI mass spectrum of the cell lysate of E. coli O157:H7 that was used to treat the model cells. Many peaks derived from the cell lysate were observed in the mass spectrum. The peaks at m/z 9744 and m/z 4873 that were derived from the singly and doubly charged SLT-1B (MW = 9743 Da), respectively, appeared in the mass spectrum [Citation27]. shows the mass spectrum of the same sample that was investigated at a high mass region (> m/z 15000). The two peaks appearing at m/z ∼17,000 and ∼35,000 corresponded to the doubly and singly charged ions of the SLT-1 A subunit, respectively. The results indicate that the cell lysate was mainly dominated by SLT-1.
Figure 8. MALDI mass spectra of the cell lysate derived from E. coli O157:H7 obtained (A) in a low mass region and (B) in a high mass region. The molecular weight of SLT-1B is 9743 Da, while that of SLT-1 A is ∼35 kDa.
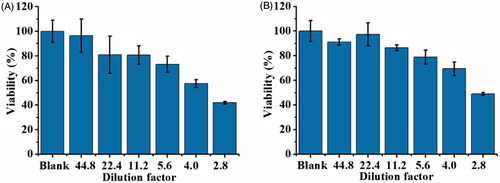
When conducting the cell infection experiment, the cell lysate was diluted with different factors for cell treatment and the cell toxicity was examined. The cell lysate derived from E. coli O157LH7 (OD600 = ∼1) contained ∼11.1 nM of SLT-1 based on our estimation by enriching SLT-1 from the cell lysate using our nanoprobes as the enrichment probes. shows the cell viability of L929 cells treated with SLT-1-containing cell lysates that were diluted with different factors. No apparent toxicity was observed when the cell lysate was diluted 44.8 fold (∼0.25 nM SLT-1), whereas the cell toxicity became apparent when the cell lysate was diluted less than 4-fold, which was estitamted to contain ∼2.78 nM SLT-1. Furthermore, the cell viability became less than 50% after the cells were infected by 2.8-fold diluted cell lysate, which was estimated to have ∼3.96 nM of SLT-1. shows the cell viability of Hep G2 cells that were infected by the SLT-containing cell lysates. The results were similar to and the cell viability was ∼50% after the Hep G2 cells were infected by 2.8-fold diluted cell lysate (∼3.96 nM of SLT-1). The results showed that the prepared cell lysate can suppress the cell growth of the model cells at the concentration of a low nanomolar.
Figure 9. Examination of the cell viability of the cells infected by SLT-1-containing cell lysate derived from E. coli O157:H7 (OD600 = ∼1), which was estimated to have SLT-1 with the concentration of ∼11.1 nM. Thus, 2.8-fold diluted cell lysate contained ∼3.96 nM of SLT-1. (A) L929 cells cultured in DMEM medium (∼4 × 104 cells mL−1, 100 μL) were incubated with the cell lysates (100 μL), which were diluted with different factors, for 1 h. The cell lysate was diluted by DMEM medium. (B) Hep G2 cells cultured in MEM medium (∼4 × 104 cells mL−1, 100 μL) were incubated with the cell lysates (100 μL), which were diluted with different factors, for 1 h. The cell lysate was diluted by MEM medium. Blank stands for the control sample that was not treated with SLT-1 containing cell lysate. Three replicates were conducted.
Examination of the toxicity of the conjugates of POA-Au NPs and SLT-1B
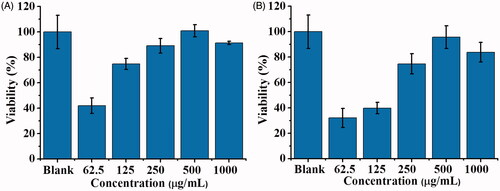
After demonstrating that the cell viability of the model cells that can reach ∼50% by incubating in a 2.8-fold diluted cell lysate (∼3.96 nM of SLT-1), derived from E. coli O157:H7, we then examine if our POA-Au NPs can be used to reduce the toxicity of SLT-1 in the SLT-1 infected model cells. POA-Au NPs were first mixed with the 2.8-fold diluted cell lysate and the resultant mixture was used to incubate with the model cells to examine the detoxification capability of POA-Au NPs. The 2.8 fold-diluted cell lysate (100 µL) was added with POA-Au NPs at different concentrations (0, 62.5, 125, 250, 500 and 1000 µgmL−1) under microwave heating (power: 900 W) for 90 s. Microwave-heating [Citation41] was used to accelerate the binding of SLT-1 on the surface of POA-Au NPs. Without further washing with buffer, the cell lysate, including different concentrations of POA-Au NPs, were used to treat L929 cells for 2 h. shows the cell viability of L929 cells that were obtained by treating with the cell lysate that contains different amounts of POA-Au NPs for 2 h. The results showed that the cell viability was lower if a lower amount of POA-Au NPs was initially present in the cell lysate. When the concentration of POA-Au NPs in the cell lysate was increased to 500 μgmL−1, the cell viability was similar to that obtained in the control sample without being treated with the cell lysate. That is, if a sufficient amount of POA-Au NPs were used to bind with the B subniuts in SLT-1 in the cell lysate first, the cells would not suffer from the toxicity derived from SLT-1. SLT-1 was blocked to infect the host cells when the cell binding moiety on SLT-1B was obstructed by POA-Au NPs because SLT-1B plays a critical role in binding with the cell membrane of host cells. shows the results of using HepG2 cells as the model cells. The results were similar to those shown in . The results suggested that POA-Au NPs can play as protective agents to inhibit SLT-1 toxicity in these SLT-1 infected model cell systems.
Figure 10. Examination of the protective roles of POA-Au NPs toward L929 cells and Hep G2 cells. Bar graphs representing the cell viability obtained from (A) L929 cells (100 μL, ∼4 × 104 cells mL−1) and (B) Hep G2 cells (100 μL, ∼4 × 104 cells mL−1) that were treated by the cell lysate containing the conjugate of SLT-1B and POA-Au NPs with different concentrations (0–1000 μgmL−1) for 2 h. Three replicates were conducted for the sample at each concentration. Blank stands for the control sample that was not treated with the cell lysate and POA-Au NPs.
Examination of the protective roles of POA-Au NPs toward SLT-1-infected cells
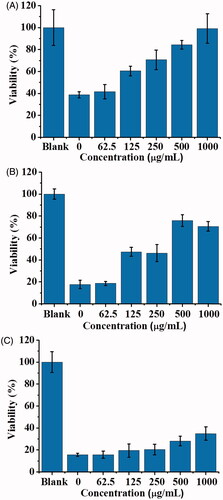
In addition, we further examined whether POA-Au NPs could be used to rescue the model cells that have been infected by the cell lysate containing SLT-1. show the cell viability of L929 cells that were infected by 2.8-fold diluted cell lysate (100 μL, ∼3.96 nM of SLT-1), derived from E. coli O157:H7 for 1, 2 and 4 h, respectively, followed by treated with POA-Au NPs at different concentrations. The cell viability was ∼40% when the model cells were incubated with the cell lysate for 1 h (, the bar graph labeled as 0). The viability dropped to ∼20% when the cells were further incubated to 2 and 4 h (). That is, the cell viability was declined as the increase of the incubation time if the cells were not treated with any POA-Au NPs. Nevertheless, the cell viability was improved after POA-Au NPs treatment. Furthemore, the cell viability was increased as the concentration of POA-Au NPs was increased. When 1000 μgmL−1of POA-Au NPs were added to the cells that had been infected by SLT-1 containing cell lysate for 1 h, the cells could be ∼100% rescued (). The cell viability declined to ∼80% when the SLT-1-infected cells were added with POA-Au NPs (1000 μgmL−1) after the cells were incubated with SLT-1 containing cell lysate for 2 h. The cell viability declined even more when POA-Au NPs (1000 μgmL−1) were added to the cells that had been incubated with SLT-1 containing cell lysate for 4 h. Only ∼40% of cells could be saved. The results indicated that POA-Au NPs have the capacity to neutralize the toxicity of SLT-1 through binding interactions. Furthermore, when a sufficient amount of POA-Au NPs was promptly added to the model cells that were only incubated with SLT-1 containing cell lysate within 1 h, the cell viability could be seen to reach ∼100%. However, the cell cannot be completely rescued if the addition of POA-Au NPs to the cells that had been incubated with SLT-1 containing cell lysate were incubated longer than 1 h. That is, timing is important in rescuing the cells that were infected by SLT-1.
Figure 11. Examination of the protective roles of POA-Au NPs toward SLT-1-infected L929 cells. Bar graphs obtained from L929 cells (100 μL, ∼4 × 104 cells mL−1) that were infected by the SLT-containing cell lysate derived from E. coli O157:H7 for (A) 1 h, (B) 2 h and (C) 4 h followed by treated with POA-Au NPs at different concentrations (0, 62.5, 125, 250, 500 and 1000 µgmL−1) for 2 h, respectively. Blank stands for the control sample that was not treated with the cell lysate and POA-Au NPs. Three replicates were conducted for each concentration.
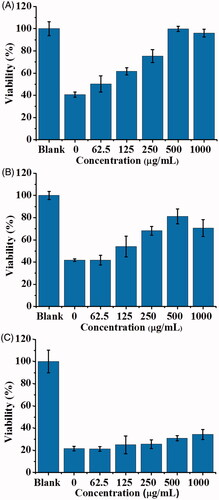
Moreover, Hep G2 cells were also selected as the model sample to further confirm the protective roles of POA-Au NPs toward SLT-1-infected cells. Hep G2 cells were infected by SLT-1 by using the same cell lysate, which was estimated to contain ∼3.96 nM of SLT-1, as used in the samples in . show the cell viability results obtained by using Hep G2 cells as the samples that were incubated with SLT-1-containing cell lysate for 1, 2 and 4 h, respectively, followed by the treatment of POA-Au NPs with different concentrations. The cell viabilities of Hep G2 cells obtained after the incubation of SLT-1-containing cell lysate with Hep G2 cells for 1 h (), 2 h () and 4 h () were ∼42, ∼40 and ∼21%, respectively, obtained without further being treated by POA-Au NPs. However, ∼100% cell viability can be achieved when the cells were incubated with the SLT-1 containing cell lysate for 1 h followed by immediate addition of POA-Au NPs with the concentration of 500 μgmL−1 (). Moreover, ∼80% cell viability can be achieved when the cells were incubated with the SLT-1 containing cell lysate for 2 h followed by immediatetreatment with POA-Au NPs (500 μgmL−1; ). Similar to what was shown in , the cell viability was hard to be improved when POA-Au NPs were added to the cells that had been incubated with SLT-1-containing cell lysate for 4 h. The cell viability was only ∼35%. These results were similar to those obtained when L929 cells were used as the model cells (). On the basis of these results, it is clear that POA-Au NPs can be used to neutralize the toxicity of SLT-1 in these model cells although the mechanism is yet unclear. Nonetheless, POA-Au NPs possess the capability to protect cells from SLT-1 damage. Furthermore, the timing of the addition of sufficient amount of POA-Au NPs to the cells infected by SLT-1 is important. The cells can be completely rescued if the administration of POA-Au NPs can be implemented after the cells were infected by SLT-1 within 1 h.
Figure 12. Examination of the protective roles of POA-Au NPs toward SLT-1-infected Hep G2 cells. Bar graphs obtained from Hep G2 cells (100 μL, ∼4 × 104 cells mL−1) that were infected by the SLT-containing cell lysate derived from E. coli O157:H7 for (A) 1 h, (B) 2 h and (C) 4 h followed by treated with POA-Au NPs at different concentrations (0, 62.5, 125, 250, 500, and 1000 µgmL−1) for 2 h, respectively. Blank stands for the control sample that was not treated with the cell lysate and POA-Au NPs. Three replicates were conducted for each concentration.
Conclusion
SLT-1 infections can cause death. However, many medicines are not avaialbe for direct inhibition of the activity of SLT-1 yet. Thus, it is important to explore effective agents that are able to inhibit the toxicity of SLT-1. In this study, the generated POA-Au NPs have been demonstrated to have the capability to neutralize the toxicity of SLT-1 in SLT-1 infected cells. According to our results, a sufficient amount of POA-Au NPs can be used to promptly rescue the SLT-infected cells and save the cell viability up to ∼100% if the administration of POA-Au NPs can be realized within 1 h after SLT-1 infections. In addition, the generated POA-Au NPs possess good cell biocompatibility. The cell toxicity was not apparent until the concentration of POA-Au NPs reached 1000 μgmL−1 and incubation time was over 4 h based on the in vitro experiments. POA-Au NPs with the concentration of 500 μgmL−1 were sufficient to save the cell viability of the two model cells to >80%. Nonetheless, the administration timing is a key point to neutralize the SLT-1 toxicity in the cell models. The administration of POA-Au NPs should not be later than 2 h to have the cell viability recovered at >80%. Given that pigeon eggs are affordable and easily available, the preparation of POA-Au NPs is simple and straightforward. It can be simply conducted by one-pot reactions. POA-Au NPs may have potential to be used in clinical treatment for SLT-1 caused illnesses. However, in vivo studies should be investigated further to demonstrate the usefulness of POA-Au NPs as the protective agents to inhibit the toxicity of SLT-1.
Acknowledgements
We are very grateful to Professor Yu-Chen Chen (Tajen University, Taiwan), Mr. Chao-Ming Su, Ms. Ling-Tun Kung and Mr. Lung-Hsing Liu for their kind help in finding fresh pigeon eggs.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Motarjemi Y, Kaferstein F, Moy G, et al. Contaminated weaning food: a major risk factor for diarrhea and associated malnutrition. Bull World Health Organ. 1993;71:79–92.
- Read SC, Gyles CL, Clarke RC, et al. Prevalence of verocytotoxigenic Escherichia coli in ground beef, pork, and chicken in southwestern Ontario. Epidemiol Infect. 1990;105:11–20.
- Slutsker L, Ries AA, Maloney K, et al. A nationwide case-control study of Escherichia coli O157:H7 infection in the United States. J Infect Dis. 1998;177:962–966.
- Flores FX, Jabs K, Thorne GM, et al. Immune response to Escherichia coli O157:H7 in hemolytic uremic syndrome following salmonellosis. Pediatr Nephrol. 1997;11:488–490.
- Boyce TG, Swerdlow DL, Griffin PM. Current concepts: Escherichia coli O157:H7 and the hemolytic-uremic syndrome. N Engl J Med. 1995;333:364–368.
- Kitova EN, Kitov PI, Bundle DR, et al. The observation of multivalent complexes of Shiga-like toxin with globotriaoside and the determination of their stoichiometry by nanoelectrospray Fourier-transform ion cyclotron resonance mass spectrometry. Glycobiology. 2001;11:605–611.
- Franz E, Klerks MM, De Vos OJ, et al. Prevalence of Shiga toxin-producing Escherichia coli stx1, stx2, eaeA, and rfbE genes and survival of E. coli O157:H7 in manure from organic and low-input conventional dairy farms. Appl Environ Microbiol. 2007;73:2180–2190.
- Rodrigue DC, Mast EE, Greene KD, et al. A university outbreak of Escherichia coli O157: H7 infections associated with roast beef and an unusually benign clinical course. J Infect Dis. 1995;172:1122–1125.
- Orden JA, Cid D, Ruiz‐Santa‐Quiteria JA, et al. Verotoxin‐producing Escherichia coli (VTEC), enteropathogenic E. coli (EPEC) and necrotoxigenic E. coli (NTEC) isolated from healthy cattle in Spain. J Appl Microbiol. 2002;93:29–35.
- Wells JG, Shipman LD, Greene KD, et al. Isolation of Escherichia coli serotype O157: H7 and other Shiga-like- toxin-producing E. coli from dairy cattle. J Clin Microbiol. 1991;29:985–989.
- Lahti E, Eklund M, Ruutu P, et al. Use of phenotyping and genotyping to verify transmission of Escherichia coli O157:H7 from dairy farms. Eur J Clin Microbiol Infect Dis. 2002;21:189–195.
- O’Brien AD, Newland JW, Miller SF, et al. Shiga-like toxin-converting phages from Escherichia coli strains that cause hemorrhagic colitis or infantile diarrhea. Science. 1984;226:694–696.
- Jackson MP, Neill RJ, O’Brien AD, et al. Nucleotide sequence analysis and comparison of the structural genes for Shiga-like toxin I and Shiga-like toxin II encoded by bacteriophages from Escherichia coli 933. FEMS Microbiol Lett. 1987;44:109–114.
- Donohue-Rolfe A, Acheson DW, Kane AV, et al. Purification of Shiga toxin and Shiga-like toxins I and II by receptor analog affinity chromatography with immobilized P1 glycoprotein and production of cross-reactive monoclonal antibodies. Infect Immun. 1989;57:3888–3893.
- Fraser ME, Fujinaga M, Cherney MM, et al. Structure of Shiga toxin type 2 (Stx2) from Escherichia coli O157:H7. J Biol Chem. 2004;279:27511–27517.
- Lingwood CA, Law H, Richardson S, et al. Glycolipid binding of purified and recombinant Escherichia coli produced verotoxin in vitro. J Biol Chem. 1987;262:8834–8839.
- Cherian RM, Gaunitz S, Nilsson A, et al. Shiga-like toxin binds with high avidity to multivalent O-linked blood group P1 determinants on mucin-type fusion proteins. Glycobiology. 2014;24:26–38.
- Ching JC, Jones NL, Ceponis PJ, et al. Escherichia coli Shiga-like toxins induce apoptosis and cleavage of poly(ADP-ribose) polymerase via in vitro activation of caspases. Infect Immun. 2002;70:4669–4677.
- Tesh VL, Samoel JE, Perera LP, et al. Evaluation of the role of Shiga and Shiga-like toxins in mediating direct damage to human vascular endothelial cells. J Infect Dis. 1991;164:344–352.
- Karmali MA. Prospects for preventing serious systemic toxemic complications of Shiga toxin-producing Escherichia coli infections using Shiga toxin receptor analogues. J Infect Dis. 2004;189:355–359.
- Walterspiel JN, Morrow AL, Cleary TG, et al. Effect of subinhibitory concentrations of antibiotics on extracellular Shiga-like toxin I. Infection. 1992; 20:25–29.
- Donta ST, Tomicic TK, Donohue-Rolfe A. Inhibition of Shiga-like toxins by brefeldin A. J Infect Dis. 1995;171:721–724.
- Dong J, Zhang Y, Chen Y, et al. Baicalin inhibits the lethality of Shiga-like toxin 2 in mice. Antimicrob Agents Chemother. 2015;59:7054–7060.
- Miyashita S, Matsuura Y, Miyamoto D, et al. Development of recombinant B subunit of Shiga-like toxin 1 as a probe to detect carbohydrate ligands in immunochemical and flow cytometric application. Glycoconj J. 1999;16:697–705.
- Kitov PI, Sadowska JM, Mulvey G, et al. Shiga-like toxins are neutralized by tailored multivalent carbohydrate ligands. Nature. 2000;403:669–672.
- Jacewicz MS, Acheson DW, Mobassaleh M, et al. Maturational regulation of globotriaosylceramide, the Shiga-like toxin 1 receptor, in cultured human gut epithelial cells. J Clin Invest. 1995;96:1328–1335.
- Kuo FY, Chang BY, Wu CY, et al. Magnetic nanoparticle-based platform for characterization of Shiga-like Toxin 1 from complex samples. Anal Chem. 2015;87:10513–10520.
- Lin PC, Yu CC, Wu HT, et al. A chemically functionalized magnetic nanoplatform for rapid and specific biomolecular recognition and separation. Biomacromolecules. 2012;14:160–168.
- Wang B, Boons G-J. Carbohydrate recognition: biological problems, methods, and applications. New Jersey: John Wiley & Sons; 2011.
- Suzuki N, Khoo KH, Chen HC, et al. Isolation and characterization of major glycoproteins of pigeon egg white ubiquitous presence of unique N-glycans containing Galα(1–4)Gal. J Biol Chem. 2001;276:23221–23229.
- Bock K, Breimer ME, Brignole A, et al. Specificity of binding of a strain of uropathogenic Escherichia coli to Gal alpha 1—4 Gal-containing glycosphingolipids. J Biol Chem. 1985;260:8545–8551.
- Takahashi N, Khoo KH, Suzuki N, et al. N-glycan structures from the major glycoproteins of pigeon egg white predominance of terminal Galα (1) Gal. J Biol Chem. 2001;276:23230–23239.
- Sequence of pigeon ovalbumin information [Internet]. [cited 2018 Feb 12]. Available from: http://www.uniprot.org/uniprot/H9CJM6
- Huang WC, Tsai PJ, Chen YC. Functional gold nanoparticles as photothermal agents for selective-killing of pathogenic bacteria. Nanomedicine. 2007;2:777–787.
- Ho KC, Tsai PJ Lin YS, et al. Using biofunctionalized nanoparticles to probe pathogenic bacteria. Anal Chem. 2004;76:7162–7168.
- Lin CC, Yeh YC, Yang CY, et al. Selective binding of mannose-encapsulated gold nanoparticles to type 1 pili in Escherichia coli. J Am Chem Soc. 2002;124:3508–3509.
- Lai HZ, Chen WY, Wu CY, et al. Potent antibacterial nanoparticles for pathogenic bacteria. ACS Appl Mater Interfaces. 2015;7:2046–2054.
- Das SK, Dickinson C, Lafir F, et al. Synthesis, characterization and catalytic activity of gold nanoparticles biosynthesized with Rhizopus oryzae protein extract. Green Chem. 2012;14:1322–1334.
- Leifert A, Pan-Bartnek Y, Simon U, et al. Molecularly stabilised ultrasmall gold nanoparticles: synthesis, characterization and bioactivity. Nanoscale. 2013;2:6224–6242.
- Li CH, Bai YL, Selvaprakash K, et al. Selective detection of Shiga-like toxin 1 from complex samples using pigeon ovalbumin functionalized gold nanoparticles as affinity probes. J Argic Food Chem. 2017;65:4359–4365.
- Chen WY, Chen YC. Acceleration of microwave-assisted enzymatic digestion reactions by magnetite beads. Anal Chem. 2007;79:8061–8066.