?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Due to size- and shape-dependent properties, a shape-controlled synthesis of silver nanoparticles (AgNPs) is one of the research challenging topics for their production and potential applications. This work reported the simple eco-friendly syntheses of different shaped AgNPs controlled by the plasmid DNA content and light emitting diodes (LEDs) irradiation. The synthesized AgNPs appeared as yellow, orange and green colloidal AgNPs, which transmission electron microscope (TEM) images revealed their different shapes; spherical AgNPs, a mixture of spherical and hexagonal AgNPs, and a mixture of spherical, hexagonal and corner-truncated triangle AgNPs, respectively. The average sizes of spherical, hexagonal and corner-truncated triangle AgNPs in the green colloidal solution were 12.32 ± 2.22, 23.03 ± 6.62 and 15.84 ± 4.31 nm, respectively. The analyses of X-ray diffraction, selected area electron diffraction and high-resolution TEM indicated the crystalline nature of the synthesized particles as the face-centred cubic silver. All synthesized AgNPs exhibited antioxidant activities similarly, whereas the yellow colloidal AgNPs exhibited the strongest antibacterial activity against both Gram-negative Escherichia coli and Gram-positive Staphylococcus aureus as compared with the green and orange colloidal AgNPs.
Introduction
With their excellent and unique properties, silver nanoparticles (AgNPs) have received many research interests for their potential applications in various fields such as biosensors, catalysts, electronics, photonics, information storages, optoelectronics, antimicrobial agents, antioxidant agents and anticancer agents [Citation1]. Interestingly, the optical, electronic, magnetic and catalytic properties of AgNPs greatly depend on their size and shape [Citation2], thus a production of size- and shape-controlled AgNPs is one of the challenging research topics to achieve the specific characteristics for their advantageous applications. Many methods have been proposed to produce non-spherical AgNPs such as bar, cube, triangle, hexagon, wire and pyramid. One of the common approaches to synthesize different shaped AgNPs is the seed-mediated chemical reaction, which the seeds are formed through the reduction of silver ions by a strong reducing agent, followed by a shape-growth step [Citation3]. Several parameters are adjusted to control the formation of certain shapes of AgNPs, including the reduction rates [Citation4], types of capping agents [Citation5,Citation6], the molar ratios of reducing agent and metal precursor, and the molar ratios of capping agent and metal precursor [Citation7].
The photo-induction process has received the increasing interest as a simple, effective approach to mediate a formation of different-shaped AgNPs [Citation8]. Several light sources, including fluorescent light, UV light, light emitting diodes (LEDs), laser and sunlight, were reported to drive the photo-reduction of metal salts and induce the growth of metal seeds into anisotropic shapes [Citation8–13]. The shape conversion direction of AgNPs was proposed to greatly depend on the excitation wavelength of a specific plasmon. Stamplecoskie et al. reported that the 3-nm citrate-capped seeds of AgNPs were transformed into the larger spheres, dodecahedra, triangular plates and nanorods via the exposure to LED irradiation at 405, 455, 627 and 720 nm, respectively [Citation9]. Under the exposure to LED irradiation at 520 and 562 nm, the citrate-capped seeds of AgNPs were also reported to transfigure to the triangular prism and circular plate, respectively [Citation14]. Alternatively, the citrate-capped seeds of AgNPs were reported for a conversion to nanoprisms by LED activation in a range of 467–630 nm [Citation15].
With increasing concerns on environmental safety, green synthesis of AgNPs has received more interests, especially the uses of natural biomacromolecules derived from plant extracts [Citation16]. Various species and parts of plants were used to mediate a formation of AgNPs such as roots of red ginseng [Citation17], barks of Terminalia arjuna [Citation18], leaves of Euphrasia officinalis [Citation19] and calluses of Catharanthus roseus [Citation20]. Phytochemicals such as terpenoids, flavones, ketones, aldehydes, amides and carboxylic acids were proposed to function to reduce Ag+ to Ag0, while complex carbohydrates in the plant extracts stabilized the produced AgNPs [Citation21]. Among these biological molecules, DNA is one of the abundant molecules that are potentially used for mediating a formation of AgNPs via a green synthesis approach but very few works were reported. Herring sperm and salmon milt DNA, for examples, were reported to facilitate a formation of spherical AgNPs under an alkaline pH with a strong reducing agent, sodium borohydride [Citation22]. Recently, we reported the use of bacterial genomic DNA for assisting a formation of spherical AgNPs via a green synthesis approach without the use of alkaline solution [Citation23]. Nevertheless, the green synthesis of non-spherical AgNPs mediated by DNA molecules under a neutral condition is still a challenge. Thus, this work is interesting to investigate the use of small size DNA, a plasmid DNA, for assisting a green production of non-spherical AgNPs via the LED-irradiation activation. Moreover, the antibacterial and antioxidant activities of the different-shaped AgNPs are investigated.
Materials and methods
Materials
Silver nitrate (AgNO3) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) were purchased from Sigma-Aldrich (St. Louis, MO). Sodium borohydride (NaBH4) was purchased from Fluka (St. Louis, MO). Muller Hinton (MH) medium was purchased from Merck (Darmstadt, Germany). Sodium chloride, yeast extract and tryptone were purchased from VWR chemical (Leicestershire, UK), CONDA (Madrid, Spain) and Criterion™ (Santa Maria, CA), respectively. All chemical used were of analytical grade.
Plasmid DNA extraction and purification
The plasmid DNA was prepared by alkaline lysis and phenol–chloroform extraction based on Sambrook et al. [Citation24]. A single colony of Escherichia coli containing the plasmid DNA, pGADT7 AD (Clontech, Mountain View, CA), was cultured in 4 ml Luria–Bertani (LB) broth containing 50 µg/ml of ampicillin at 37 °C for 6 h before transferring to culture in 200 ml fresh medium for another 18 h. The bacterial cells were harvested by centrifugation at 8000g for 2 min. The cell pellet was resuspended in 1 ml GTE buffer (50 mM glucose, 25 mM Tris-HCl pH 8.0 and 10 mM EDTA pH 8.0) and lysed in 2 ml lysis buffer (0.2 N NaOH, 1% SDS) on ice for 5 min. After adding 1.5 ml neutralization buffer (5 M potassium acetate pH 5.2), the mixture was centrifuged at 12,000g at 4 °C for 5 min to remove cell debris. The collected supernatant was mixed with one volume of isopropanol to precipitate the DNA. After DNA pellet was collected by centrifuging, it was washed once with 70% ethanol and subsequently air-dried. The DNA was treated with RNase (300 µg/ml) at 37 °C for 1 h before purifying by adding an equal volume of phenol–chloroform solution (25:25, v/v). After a centrifugation, the aqueous phase was extracted once with chloroform. The aqueous phase was collected to precipitate the DNA by adding 2.5 volumes of ethanol and 0.1 volume of 3 M sodium acetate. The DNA pellet was collected by a centrifugation and washed once with 70% ethanol, before dissolving in sterile deionized water. The DNA concentration was determined by a Nanodrop® ND-1000 Spectrophotometer (Nanodrop Technologies, Wilmington, DE). The purified plasmid DNA was visualized on a 0.8% the agarose gel.
Synthesis of AgNPs
To synthesize AgNPs, the plasmid DNA was heat-denatured in boiling water for 5 min and immediately put on ice before using. The seeds of AgNPs were prepared in the reaction containing 1.0 mM silver nitrate (2 ml), 2.0 mM NaBH4 (6 ml) and the DNA (20 µg) at room temperature for 5 min. The reaction was then exposed to different wavelengths of LED lights for 24 h; blue light (λmax = 460 nm, 20 W), green light (λmax = 520 nm, 20 W) and red light (λmax = 620 nm, 20 W). The reactions with no light exposure and no DNA were used as the controls. The formation of AgNPs was monitored by measuring the absorbance at 300–900 nm by a spectrophotometer (Analytik Jena, Jena, Germany). To study the effect of DNA content, the reactions contained different amounts of DNA (10, 20 and 40 μg) were also prepared before exposing to the 460-nm LEDs for 24 h. In addition, the formation of AgNPs in a time course of 24 h was studied by sampling the reactions using 20 and 40 μg DNA at 3, 6, 12 and 24 h.
Characterization of the synthesized AgNPs
The taken images of transmission electron microscope (TEM; FEI, Hillsboro, OR) were used to determine the morphology and size of the synthesized AgNPs. The samples were deposited on a carbon-coated copper holder and dried at room temperature before observing under TEM operating at 200 kV. The particle size was analyzed from TEM images using the Image J software (NIH, Bethesda, MD). In addition, the selected area electron diffraction (SAED) pattern and high-resolution transmission electron microscope (HRTEM) were analyzed by a conventional transmission mode of TEM operating at 200 kV. The crystalline nature of the synthesized AgNPs was analyzed by an X-ray diffractometer (XRD; Bruker, Bremen, Germany) using a Cu Kα radiation (λ = 1.5406 Å). The samples were prepared by dropping on a glass slide and air-dried before transferring to XRD analysis. The XRD measurement conditions were set as follows; a diffraction angle 2θ between 30° and 80°, a step-width Δ2θ of 0.02° and a sampling time of 0.2 s/step.
Determination of antioxidant activity
To evaluate the antioxidant activity of the synthesized AgNPs, the DPPH free radical scavenging assay was conducted according to Ajayi et al. [Citation25]. The reactions composed of 0.135 mM DPPH in absolute methanol (100 µl) and various concentrations of AgNPs (1.25, 2.5, 5, 10, 20 and 40 µg/µl) in absolute methanol (100 µl). The reaction mixtures were incubated in the dark at room temperature for 30 min before measuring the absorbance at 517 nm by a microplate reader (BioTek Instruments, Winooski, VT). The ascorbic acid (1.25–80.00 µg/µl) was used as the standard. The scavenging abilities of the synthesized AgNPs were calculated by the following equation [Citation25], where Ac is the absorbance of the DPPH control solution and As is the absorbance of the mixture of DPPH and sample solution.
Determination of antibacterial activity
Antibacterial activities of the synthesized AgNPs were determined by the minimal inhibitory concentration (MIC), the lowest concentration of AgNPs to inhibit the visible growth of the bacteria and the minimal bactericidal concentration (MBC), the lowest concentration of AgNPs to completely kill the bacteria. In this work, E. coli (ATCC 25922) and Staphylococcus aureus (ATCC 25923) were used as the representatives of Gram-negative and Gram-positive bacteria, respectively. The antibacterial assay followed the standard broth dilution method [Citation26]. Briefly, the synthesized AgNPs were twofold serially diluted before mixing with the tested bacteria at a concentration of 1 × 106 CFU/ml to obtain the final concentration of 5 × 105 CFU/ml. The bacterial cultures were incubated at 37 °C with a constant shaking at 80 rpm and the optical density (OD) at 600 nm were recorded every 6 h for 24 h in order to observe the bacterial growth. The lowest concentration of AgNPs showing no visible growth of bacteria was determined as the MIC. To determine MBC, the cultures at the MIC value and two higher concentrations were spread and cultured on MH agar plates at 37 °C for 24 h. The lowest concentration of AgNPs showed no bacterial growth on the culture plates was determined as the MBC [Citation27].
Statistical analysis
Five sample replicates were used for each experimental condition and at least two independent experiments were performed. The data were displayed as means ± standard deviations. The comparison of means was analyzed by one-way ANOVA and Tukey HSD with SPSS program (SPSS, Chicago, IL). The p values of less than .05 represented the different statistical significance.
Results and discussion
Syntheses of different-shaped AgNPs
In this work, the formation of AgNPs was assisted by the 8-kb plasmid DNA, pGADT7 AD. The plasmid was extracted and purified by the alkaline lysis and phenol–chloroform extraction methods, respectively, which 1.32 ± 0.01 mg plasmid were obtained per 200 ml culture. The absorbance ratio at 260 and 280 nm of the extracted plasmid DNA was at 1.85, which was the acceptable value as a pure DNA [Citation28]. shows the pattern of the extracted plasmid DNA as three conformations on a 0.8% agarose gel; open circular dimer, open circular and supercoiled DNA [Citation29].
Figure 1. Image of the extracted plasmid DNA visualized on an agarose gel (A) and UV–VIS spectra of the formed AgNPs under different conditions (B).
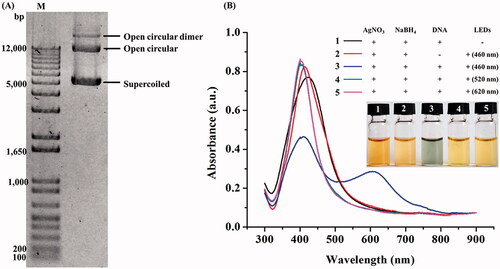
To synthesize AgNPs, the plasmid DNA (20 µg) was heat-denatured before using in the reactions composing of AgNO3 and NaBH4, before exposing to the blue (460 nm), green (520 nm) and red (620 nm) LEDs for 24 h. As seen from , the reactions exposed to green and red LEDs appeared as yellow colour with the surface plasmon resonance (SPR) peaks at 406 and 404 nm, respectively. Based on the reaction colour and the characteristic SPR peaks, it was likely that the formed AgNPs were nanospheres [Citation30]. In contrast, the reaction exposing to the blue LED appeared in green colour with the presence of two SPR peaks at 412 and 595 nm, suggesting the formation of non-spherical AgNPs [Citation31]. Although the right-shifted SPR peak may indicate the formation of larger sizes of AgNPs [Citation30], the newly emerged SPR peak in the range of 495–630 nm aside from the original SPR peak at 416–418 nm is the characteristics of non-spherical AgNPs [Citation32]. The control reactions with no light exposure and no DNA remained in yellow colour with the SPR peaks at 420 and 413 nm, respectively, indicating the presence of spherical AgNPs [Citation30]. The synthesized AgNPs in the reaction without a capping agent could be stable for few weeks since the absorbed borohydride ions on the particle surface provided the repulsive electrostatic forces to prevent the particle aggregation [Citation33]. Taken all results together, the optimal LED light and DNA were considered as the key factors for transforming the spherical seeds of AgNPs to the other particle shapes.
Since the concentration of the capping agent is one of the factors controlling the shape conversion of AgNPs [Citation8], a formation of different-shaped AgNPs was monitored in the reactions containing different DNA amounts (10–40 µg) under a blue LED irradiation for 24 h. As seen in , the reaction containing 10 µg DNA could induce a formation of spherical AgNPs but not for a shape-conversion as indicated by the remained yellow colour and the characteristic SPR peak at 413 nm [Citation30]. In contrast, the increased DNA amount to 20 µg was sufficient for inducing the formation of non-spherical AgNPs as determined by the changed colour to green and the characteristic SPR peaks at 412 and 595 nm, which corresponded to the in-plane dipole plasmon resonance of possibly formed hexagonal and triangle AgNPs [Citation32]. Similarly, the reaction containing 40 µg DNA could induce a formation of non-spherical AgNPs but the reaction turned to orange colour and its SPR peaks were at 424 and 562 nm, which was likely attributed to the formed hexagonal AgNPs [Citation32].
Figure 2. UV–VIS spectra of the synthesized AgNPs in the reactions containing different DNA contents at 24 h (A) and 20 µg DNA in a time course of 24 h (B) under the blue light irradiation.
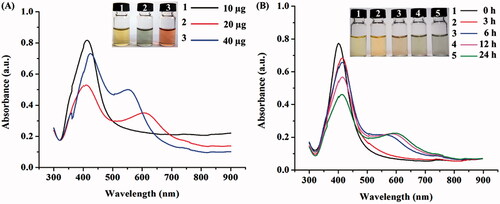
The UV–Vis spectra of the formed AgNPs in the reactions containing 20 and 40 µg DNA under blue light irradiation were also monitored in a time course of 24 h. In the reactions using 20 µg DNA, the formation of non-spherical AgNPs was first detected at 6 h as determined by the changes from yellow to orange and green colours and the SPR characteristic peaks at 412 and 595 nm (). When the irradiation times were increased, the SPR peaks were slightly right-shifted to the longer wavelengths. Similarly, in the reactions using 40 µg DNA, the formation of non-spherical AgNPs was first detected at 6 h but the reaction colour turned to orange, while the SPR peaks were observed at 424 and 562 nm (data not shown). These results confirmed the effect of DNA content on the induction of non-spherical AgNPs upon a blue light exposure.
Characterization of the synthesized AgNPs
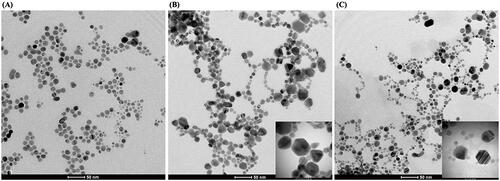
TEM images revealed the morphologies of the synthesized AgNPs as seen in . The formed AgNPs in the reaction using 10 µg DNA were all spherical with the average diameter of 11.66 ± 4.11 nm (), which was in agreement with the detection of single characteristic SPR peak at 413 nm. For the reaction using 20 µg DNA, in addition to spherical (66%) AgNPs, the hexagonal (26%) and corner-truncated triangle (8%) AgNPs were detected (), which were correlated to the characteristic SPR peaks of AgNPs at 412 and 595 nm [Citation32]. Under this condition, the average sizes of spherical, hexagonal and corner-truncated triangle AgNPs were 12.32 ± 2.22, 23.03 ± 6.62 and 15.84 ± 4.31 nm, respectively. For the reaction using 40 µg DNA, only two particle shapes were detected (); spherical (80%) and hexagonal (20%), which were also correlated to the characteristic SPR peaks of AgNPs at 424 and 562 nm [Citation32]. Under this condition, the average sizes of spherical and hexagonal AgNPs were 12.09 ± 3.24 and 16.28 ± 3.63 nm, respectively. This result suggested that the ratio of Ag+ and DNA affected the formation of non-spherical AgNPs. The percentage of obtained non-spherical AgNPs in this work was not high, the adjustment of some factors might increase the production of non-spherical AgNPs, such as pH [Citation34] and photon flux [Citation35], which the further investigation is needed. In this work, we hypothesize that the possible mechanism to produce non-spherical AgNPs involved two major steps; the small seed formation and the light-induced growth of non-spherical shape. The Ag+ ions are reduced to Ag0 atoms rapidly by the strong reducing agent, which were wrapped around by the ssDNA strands to form the small cluster of silver seeds [Citation23]. Their growth into the anisotropic shape is assisted by the LED-light activation and the selective binding of nitrogenous bases on particular planes of nanoparticles [Citation36]. The electromagnetic (EM) field produced by LED-light can excite and cause the coalescence of the nanoparticle seeds [Citation9], which the pattern of coalescence may be related to the bindings between DNA and AgNPs. Due to the negatively charged functional groups of DNA chain, it is likely that DNA may preferentially bind to the [111] planes of planar seeds, thus covering the top and bottom surfaces [Citation37]. As the result, the addition of silver atoms on these planes is blocked; therefore, the silver atoms preferentially deposit on the [1 0 0] plane leading to the growth of triangular and hexagonal plates [Citation36]. In this work, the blue LED is the only light source that provides enough energy to induce the formation of non-spherical shapes of AgNPs. In contrast, the green and red LEDs, which have the lower energy, could not provide enough energy to drive the shape transformation of AgNPs as analyzed by TEM images (data not shown).
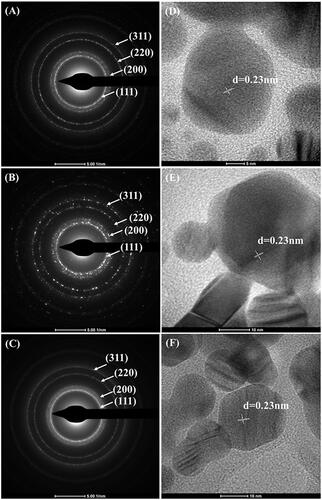
Figure 3. Representative TEM images of the synthesized yellow (A), green (B) and orange (C) colloidal AgNPs.
The identity of all synthesized AgNPs of the reactions using 10, 20 and 40 µg DNA was further analyzed by SEAD-TEM and HR-TEM. The SEAD-TEM patterns of all synthesized AgNPs demonstrated four diffraction rings, which were indexed as [111], [200], [220] and [311] planes ()), well corresponding to the Bragg's planes of face-centred cubic (fcc) structure of silver [Citation38]. These concentric rings revealed that the synthesized AgNPs were polycrystalline in nature. The HR-TEM images of the synthesized AgNPs as seen in ) revealed their d-spacing value of 0.23 nm, in a good agreement with the lattice fringe [111] plane of fcc structure of silver [Citation39].
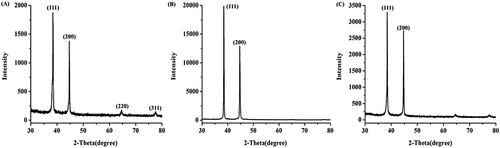
In addition, XRD analysis was conducted to confirm the crystalline nature of the synthesized AgNPs. shows the XRD pattern of the yellow-colloidal AgNPs, four diffraction peaks in the 2θ range of 30°–80° were detected, corresponding to the [111], [200], [220] and [311] planes of fcc structure of metallic silver (JCPDS file no. 89-3722) [Citation40]. On the other hand, the XRD patterns of the green and orange colloidal AgNPs, which contained the additional forms of hexagonal and corner-truncated triangle AgNPs, showed two major peaks at 38.4° and 44.7°, corresponding to the [111] and [200] facets, respectively ()). In general, the high population of hexagonal and corner-truncated triangle AgNPs exhibits the intense [111] peak due to their [111]-covering faces [Citation41]. The presence of the peak corresponding to the [200] facet in this result possibly attributed to the population of spherical AgNPs in the reactions.
Antioxidant activity of the synthesized AgNPs
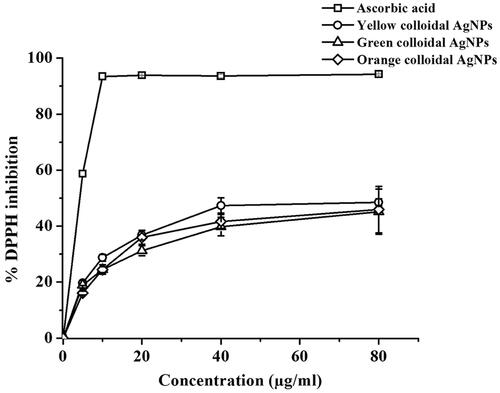
The antioxidant activity of the synthesized AgNPs was also evaluated by the DPPH scavenging assay based on the activity of tested samples to reduce DPPH radicals, which is expressed as % DPPH inhibition [Citation42]. The maximum absorption of the DPPH radicals at 517 nm is decreased upon the reduction by the tested sample together with the decolourization of the DPPH solution from violet colour (the free radical form, diphenyl picrylhydrazyl) to a yellow colour (the non-free radical form, diphenyl picryhydrazine). The DPPH radical scavenging activities of the positive control, ascorbic acid and the synthesized (yellow, green and orange colloidal) AgNPs at 5–40 μg/ml are shown in . With increasing in concentrations, all synthesized AgNPs exhibited the antioxidant activity in a similar dose-dependent response. The inhibition concentration 50% (IC50) values of the yellow, green and orange colloidal AgNPs were similar at 7.84 ± 0.43, 7.34 ± 0.76, and 7.84 ± 0.32 μg/ml, respectively. Based on the IC50 values, the antioxidant activities of the synthesized AgNPs were lower than the control ascorbic acid (4.41 ± 0.13 μg/ml). Nevertheless, their antioxidant activities were greater than many reported activities of AgNPs, which were in a range of 70–400 μg/ml [Citation43–45]. In contrast, some synthesized AgNPs were reported to exhibit the greater antioxidant activity than the AgNPs in this work, especially ones synthesized using plant extracts as capping agents, which the strong antioxidant activity of these synthesized AgNPs partially attribute to the antioxidant activity of the capping agents [Citation46]. Since the plasmid DNA, the capping agent, in this work exhibited less or no antioxidant activity, the synthesized AgNPs in this work exhibited the antioxidant activity not as high as some reported AgNPs using capping agents possessing antioxidant activity.
Antibacterial activity of the synthesized AgNPs
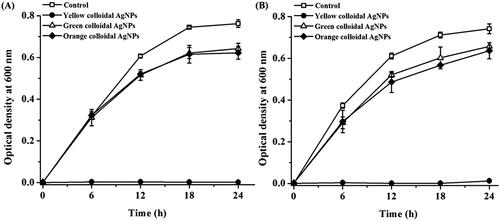
One of the interesting properties of AgNPs is their broad-spectrum antibacterial activity and this property is depended on their size and shape [Citation47]. In this work, a micro-broth dilution method was performed to investigate the antibacterial activity of the synthesized AgNPs. The growths of the representative Gram-negative and Gram-positive bacteria in response to various concentrations (5–80 μg/ml) of synthesized AgNPs in a time course of 24 h were monitored. The results showed that all synthesized AgNPs exhibited the antibacterial activity against both bacterial strains in a dose-dependent response. shows the representative graphs demonstrating the growths of E. coli and S. aureus in response to the yellow, green and orange colloidal AgNPs at 20 µg/ml in a time course of 24 h. Among them, the yellow colloidal AgNPs exhibited the strongest antibacterial activity against both bacterial strains. The antibacterial activities of the green and orange colloidal AgNPs were similar and lower than that of the yellow colloidal AgNPs. The minimum inhibition concentration (MIC) values of the yellow, green and orange AgNPs against E. coli and S. aureus were equal at 20, 40 and 40 μg/ml, respectively. Their minimum bactericidal concentration (MBC) values of the yellow, green and orange AgNPs against E. coli were 20, 40 and 40 μg/ml, respectively, while the MBC values against S. aureus were higher at 40, 80 and 80 μg/ml, respectively. The less sensitivity of S. aureus to the AgNPs likely attributed to the thicker peptidoglycan layer of its cell wall [Citation48]. In this work, spherical (yellow colloidal) AgNPs exhibited the greater antibacterial activity than the green and orange colloidal AgNPs, which contained the addition of formed hexagonal and corner-truncated triangle AgNPs. Unlike this result, El-Zahry et al. [Citation49] reported that the hexagonal AgNPs exhibited the greater antibacterial activity than the spherical AgNPs at the same size (40 nm). Since the antibacterial activity of AgNPs was size-dependent, we hypothesized that due to their larger sizes, hexagonal and corner-truncated triangle AgNPs exhibited the lower antibacterial activity than the smaller spherical AgNPs in this work. Nevertheless, the yellow, green and orange colloidal AgNPs exhibited the strong antibacterial activity against both E. coli and S. aureus with the MBC values in the range of 40–80 μg/ml. Due to their small sizes (7.44–29.05 nm), the antibacterial activity of the synthesized AgNPs likely attributed via both the accumulated particles at the bacterial cell membrane and the penetrated particles inside the bacterial cells. The attached AgNPs can interfere the normal function of the cell membrane, for instant, permeability and cellular respiration [Citation50]. The penetrated AgNPs can interact with DNA and proteins, resulting in the disruption of DNA replication, protein denaturation and activity of enzymes. In addition, AgNPs can induce a formation of cellular free radicals, eventually leading to the death of cells [Citation51].
Conclusions
This work demonstrated the photo-induced shape conversion of AgNPs using the 8.0-kb plasmid DNA, pGADT7, to assist the formation of non-spherical AgNPs for the first time. The DNA content, the wavelength of LEDs and irradiation time were the key factors to convert the seeded AgNPs into anisotropic shapes. In addition to the blue LEDs, the different DNA contents affected the formation of spherical, hexagonal and corner-truncated AgNPs. The yellow colloidal AgNPs were all spherical shape, while the green colloidal AgNPs were 66% spherical, 26% hexagonal and 8% corner-truncated triangle shapes. In addition, the orange colloidal AgNPs were 80% spherical and 20% hexagonal shapes. The identity of all synthesized AgNPs was confirmed by SEAD-TEM, HR-TEM and XRD analyses, which suggested their polycrystalline in nature and purity. All synthesized AgNPs exhibited the antioxidant and antibacterial activities in a dose–response manner. Among them, the yellow colloidal AgNPs exhibited the greatest antibacterial activity against both E. coli and S. aureus, suggesting their potential applications as an antibacterial agent.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Beyene HD, Werkneh AA, Bezabh HK, et al. Synthesis paradigm and applications of silver nanoparticles (AgNPs), a review. SM&T. 2017;13:18–23.
- González A, Noguez C, Beránek J, et al. Size, shape, stability, and color of plasmonic silver nanoparticles. J Phys Chem C. 2014;118:9128–9136.
- Sajanlal PR, Sreeprasad TS, Samal AK, et al. Anisotropic nanomaterials: structure, growth, assembly, and functions. Nano Rev. 2011;2:5883–5945.
- Mansouri SS, Ghader S. Experimental study on effect of different parameters on size and shape of triangular silver nanoparticles prepared by a simple and rapid method in aqueous solution. Arab J Chem. 2009;2:47–53.
- Zeng J, Zheng Y, Rycenga M, et al. Controlling the shapes of silver nanocrystals with different capping agents. J Am Chem Soc. 2010;132:8552–8553.
- Xia Y, Xia X, Peng H-C. Shape-controlled synthesis of colloidal metal nanocrystals: thermodynamic versus kinetic products. J Am Chem Soc. 2015;137:7947–7966.
- Rivero PJ, Goicoechea J, Urrutia A, et al. Effect of both protective and reducing agents in the synthesis of multicolor silver nanoparticles. Nanoscale Res Lett. 2013;8:101–109.
- Tang B, Sun L, Li J, et al. Sunlight-driven synthesis of anisotropic silver nanoparticles. Chem Eng J. 2015;260:99–106.
- Stamplecoskie KG, Scaiano JC. Light emitting diode irradiation can control the morphology and optical properties of silver nanoparticles. J Am Chem Soc. 2010;132:1825–1827.
- Zheng X, Xu W, Corredor C, et al. Laser-induced growth of monodisperse silver nanoparticles with tunable surface plasmon resonance properties and a wavelength self-limiting effect. J Phys Chem C. 2007;111:14962–14967.
- Khamhaengpol A, Siri S. Fluorescent light mediated a green synthesis of silver nanoparticles using the protein extract of weaver ant larvae. J Photochem Photobiol B Biol. 2016;163:337–344.
- Lee J-H, Lim J-M, Velmurugan P, et al. Photobiologic-mediated fabrication of silver nanoparticles with antibacterial activity. J Photochem Photobiol B Biol. 2016;162:93–99.
- Verma S, Rao BT, Srivastava A, et al. A facile synthesis of broad plasmon wavelength tunable silver nanoparticles in citrate aqueous solutions by laser ablation and light irradiation. Colloids Surf A Physicochem Eng Asp. 2017;527:23–33.
- Krajczewski J, Joubert V, Kudelski A. Light-induced transformation of citrate-stabilized silver nanoparticles: photochemical method of increase of SERS activity of silver colloids. Colloids Surf A Physicochem Eng Asp. 2014;456:41–48.
- Saade J, de Araújo CB. Synthesis of silver nanoprisms: a photochemical approach using light emission diodes. Mater Chem Phys. 2014;148:1184–1193.
- Jayaprakash N, Vijaya JJ, Kaviyarasu K, et al. Green synthesis of Ag nanoparticles using Tamarind fruit extract for the antibacterial studies. J Photochem Photobiol B Biol. 2017;169:178–185.
- Singh P, Kim YJ, Wang C, et al. Biogenic silver and gold nanoparticles synthesized using red ginseng root extract, and their applications. Artif Cells Nanomed Biotechnol. 2016;44:811–816.
- Ahmed Q, Gupta N, Kumar A, et al. Antibacterial efficacy of silver nanoparticles synthesized employing Terminalia arjuna bark extract. Artif Cells Nanomed Biotechnol. 2017;45:1192–1200.
- Singh H, Du J, Singh P, et al. Ecofriendly synthesis of silver and gold nanoparticles by Euphrasia officinalis leaf extract and its biomedical applications. Artif Cells Nanomed Biotechnol. 2017;45:1–8.
- Osibe DA, Chiejina NV, Ogawa K, et al. Stable antibacterial silver nanoparticles produced with seed-derived callus extract of Catharanthus roseus. Artif Cells Nanomed Biotechnol. 2017;45:1–8.
- Upadhyay LSB, Verma N. Recent developments and applications in plant-extract mediated synthesis of silver nanoparticles. Anal Lett. 2015;48:2676–2692.
- Nithyaja B, Misha H, Nampoori V. Synthesis of silver nanoparticles in DNA template and its influence on nonlinear optical properties. J Nanosci Nanotechnol. 2012;2:99–103.
- Chumpol J, Siri S. Simple green production of silver nanoparticles facilitated by bacterial genomic DNA and their antibacterial activity. Artif Cells Nanomed Biotechnol. 2017;45:1–7.
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. New York (NY): Cold Spring Harbor Laboratory Press; 1989.
- Ajayi E, Afolayan A. Green synthesis, characterization and biological activities of silver nanoparticles from alkalinized Cymbopogon citratus Stapf. Adv Nat Sci: Nanosci Nanotechnol. 2017;8:1–7.
- Wiegand I, Hilpert K, Hancock RE. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc. 2008;3:163–175.
- Krishnan R, Arumugam V, Vasaviah SK. The MIC and MBC of silver nanoparticles against Enterococcus faecalis – a facultative anaerobe. J Nanomed Nanotechnol. 2015;6:285–288.
- Boesenberg-Smith KA, Pessarakli MM, Wolk DM. Assessment of DNA yield and purity: an overlooked detail of PCR troubleshooting. Clin Microbiol Newsl. 2012;34:1–6.
- Dixit K, Ali R. Antigen binding characteristics of antibodies induced against nitric oxide modified plasmid DNA. Biochim Biophys Acta. 2001;1528:1–8.
- Agnihotri S, Mukherji S, Mukherji S. Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv. 2014;4:3974–3983.
- Tao AR, Habas S, Yang P. Shape control of colloidal metal nanocrystals. Small. 2008;4:310–325.
- Liu M, Leng M, Yu C, et al. Selective synthesis of hexagonal Ag nanoplates in a solution-phase chemical reduction process. Nano Res. 2010;3:843–851.
- Mulfinger L, Solomon SD, Bahadory M, et al. Synthesis and study of silver nanoparticles. J Chem Educ. 2007;84:322–325.
- Yu P, Huang J, Tang J. Observation of coalescence process of silver nanospheres during shape transformation to nanoprisms. Nanoscale Res Lett. 2011;6:46–53.
- Lee GP, Shi Y, Lavoie E, et al. Light-driven transformation processes of anisotropic silver nanoparticles. ACS Nano. 2013;7:5911–5921.
- Li J, Zhu Z, Liu F, et al. DNA‐mediated morphological control of silver nanoparticles. Small. 2016;12:5449–5487.
- Yi Z, Xu X, Wu X, et al. Silver nanoplates: controlled preparation, self-assembly, and applications in surface-enhanced Raman scattering. Appl Phys A. 2013;110:335–342.
- Szeremeta J, Nyk M, Samoc M. Photocurrent enhancement in polythiophene doped with silver nanoparticles. Opt Mater. 2014;37:688–694.
- Al-Ghamdi HS, Mahmoud WE. Synthesis of self-assembly plasmonic silver nanoparticles with tunable luminescence color. J Lumin. 2014;145:880–883.
- Anandalakshmi K, Venugobal J, Ramasamy V. Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl Nanosci. 2016;6:399–408.
- Park YM, Lee BG, Weon J-I, et al. One-step synthesis of silver nanoplates with high aspect ratios: using coordination of silver ions to enhance lateral growth. RSC Adv. 2016;6:95768–95773.
- Patra JK, Das G, Baek K-H. Phyto-mediated biosynthesis of silver nanoparticles using the rind extract of watermelon (Citrullus lanatus) under photo-catalyzed condition and investigation of its antibacterial, anticandidal and antioxidant efficacy. J Photochem Photobiol B Biol. 2016;161:200–210.
- Kharat SN, Mendhulkar VD. Synthesis, characterization and studies on antioxidant activity of silver nanoparticles using Elephantopus scaber leaf extract. Mater Sci Eng C. 2016;62:719–724.
- Huo Y, Singh P, Kim YJ, et al. Biological synthesis of gold and silver chloride nanoparticles by Glycyrrhiza uralensis and in vitro applications. Artif Cells Nanomed Biotechnol. 2018;46:303–312.
- Moteriya P, Chanda S. Synthesis and characterization of silver nanoparticles using Caesalpinia pulcherrima flower extract and assessment of their in vitro antimicrobial, antioxidant, cytotoxic, and genotoxic activities. Artif Cells Nanomed Biotechnol. 2017;45:1556–1567.
- Khan FU, Chen Y, Khan NU, et al. Antioxidant and catalytic applications of silver nanoparticles using Dimocarpus longan seed extract as a reducing and stabilizing agent. J Photochem Photobiol B Biol. 2016;164:344–351.
- Hong X, Wen J, Xiong X, et al. Shape effect on the antibacterial activity of silver nanoparticles synthesized via a microwave-assisted method. Environ Sci Pollut Res. 2016;23:4489–4497.
- Janthima R, Khamhaengpol A, Siri S. Egg extract of apple snail for eco-friendly synthesis of silver nanoparticles and their antibacterial activity. Artif Cells Nanomed Biotechnol. 2018;46:361–367.
- El-Zahry MR, Mahmoud A, Refaat IH, et al. Antibacterial effect of various shapes of silver nanoparticles monitored by SERS. Talanta. 2015;138:183–189.
- Guzman M, Dille J, Godet S. Synthesis and antibacterial activity of silver nanoparticles against gram-positive and gram-negative bacteria. Nanomed Nanotech Biol Med. 2012;8:37–45.
- Pandey JK, Swarnkar R, Soumya K, et al. Silver nanoparticles synthesized by pulsed laser ablation: as a potent antibacterial agent for human enteropathogenic gram-positive and gram-negative bacterial strains. Appl Biochem Biotechnol. 2014;174:1021–1031.