?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The present study aimed to evaluate the efficacy of cellulose acetate/gelatin/nanohydroxyapatite (CA/Gel/nHA) nanocomposite mats as the wound dressing. The dressings were prepared with electrospinning of CA/Gel solutions containing 12.5, 25 and 50 mg nHA. The dressings were evaluated regarding their water uptake capacity, morphology, tensile strength, water vapour transmission rate, wettability and cellular response with L929 cell line. The results showed that the concentration of nHA had a direct correlation with porosity, water contact angle, water uptake, water vapor transmission rate and proliferation. In vivo studies showed that all dressings had higher wound closure percent than the sterile gauze, as the control. The highest wound closure value was achieved in the CA/Gel +25 mg nHA group, which showed 93.5 ± 1.6%. The histological and the histomorphometric examinations of the wounds revealed that the CA/Gel +25 mg nHA dressing had the greatest collagen synthesis, re-epithelialization, neovascularization and also the best cosmetic appearance. Based on our finding, it could be concluded the applicability of electrospun nanofibrous CA/Gel/nHA dressings for successful wound treatment.
Introduction
Skin is a multifunctional and the largest organ of the human body and any disruption in its continuity resulting from thermal or physical damage is defined as the wound. Wound healing is a very complex and dynamic process involving various cells, cytokines, growth factors, vitamins and minerals [Citation1–3]. The suitable wound dressing is a key factor in wound care and subsequently in the healing process [Citation4,Citation5]. A good wound dressing should be able to provide or maintain a moist environment, promote connective tissue synthesis and angiogenesis, enhance epidermal migration, allow gas exchange between environment and wounded tissue and also must be non-allergic, sterile and non-toxic [Citation4,Citation6]. Different kinds of dressing are being used to study the healing process of the skin wound including decellularized porcine, hydrogels, freeze-dried, gas-foaming and dermal matrix-based structures [Citation5,Citation7].
Recently, nanofibrous electrospun wound dressings have attracted a lot of attention in wound healing due to their promising properties [Citation8–10]. The fibrous structure of electrospun nanofibres mimics the extracellular matrix (ECM) of skin, their high surface to volume ration allows incorporation of various drugs and bioactive agents into and on the surface of these structures [Citation11,Citation12]. Moreover, the porosity of the electrospun nanofibres allows gas exchange between wounded tissue and environment, on the other hand, prevent penetration of infections form environment to wound site [Citation7,Citation13,Citation14].
Electrospinning is enabling technology capable to produce micro and nanofibres from a variety of synthesis and natural sources with biologically relevant features. Generally, an electrospinning apparatus comprises of three main parts, an injection system as the nozzle, a collector and a high-voltage power supply connected to nozzle and collector [Citation15–18]. Applied high voltage as the main driving force of fibres formation charges the polymer solution fed into the nozzle toward the collector [Citation19,Citation20,Citation21].
Cellulose acetate (CA), a soluble and low-cost derivative of cellulose, can be efficiently processed into membranes, films and fibres from either solution or melts [Citation22]. Electrospun CA nanofibres have various applications such as sensors, catalyst, drug delivery carriers, filter, and scaffolds for tissue engineering [Citation23,Citation24]. In recent years, some researchers blended CA with the other polymers for wound dressing applications [Citation25–27]. Gelatin (Gel) is another commercially available biopolymer that is widely used in wound dressing applications due to its excellent biocompatibility, non-immunogenicity and biodegradability [Citation28,Citation29].
Incorporation of bioactive agents in wound dressing could help the healing process. Several studies have shown the established role of calcium in the normal haemostasis of skin, keratinocyte differentiation and proliferation [Citation30–33]. The local calcium can modulate epidermal lipid barrier function, cell proliferation, maturation and motility which are vital for dermal reconstruction and epidermal regeneration in healing process [Citation32,Citation34–36].
Nanocrystalline hydroxyapatite (nHA), a calcium-phosphate based bioceramic, is a major component of healthy bone [Citation37,Citation38]. These crystals are a large source of calcium and phosphate for the body along with their biological and mechanical functions. nHA has many diverse biomedical applications due to its biocompatibility, bioactivity, osteoconductivity, non-toxicity and non-inflammatory nature [Citation39–41].
In the present study, we combined the nanofibrous structure of the electrospun cellulose acetate/gelatin membrane with bioactivity and biocompatibility of nHA for wound dressing application. The gelatin was blended with the CA to obtain a composite with improved cytocompatibility. Moreover, nHA was used as the bioactive agent not only to improve the biocompatibility of the dressing but also to provide a local concentration of calcium.
Materials and methods
Materials
The solvents and materials were purchased from Merck (Darmstadt, Germany) and Sigma-Aldrich (St. Louis, MO, USA), respectively, unless otherwise noted.
Methods
Synthesis of nHA
A modified wet chemical precipitation method was used for the synthesis of nHA as follows. Ca(OH)2 (7.48 g) was dissolved in a 100 ml volume of ethanol–water solution mixture (50:50%, v/v) at 33 °C via vigorous magnetic stirring for 3 h. At 35 °C, 6.7 g of ammonium dihydrogen phosphate ((NH4)H2PO4) was dissolved in 100 ml volume water and then added to the Ca(OH)2 solution over a period of 24 h. NaOH 1 M was used to adjust the pH of the solution at 11 and monitored digitally during the precipitation reaction. The slurry finally was frozen at −80 °C for 24 h and freeze-dried (Telstar, Terrassa, Spain) for 48 h [Citation42].
Electrospinning of nanofibres
Cellulose acetate [CA; white powder; Mw = 30,000 Da; acetyl content =39.7% (w/w)] and gelatin powder (bovine skin, type B) with a weight ratio of 25:75 (wt%) was dissolved in HFP to obtain the final concentration of 6% w/v. After stirring for 24 h and obtaining a homogeneous polymer solution, nHA with a series of 12.5, 25 and 50 mg/10 cc solution were dispersed in the prepared polymer solution.
The polymer solution was loaded into a 10-ml disposable syringe and feed to the surface of the nozzle, an 18 gauge stainless steel needle, with a flow rate of 0.8 ml/h via a syringe pump (Fanavaran Nano-Meghyas, Tehran, Iran). A high-voltage source (Fanavaran Nano Meghyas, Tehran, Iran) was used to apply 18 kV voltages between the needle and the collector. The distance between the tip of the nozzle and the surface of the collector, a steel drum covered with aluminium foil, was set at 13 cm. Accessed nanofibrous mats were cross-linked by the vapour of glutaraldehyde solution 25% for 18 h followed by extensive washing with distilled water and drying at ambient temperature.
The size of the prepared nHA was determined by dynamic light scattering device (DLS; Malvern Instruments, Worcestershire, UK). The nHA morphology was observed by Scanning Electron Microscopy (SEM; KYKY Technology Development, Beijing, China). Before the observation, the suspension was frozen at −80 °C for 24 h and freeze-dried (Telstar, Terrassa, Spain) for 48 h, then sputtered with gold and examined at an accelerating voltage of 26 kV.
The surface morphology of the nHA-incorporated nanofibres was observed by the SEM. The mates were sputtered with a thin layer of gold and examined at an accelerating voltage of 18 kV. The diameter of electrospun nanofibres was statistically calculated using Fiji/ImageJ (National Institutes of Health, Bethesda, MD, USA) by measuring 20 fibres at random.
The wettability of the nHA-incorporated nanofibres was evaluated via a static contact-angle measuring device (KRUSS, Hamburg, Germany) under ambient temperature and humidity. In order to mechanical property evaluation, five pieces of rectangular mates were cut with the dimensions of 20 mm ×60 mm and evaluated by a universal testing machine (Santam, Karaj, Iran) at a displacement rate of 10 mm/min under a load of 10 N.
Water-uptake and water vapour transmission rate
EquationEquation (1)(1)
(1) was used to calculate the water-uptake capacity which shows the capacity of the wound dressing to absorb wound exudates [Citation43,Citation44]:
(1)
(1)
where W0 is the weight of dry samples and W1 is the weight of samples after immersion in PBS at room temperature for 24 h.
The water vapour transmission rate (WVTR) across the dressing was determined according to our previous work. Briefly, the opening of a round container with the diameter of 1.23 cm was fixed with the nanofibrous mat. The containers were filled with 5 ml of distilled water and placed in an oven at 37 °C for 48 h and EquationEquation (2)(2)
(2) was used to calculate WVTR [Citation43]. Containers without the nanofibrous mat on their openings were used as the control:
(2)
(2)
where ΔW is the water weight (g) change and A is the exposure area (m2).The average value of three samples for each mat was reported.
Porosity measurement
The liquid displacement method was used to evaluate the porosity of the mats, using the following equation [Citation45]:
(3)
(3)
where V1, V, and V3 are initial volume of 96% ethanol, its volume after a scaffold was soaked in (and ethanol filled the pores) and volume of ethanol after the scaffold removal, respectively.
Cell culture studies
L929 murine fibroblastic cell line was obtained from the National Cell Bank of Iran (NCBI), Pasteur Institute of Iran, Tehran, Iran, and cultured in Dulbecco’s modified Eagle’s medium: nutrient mixture F-12 (DMEM/F12; Gibco, Grand Island, NY, USA) supplemented with 10% (v/v) foetal bovine serum (FBS; Gibco, Grand Island, NY, USA), 100 unit/mL of penicillin and 100 mg/mL of streptomycin in a humidified incubator at 37 °C with 5% CO2. The cell media was changed every other day.
The cells proliferation on the wound dressings was evaluated using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). Nanofibrous mats were cut circularly and put on the bottom of the 96-well plates so that the entire surface of the well was covered. The sterilization was performed via UV light for 60 min followed by twice washing with phosphate-buffered saline (PBS, pH = 7.4) and once with DMEM/F12. After the sterilization, a density of 1 × 104 cells/well was seeded on the mats.
In vivo wound healing study
The wound healing efficacy of the dressings was evaluated via a full-thickness excision wound model. 36 healthy adult male Wistar rats (3 months old, weighing 250–270 g) were purchased from Pasteur Institute (Tehran, Iran) and housed with access to diet and water ad libitum for 3 d before experimentation. The rats were anesthetized by intraperitoneal injection of Ketamine 5%/Xylazine 2% [1:4 (v/v), 0.10 ml/100 g body weight]. The dorsal hair of each rat was shaved and disinfected with ethanol at the operation site, and then a full-thickness 1.5 × 1.5 cm2 wound to the depth of all the skin layers was excised using a scalpel blade.
The animals were divided randomly into six groups (six rats per group) as follows: four test group and two control (positive and negative) groups. The test groups were treated with CA/Gel, CA/Gel +125 mg nHA, CA/Gel +25 mg NHA and CA/Gel +50 mg nHA. The groups without any wound and the wound treated with the sterile gauze were positive and negative controls, respectively.
Animal experiments were approved by the ethical committee of Tehran University of Medical Sciences and were carried out in accordance with the university guidelines. An elastic adhesive bandage was used to fix the dressing on the wounded area. The wound closure was evaluated 7 and 14 d post-wounding via macroscopic changes in the wound area recorded by a digital camera (Canon Inc., Tokyo, Japan) and measured using an image analysing program (Digimizer, Ostend, Belgium). The following equation is used to calculate the wound closure:
(4)
(4)
Histopathology study
The animals were euthanized 14 d post-treatment and the harvested tissue specimens (containing the entire wound and adjacent normal skin) have fixed in the 10% neutral buffered formalin (pH 7.26) for 48 h, and after processing and embedding in paraffin were cross-sectioned and stained with haematoxylin–eosin (H&E) and Masson's Trichrome (MT). The histological slides were evaluated by the independent reviewer using light microscopy (Olympus BX51; Olympus, Tokyo, Japan). Epithelialization, inflammatory cell infiltration, fibroplasia, neovascularization, granulation tissue formation and collagen deposition have assessed in different groups, comparatively.
Statistical analysis
All results were compared using Student's t-tests or one-way analysis of variance (ANOVA). Results with p values of less than .05 were considered statistically significant. Statistical analyses were performed using the SPSS software, version 20.0 (SPSS, Inc, Chicago, IL, USA).
Results
Characterization of nHA and nanofibrous dressings
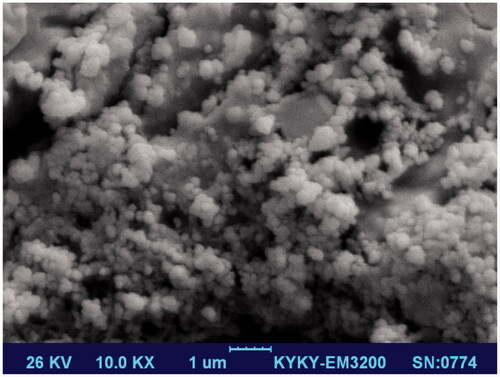
The morphology and the size of nHA were observed via SEM and DLS, respectively. The results of DLS show that the average size of HA nanoparticles is 540.5 ± 38.5 nm with a polydispersity index (PDI) of 0.32. As shown in , nHA particles had spherical morphology with a smooth surface.
Among the various methods for fabrication of nHA, wet chemical precipitation has been widely used due to its simplicity and efficacy. The results of the present study show that the wet chemical precipitation has this ability to produce monodisperse and uniform nHA.
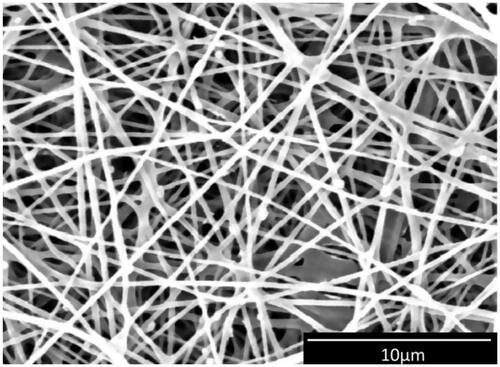
Incorporation of nHA into the electrospun nanofibres is a simple and effective method to fabricate bioactive structures. As shown in , SEM micrograph of CA/Gel +25 mg nHA, nanofibres are oriented in a random, dispersive manner, forming a non-woven porous structure. The diameter of the nanofibres was 316 ± 115 nm, calculated using ImageJ (NIH Image J system, Bethesda, MD, USA).
Hydrophilicity
A proper wound dressing should be hydrophilic to absorb the wound exudates and also retain the wound bed moist. The hydrophilicity of the prepared dressing was evaluated via the water contact angles and the results show that incorporating nHA into CA/Gel nanofibres decrease the water contact angles (). The water contact angles value for AC-Gel, AC-Gel +12.5 nHA, AC-Gel+ 25 nHA and AC-Gel+ 50 nHA were 61.25 ± 0.75, 58.5 ± 1.5, 57.25 ± 0.75 and 56.5 ± 0.5, respectively. These results show that each dressing has good hydrophilic nature as the wound dressing. Comparison between each dressing shows that the differences are not statistically significant.
Table 1. Characterization of the wound dressings.
Porosity (%)
The porosity of the mats was estimated based on the liquid displacement method and the results show that in all prepared nanofibres the porosity is high enough (>65%) to be suitable for wound dressing applications [Citation46]. As shown in , increasing the concentration of nHA enhanced the porosity of AC-Gel. However, the differences between porosity values are not statistically significant.
Tensile strength
The mechanical properties as the important characteristic determine the applicability of dressing. The tensile strength and flexibility of dressing should be enough to withstand the handling and replacement of the dressing during the wound healing period. The results show that incorporating nHA into CA/Gel nanofibres reduce the ultimate tensile strength from 3.01 ± 0.07 to 2.98 ± 0.05, 2.82 ± 0.04, 2.68 ± 0.06 MPa, for AC-Gel +12.5 nHA, AC-Gel+ 25 nHA and AC-Gel+ 50 nHA, respectively (). The differences between AC-Gel and AC-Gel+ 25 nHA and AC-Gel+ 50 nHA were statistically significant (p < .05).
Water vapour transmission rate and water-uptake capacity
The wound healing process is closely related to WVTR of dressing and a proper wound dressing should be able to control the gas exchange through the dressing. A dressing with the high WVTR dehydrates the wound and results in scar formation, on the other hand, low WVTR increases the risk of infection and delays the healing process due to the accumulation of exudates. The results of our study show that increasing nHA concentration has a direct effect on WVTR and water-uptake capacity of the nanofibrous mat ().
The WVTR of CA/Gel nanofibres is 8.93 ± 0.98 mg/cm2 h and the addition of 12.5, 25 and 50 mg nHA increased WVTR to 9.6 ± 0.66, 11.33 ± 0.62 and 11.63 ± 1.11 mg/cm2 h, respectively. The results also show that WVTR for all dressing is statistically significant in comparison with the control (an open container) (p < .005). Water uptake as the other valuable property of a wound dressing was investigated and the results show that increasing the concentration of nHA enhanced water uptake value (). The differences between water uptake values are not statistically significant.
In vitro proliferation study
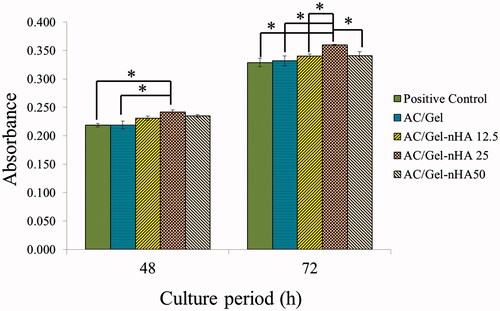
To further explore L929 cells proliferation on nanofibrous dressings, MTT assay was carried out at 48 and 72 h.
As shown in , the CA/Gel dressings could promote the cells proliferation in an obvious dose-dependent tendency at 48 and 72 h. The results show that 48 h after cell seeding the proliferation of L929 cells on AC/Gel +25 mg nHA membrane was higher than control and the other groups (). The proliferation of this group was only statistically significant compared with the control and AC/Gel (p < .05). The cells proliferation on AC/Gel +25 mg nHA membrane was significantly higher than the other groups 72 h after cell seeding (p < .05). The results show that the highest OD value was detected in the AC/Gel +25 mg nHA group at each time point. Overall, the results of cell proliferation assay showed that due to its cytocompatible nature, the incorporation of nHA mad CA/Gel more suitable for cell proliferation.
Figure 3. In vitro cell culture results: histogram comparing the proliferation of L929 cells on the wound dressings 48 h and 72 h after cell seeding. The positive control is tissue culture plate (TCP). The data are expressed as the mean ± SD, n = 3; *p < .05, **p < .01 (obtained by Student’s t-test).
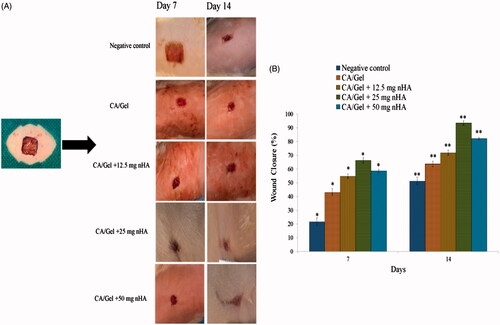
In vivo wound healing study
The healing efficacy of the prepared dressings was further investigated via in vivo study and the results are presented in . As shown in , there are no signs of inflammation or infection in any of the wounds. The wounds in the control and CA/Gel groups were fresh and still bleeding on day 7. The wound margin in the CA/Gel +25 mg nHa group was unclear which is the sign of the marginal regeneration in this group. The wound healing process was quantified by the wound closure percent measurement (). The nanofibrous wound dressings possessed higher average wound closure than the negative control group in the both time intervals (p < .05). The average wound closure for the negative control group on days 7 and 14 were 21.59 ± 2.9% and 51.23 ± 2.81%, respectively. The highest wound closure percent was observed in the CA/Gel +25 mg nHa group among the all studied groups with the average wound closure of 66.26 + 1.91% and 93.56 + 1.6% on days 7 and 14 post-wounding, respectively. The difference between the observed wound closure percent was statistically significant in all groups (p < .05).
Figure 4. In vivo wound healing results. (A) Macroscopic appearances of the treated wounds 7 and 14 d post-wounding. (B) Histograms comparing the wound closure percentages of the wound dressings at the end of 7th and 14th day post-wounding. The data are expressed as mean ± SD, n = 5. * = Significant difference from all in day 7 (p < .05). **Significant difference from all in day 14 (p < .05).
Histopathological study
Histological analysis of the skin wounds was performed by H&E and MT staining, as shown in . Normal skin tissue consists of three main layers including epidermis, dermis, and hypodermis (). The wounds in the sterile gauze group (negative control) displayed evident neutrophils infiltration (, thin arrows) and granulation tissue (GT) formation. However, the epidermal layer has not been formed yet and the wound covered by a crusty scab (, thick arrows).
Figure 5. Haematoxylin–eosin-stained (H&E stained) microscopic sections of healed incisions in rats at 14 d: D: defect; positive control: normal skin; negative control: sterile gauze-treated wound; thick arrows in E: crusty scab; thick arrows in L: epidermal proliferation; arrows head in Q and R: foreign body reaction; thin arrow in R: chronic inflammatory cells.
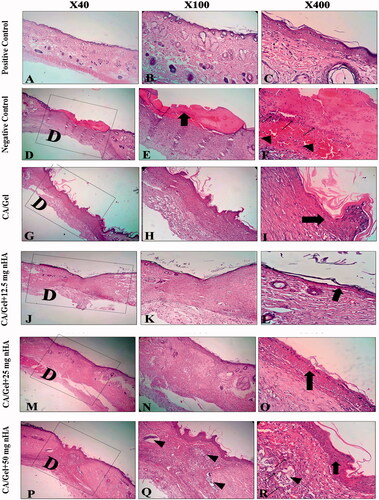
As shown in , there is the sign of epidermis and dermis formation in the CA/Gel group (, thick arrow), however, the thickness of skin was remarkably lower than the other groups. Histopathology of CA/Gel +12.5 mg nHA-treated wounds displayed the epidermal proliferation (, thick arrow) and increasing the dermis layer thickness. CA/Gel +25 mg nHA-treated group showed more resemblance to the normal skin in comparison to the other groups, with a thin epidermis (, thick arrow) and normal thickness of skin layers. Histopathological evaluation of CA/Gel +50 mg nHA treated showed hyperplasia of epidermal layer and foreign body reaction (, arrows head). It seems that increasing the percentage of nHA over 25 mg can stimulate the immune system and subsequently a foreign body reaction. Although, the epidermis layer covered the whole area of the wound in this group (, thick arrow) and chronic inflammatory cells including macrophages, lymphocytes and giant cells infiltrated in the wound site (, thin arrow) 14-d post-treatment. These results indicated that, the CA/Gel +50 mg nHA was less biocompatible than the CA/Gel, CA/Gel +12.5 and CA/Gel +25 mg nHA treatment groups.
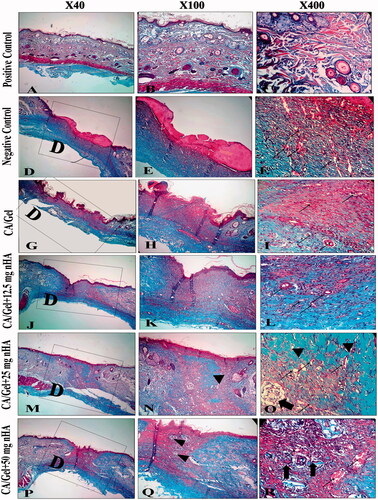
MT staining was used to evaluate the collagen formation and distribution in the wounded area during the healing process. Collagen fibres were stained blue-green in MT staining method which the intensity of these colours corresponds to the relative amount of deposited collagen and reflects the advancement of collagen synthesis and remodelling.
The results indicated that among the experimental groups, CA/Gel +25 mg nHA had the greatest collagen synthesis. On the other hand, the rate of collagen fibres synthesis and deposition in wound site were the lowest in the CA/Gel group.
Histomorphometric analysis
Histomorphometric examinations were used to quantitatively evaluate the healing process of the wound under influence of the prepared dressings after 14 d of skin injury.
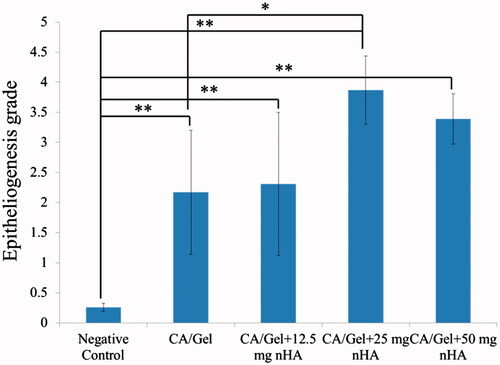
Re-epithelialization degree as the most important part of the wound healing process was investigated via histomorphometric analysis and the results are represented in . Amongst the all groups, re-epithelialization in the negative control group was minimum; and the wound area was mostly filled with immature granulation tissue. Moreover, the all prepared dressings induced the re-epithelialization statistically significant than the negative control group (p < .01). The CA/Gel +25 mg nHA dressing had the best performance and induced the highest re-epithelialization in the wound site which was significantly higher than the CA/Gel group (p < .05).
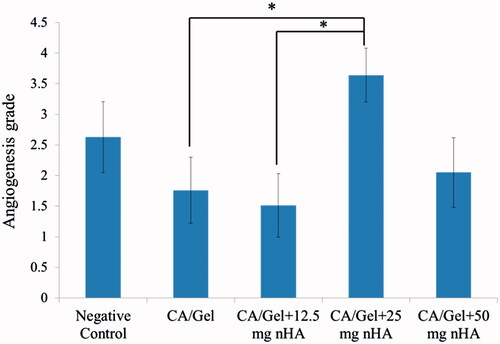
Angiogenesis as the other critical requirement of the wound repair was investigated in control and test groups and the results are represented in .
As shown in the angiogenesis index for the CA/Gel +25 mg nHA group was significantly higher than those for the CA/Gel and CA/Gel +12.5 mg nHA (p < .05). Interestingly, the angiogenesis index for the control group was higher than CA/Gel, CA/Gel +12.5 mg nHA and CA/Gel +50 mg nHA.
Overall, the healing condition of the CA/Gel +25 mg nHA-treated group was similar to that of the control negative group at day 14. Among the experimental groups, the best cosmetic appearance was observed in the 25 mg nHA group which induced the neovascularization (, thin arrows) and hair follicles had grown very fast (, thick arrows).
Figure 6. Masson’s trichrome (MT stained) microscopic sections of healed incisions in rats at 14 d. D: defect; positive control: normal skin; negative control: sterile gauze-treated wound; thick arrow in R: hair follicle; thick arrows in O: multinucleated giant cells; arrowheads in Q and R: collagen deposition; arrowheads in N: foreign body reaction; thin arrows: angiogenesis.
Discussion
The bioactive structures are promising for the wound dressing applications due to their ability to improve the wound healing process. nHA as the biocompatible, non-toxic and no-allergic bioactive-ceramic has various application in tissue engineering [Citation47,Citation48]. In the present study, we fabricated electrospun nanofibrous CA/Gel containing nHA composite and evaluated its potential as the wound dressing.
The wettability of the dressings which represent the ability of dressings to adhere to the wound bed was evaluated and results showed a direct correlation between the concentration of the nHA and the wettability degree. Increasing the concentration of nHA increased the wettability value and consequently, the water uptake capacity of the dressings due to the hydrophilic nature of nHA. The previous studies have shown that incorporating nHA could decrease the water contact angles of nanofibrous film. Xiuling Xu et al. [Citation17] reported that increasing the nHA concentration reduced the poly(l-lactide) nanofibres water contact angle. Another study showed that increasing the concentration of nHA decreased the water contact value of poly(ε-caprolactone) nanofibres [Citation49]. Mohd Izzat Hassan et al. [Citation49] reported that PCL/nHA composite has higher water uptake value than pure PCL. The other study showed that nHA improved the water-binding ability of the electrospun eri silk fibroin scaffold [Citation5].
The results of WVTR examination also showed that increasing the nHA concentration increased the WVTR which is related to the bigger pore size of the dressing with a higher concentration of nHA. The mechanical strength evaluation showed that incorporating nHA decreased the Ultimate Tensile Strength of dressings due to the increase in stiffness of the dressings via increasing the nHA concentration. The previous studies have shown the similar trend for electrospun PCL/nHA and PLA/nHA scaffolds [Citation50]. The results of MTT assay showed that nHA has the positive effect on the proliferation of fibroblast cells and the CA/Gel +25 mg nHA represent the highest proliferation. The impacts of nHA on the proliferation of various cell types have investigated in previous studies. Xiang Gao et al. [Citation51] reported that nHA had acceptable cytocompatibility at the concentration range of 0–10 wt% and beyond this range was cytotoxic due to the ROS production. The other study showed that deposition of HA onto the surface of electrospun poly(ɛ-caprolactone)–poly(ethylene glycol)–poly(ɛ-caprolactone) improve the cytocompatibility [Citation52].
The efficacy of nHA containing electrospun CA/Gel nanofibrous dressings was evaluated via the in vivo evaluation. The results showed that the prepared dressings had the higher wound closure than the gauze-treated wound (negative control) and the highest wound closure percent was obtained with CA/Gel +25 mg nHA dressing. The survival of keratinocytes and the newly formed granulation tissue depend on neovascularization which provides metabolites and oxygen to the tissues [Citation53–55]. nHA has this ability to stimulate capillary endothelium toward an angiogenic phenotype via up-regularization of fibroblast growth factor-2 (FGF-2) and endothelial nitric oxide synthase (eNOS) [Citation13]. The results of the histomorphometric analysis showed that dressings containing 25 mg nHA represent the highest angiogenesis and re-epithelialization indexes. Some studies have investigated the effect of calcium-based nanoparticles on wound healing process [Citation30,Citation31,Citation56,Citation57]. Ramana Ramya et al. [Citation58] investigated the effects of gamma irradiated agarose–gelatin/nHA composite on skin tissue regeneration. Their results showed that the agarose–gelatin/nHA composite has good biocompatibility, haemocompatibility, antimicrobial efficacy and bioactivity. They suggest that the prepared nanocomposite is suitable for skin wound dressing and soft tissue regeneration. In another study, polarized hydroxyapatite and silk fibroin (pHA/SF) composite was evaluated as the dressing gel. The results showed that pHA/SF induced promotive effects on wound healing, re-epithelization, matrix formation and advanced the maturation of fibroblast cells [Citation57].
Conclusion
In the present study, we fabricated and evaluated cellulose acetate/gelatin/hydroxyapatite nanocomposite mats as a wound dressing. The results showed that the properties of the nanocomposite mats are favourable for wound dressing applications. In vitro proliferation study showed that CA/Gel +25 mg nHA represent the highest proliferation among the control and the test groups. Along with the in vitro studies, the in vivo evaluation also confirmed the promising efficacy of CA/Gel +25 mg nHA as the wound dressing. Based on the obtained results, it could be concluded that the prepared dressing is favourable for the successful wound treatment.
Disclosure statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Robson MC, Steed DL, Franz MG. Wound healing: biologic features and approaches to maximize healing trajectories. Curr Probl Surg. 2001;38:72–140.
- Szycher M, Lee SJ. Modern wound dressings: a systematic approach to wound healing. J Biomater Appl. 1992;7:142–213.
- Singh MR, Saraf S, Vyas A, et al. Innovative approaches in wound healing: trajectory and advances. Artif Cells Nanomed Biotechnol. 2013;41:202–212.
- Karahaliloglu Z, Kilicay E, Denkbas EB. Antibacterial chitosan/silk sericin 3D porous scaffolds as a wound dressing material. Artif Cells Nanomed Biotechnol. 2017;45:1172–1185.
- Rahmani Del Bakhshayesh A, Annabi N, Khalilov R, et al. Recent advances on biomedical applications of scaffolds in wound healing and dermal tissue engineering. Artif Cells Nanomed Biotechnol. 2017 [cited Jul 12]. DOI:https://doi.org/10.1080/21691401.2017.1349778
- Dhivya S, Padma VV, Santhini E. Wound dressings – a review. Biomedicine. 2015;5:137–145.
- Chen S, Liu B, Carlson MA, et al. Recent advances in electrospun nanofibers for wound healing. Nanomedicine. 2017;12:1335–1352.
- Farzamfar S, Naseri-Nosar M, Samadian H, et al. Taurine-loaded poly (ε-caprolactone)/gelatin electrospun mat as a potential wound dressing material: in vitro and in vivo evaluation. J Bioact Compat Polym. 2017 [cited Nov 14]. DOI:https://doi.org/10.1177/0883911517737103
- Liu M, Duan X-P, Li Y-M, et al. Electrospun nanofibers for wound healing. Mater Sci Eng: C. 2017;76:1413–1423.
- Pilehvar-Soltanahmadi Y, Akbarzadeh A, Moazzez-Lalaklo N, et al. An update on clinical applications of electrospun nanofibers for skin bioengineering. Artif Cells Nanomed Biotechnol. 2016;44:1350–1364.
- Chen X, Zhao R, Wang X, et al. Electrospun mupirocin loaded polyurethane fiber mats for anti-infection burn wound dressing application. J Biomater Sci Polym Ed. 2017;28:162–176.
- Farzamfar S, Naseri-Nosar M, Vaez A, et al. Neural tissue regeneration by a gabapentin-loaded cellulose acetate/gelatin wet-electrospun scaffold. Cellulose. 2017 [cited Dec 21]. DOI:https://doi.org/10.1007/s10570-017-1632-z
- Ahmadi-Aghkand F, Gholizadeh-Ghaleh Aziz S, Panahi Y, et al. Recent prospective of nanofiber scaffolds fabrication approaches for skin regeneration. Artif Cells Nanomed Biotechnol. 2016;44:1635–1641.
- Aytimur A, Uslu İ. Promising materials for wound dressing: PVA/PAA/PVP electrospun nanofibers. Polym Plast Technol Eng. 2014;53:655–660.
- Samadian H, Zakariaee SS, Adabi M, et al. Effective parameters on conductivity of mineralized carbon nanofibers: an investigation using artificial neural networks. RSC Adv. 2016;6:111908–111918.
- Samadian H, Mobasheri H, Hasanpour S, et al. Electrospinning of polyacrylonitrile nanofibers and simulation of electric field via finite element method. Nanomed Res J. 2017;2:87–92.
- Otsuka I, Njinang CN, Borsali R. Simple fabrication of cellulose nanofibers via electrospinning of dissolving pulp and tunicate. Cellulose. 2017;24:3281–3288.
- Tungprapa S, Puangparn T, Weerasombut M, et al. Electrospun cellulose acetate fibers: effect of solvent system on morphology and fiber diameter. Cellulose. 2007;14:563–575.
- Samadian H, Mobasheri H, Hasanpour S, et al. Needleless electrospinning system, an efficient platform to fabricate carbon nanofibers. J Nano Res. 2017;50:78–79.
- Eatemadi A, Daraee H, Zarghami N, et al. Nanofiber: synthesis and biomedical applications. Artif Cells Nanomed Biotechnol. 2016;44:111–121.
- Angammana CJ, Jayaram SH. Fundamentals of electrospinning and processing technologies. Particul Sci Technol. 2016;34:72–82.
- Nosar MN, Salehi M, Ghorbani S, et al. Characterization of wet-electrospun cellulose acetate based 3-dimensional scaffolds for skin tissue engineering applications: influence of cellulose acetate concentration. Cellulose. 2016;23:3239–3248.
- Ahn HR, Tak TM, Kwon Y-N. Preparation and applications of poly vinyl alcohol (PVA) modified cellulose acetate (CA) membranes for forward osmosis (FO) processes. Desalin Water Treat. 2015;53:1–7.
- Frey MW. Electrospinning cellulose and cellulose derivatives. Polym Rev. 2008;48:378–391.
- Liu X, Lin T, Gao Y, et al. Antimicrobial electrospun nanofibers of cellulose acetate and polyester urethane composite for wound dressing. J Biomed Mater Res. 2012;100:1556–1565.
- Vatankhah E, Prabhakaran MP, Jin G, et al. Development of nanofibrous cellulose acetate/gelatin skin substitutes for variety wound treatment applications. J Biomater Appl. 2014;28:909–921.
- Tong WY, bin Abdullah AYK, binti Rozman NAS, et al. Antimicrobial wound dressing film utilizing cellulose nanocrystal as drug delivery system for curcumin. Cellulose. 2018;25:631–638.
- Wang C, Zhu F, Cui Y, et al. An easy-to-use wound dressing gelatin-bioactive nanoparticle gel and its preliminary in vivo study. J Mater Sci: Mater Med. 2017;28:10.
- Chang W-H, Chang Y, Lai P-H, et al. A genipin-crosslinked gelatin membrane as wound-dressing material: in vitro and in vivo studies. J Biomater Sci Polym Ed. 2003;14:481–495.
- Lansdown AB. Calcium: a potential central regulator in wound healing in the skin. Wound Repair Regen. 2002;10:271–285.
- Kawai K, Larson BJ, Ishise H, et al. Calcium-based nanoparticles accelerate skin wound healing. PLoS One. 2011;6:e27106.
- Magee AI, Lytton NA, Watt FM. Calcium-induced changes in cytoskeleton and motility of cultured human keratinocytes. Exp Cell Res. 1987;172:43–53.
- Motta G. Calcium alginate topical wound dressings: a new dimension in the cost-effective treatment for exudating dermal wounds and pressure sores. Ostomy Wound Manage. 1989;25:52–56.
- Dlugosz AA, Yuspa SH. Protein kinase C regulates keratinocyte transglutaminase (TGK) gene expression in cultured primary mouse epidermal keratinocytes induced to terminally differentiate by calcium. J Invest Dermatol. 1994;102:409–414.
- Lee SH, Jiang S, Choi EH, et al. Iontophoresis itself on hairless mouse skin induces the loss of the epidermal calcium gradient without skin barrier impairment. J Invest Dermatol. 1998;111:39–43.
- Trump BF, Berezesky IK, Sato T, et al. Cell calcium, cell injury and cell death. Environ Health Perspect. 1984;57:281.
- Zhu W, Wang D, Xiong J, et al. Study on clinical application of nano-hydroxyapatite bone in bone defect repair. Artif Cells Nanomed Biotechnol. 2015;43:361–365.
- Hernandez-Soria A, Yang X, Grosso MJ, et al. In vitro elution characteristics of antibiotic laden BoneSource™, hydroxyapatite bone cement. J Biomater Sci Polym Ed. 2013;24:797–806.
- Zakaria SM, Sharif Zein SH, Othman MR, et al. Nanophase hydroxyapatite as a biomaterial in advanced hard tissue engineering: a review. Tissue Eng Part B: Rev. 2013;19:431–441.
- Pepla E, Besharat LK, Palaia G, et al. Nano-hydroxyapatite and its applications in preventive, restorative and regenerative dentistry: a review of literature. Ann Stomatol. 2014;5:108.
- Prakasam M, Locs J, Salma-Ancane K, et al. Fabrication, properties and applications of dense hydroxyapatite: a review. Jfb. 2015;6:1099–1140.
- Salehi M, Naseri-Nosar M, Ebrahimi-Barough S, et al. Regeneration of sciatic nerve crush injury by a hydroxyapatite nanoparticle-containing collagen type I hydrogel. J Physiol Sci. 2017 [cited Sep 6]. DOI:https://doi.org/10.1007/s12576-017-0564-6
- Salehi M, Farzamfar S, Bastami F, et al. Fabrication and characterization of electrospun PLLA/collagen nanofibrous scaffold coated with chitosan to sustain release of aloe vera gel for skin tissue engineering. Biomed Eng Appl Basis Commun. 2016;28:1650035.
- Arslan A, Şimşek M, Aldemir SD, et al. Honey-based PET or PET/chitosan fibrous wound dressings: effect of honey on electrospinning process. J Biomater Sci Polym Ed. 2014;25:999–1012.
- Wienk I, Folkers B, van den Boomgaard T, et al. Critical factors in the determination of the pore size distribution of ultrafiltration membranes using the liquid displacement method. Sep Sci Technol. 1994;29:1433–1440.
- Xu R, Xia H, He W, et al. Controlled water vapor transmission rate promotes wound-healing via wound re-epithelialization and contraction enhancement. Sci Rep. 2016;6:24596.
- Oliveira HL, Da Rosa WL, Cuevas-Suárez CE, et al. Histological evaluation of bone repair with hydroxyapatite: a systematic review. Calcif Tissue Int. 2017;101:341–354.
- Mhaske M, Kedar P, Bansode S. Tissue engineering: a review. Tissue Eng. 2017;2:280--285.
- Hassan MI, Sultana N, Hamdan S. Bioactivity assessment of poly (ε-caprolactone)/hydroxyapatite electrospun fibers for bone tissue engineering application. J Nanomater. 2014;2014:8.
- Xu X, Chen X, Liu A, et al. Electrospun poly (L-lactide)-grafted hydroxyapatite/poly (l-lactide) nanocomposite fibers. Eur Polym J. 2007;43:3187–3196.
- Gao X, Song J, Ji P, et al. Polydopamine-templated hydroxyapatite reinforced polycaprolactone composite nanofibers with enhanced cytocompatibility and osteogenesis for bone tissue engineering. ACS Appl Mater Interfaces. 2016;8:3499–3515.
- Fu S, Yang L, Fan J, et al. In vitro mineralization of hydroxyapatite on electrospun poly (ɛ-caprolactone)–poly (ethylene glycol)–poly (ɛ-caprolactone) fibrous scaffolds for tissue engineering application. Colloids Surf B: Biointerfaces. 2013;107:167–173.
- Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736–1743.
- Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746.
- Tonnesen MG, Feng X, Clark RA, editors. Angiogenesis in wound healing. Journal of Investigative Dermatology Symposium Proceedings; 2000: Elsevier.
- Kaneko A, Hirai S, Tamada Y, et al. Evaluation of calcium phosphate-coated silk fabric produced by sol–gel processing as a wound cover material. Sen-i Gakkaishi. 2009;65:97–102.
- Okabayashi R, Nakamura M, Okabayashi T, et al. Efficacy of polarized hydroxyapatite and silk fibroin composite dressing gel on epidermal recovery from full‐thickness skin wounds. J Biomed Mater Res. 2009;90:641–646.
- Ramya JR, Arul KT, Sathiamurthi P, et al. Novel gamma irradiated agarose-gelatin-hydroxyapatite nanocomposite scaffolds for skin tissue regeneration. Ceramics Int. 2016;42:11045–11054.