Abstract
Aim
It was previously reported that the ratio of soluble fms-like tyrosine kinase-1 (sFlt-1) to placental growth factor (PlGF) can predict the clinical onset of preeclampsia. This study seeks to validate the association between ratios of sFlt-1/PlGF with preeclampsia and to identify the contribution of ethnicity across diverse populations of the Xinjiang Uygur Autonomous Region.
Methods
Pregnant women were classified into those with preeclampsia (n = 136) and healthy controls (n = 350). Serum levels of sFlt-1 and PlGF were quantified using a Roche serum instrument in both patients and controls.
Results
Compared to healthy controls, women with preeclampsia had significantly higher levels of sFlt-1 (7303.81 pg/ml vs. 2508.69 pg/ml, p < .001) and ratios of sFlt-1/PlGF (241.68 vs. 14.29, p < .001), whereas levels of PIGF were decreased (241.68 vs. 14.29, p < .001). These three values varied greatly across nationalities, and non-Han Chinese subjects (including Uygur, Kazak, Hui) were more likely to experience severe preeclampsia than Han Chinese subjects.
Conclusions
This is the first study to demonstrate that the ratio of sFlt-1/PlGF can both predict and serve as a diagnostic factor for preeclampsia in pregnant women from different populations within the Xinjiang region of China.
Keywords:
Introduction
Preeclampsia is a pregnancy-related disease that can lead to severe complications and subsequent health problems for both the mother and the baby [Citation1,Citation2]. In pregnant women, symptoms of preeclampsia include headache, impaired vision, epigastric pain, thrombocytopenia, haemolysis or liver abnormalities, renal function and even death. Preeclampsia also affects the foetus and may cause intrauterine growth restriction (IUGR), premature delivery, placental abruption and foetal/neonatal death [Citation3,Citation4]. Every year, approximately 2–8% of pregnant women are affected by preeclampsia, but this rate is higher in specific geographic regions, especially in the developing world. Globally, preeclampsia accounts for 12% of all maternal deaths [Citation5].
Development of preeclampsia is thought to result from the contribution of immunologic, economic, social, racial and genetic risk factors [Citation5,Citation6], and due to this complex etiology, the path to pathogenesis is not clearly understood. Molecular markers associated with the onset of preeclampsia represent a potential therapeutic tool to predict the risk of developing the disease. Previous studies have screened a wide-range of potential biomarkers that may help in diagnosis [Citation7,Citation8] and found elevated levels of soluble fms-like tyrosine kinase-1 (sFlt-1) and reduced levels of placental growth factor (PlGF) in the preeclampsia patients even prior to the development of clinical symptoms [Citation9–14]. sFlt-1 is an antagonist of PlGF and vascular endothelial growth factor (VEGF), which are the main angiogenic factors driving placental vascular development and maternal endothelial function. Increased levels of sFlt-1 result in higher antagonistic-activity and prevents PlGF and VEGF from interacting with endogenous receptors in the blood vessels [Citation15,Citation16]. In recent years, utilization of the Elecsys immunoassay to monitor sFlt-1/PlGF ratios in addition to clinical symptoms is strongly recommended to diagnose preeclampsia during the second half of pregnancy (week 20 to week 34, plus six days of gestation). A high ratio of sFlt-1/PlGF is associated with an increased risk of preeclampsia, and this combined measure may have more predictive power than either biomarker could achieve alone [Citation10,Citation17–21]. As a result, Roche Diagnostics (Penzberg, Germany) developed the Elecsys immunoassay for sFlt-1 and PlGF, which have received Conformité Européenne (CE) certification for use as in vitro medical devices [Citation17]. Diagnosis and prediction of preeclampsia using the automated Roche Elecsys sFlt-1/PlGF immunoassay were recently incorporated into official guidelines for many countries, yet the use of sFlt-1/PlGF ratios in preeclampsia prediction and diagnosis are only validated in predominantly European populations. Data for this method from Chinese populations are insufficient, as are data from less-represented ethnic minorities in China.
Xinjiang is an ethnically diverse region of China with a high incidence of preeclampsia, especially in the Uygur, Kazak and various other ethnic groups. The relationship between ethnicity and the risk of preeclampsia is well documented with some studies reporting the morbidity of the Uygur 2.4 times higher than the Han nationality. It is not yet known if the predictive and diagnostic value of sFlt-1/PlGF ratio is similarly related to ethnicity across these populations.
To address this, we worked with the ethnically diverse Xinjiang population in Northwest region of China to determine if acute presentation and the clinical course of preeclampsia differed among women from different ethnic groups. Utilizing the Roche serum instrument, we sought to clarify the predictive and diagnostic efficacy of sFlt-1/PlGF ratio for preeclampsia in the Xinjiang Uygur Autonomous Region to provide insight into the potential role of protein factors in the pathophysiologic condition of preeclampsia.
Materials and methods
Human subjects
A total of 486 women (136 cases and 350 controls) were recruited for this study. All participants were examined and treated at The Xinjiang Uygur Autonomous Region Maternal and Child Health Care Hospital from January 2016 to October of 2016. Diagnostic criteria for preeclampsia were based on international guidelines provided in Table S1 [Citation22,Citation23]. Participants in the control group were randomly selected from the hospital during the same period, and only included those with no history of chronic disease or pregnancy-related complications. This study was approved by the Ethics Committee of Northwest University and the Ethics Committee of Xinjiang Uygur Autonomous Region Maternal and Child Health Care Hospital. Informed consent was obtained from all participants.
Table 1. Maternal characteristics in women with preeclampsia and control subjects.
Blood sampling
All samples were collected at a gestational period of >20 weeks prior to diagnosis of preeclampsia. A total of 4 ml of peripheral blood was collected from the participants’ vein in a disposable vacuum blood tube. Within 2 h, after blood collection, samples were centrifuged at 2000 × g for 10 min to isolate serum. In both cohorts, blood samples were transported in a cooler with dry ice within 1–4 days to the Test Center of the National Engineering Research Center.
Serum level detection
Measurements of sFlt-1 and PlGF using the fully automated Roche Elecsys Systemand (Penzberg, Germany) were immediately performed at the Test Center of the National Engineering Research Center upon sample arrival, and the sFlt-1/PlGF ratio was calculated for each sample immediately after.
Statistical analysis
Data analysis was performed using SPSS version 20.0 (SPSS, Chicago, IL). Continuous variables were described using mean ± standard deviation (SD) and mean (range), and the independent sample t-test was used for continuous variables to compare patients and healthy controls. Logistic regression analyses were employed to assess the maternal characteristics, which included age, BMI, nationalities and medical history. In parallel, we performed subgroup analyses of the three values (sFlt-1, PlGF and sFlt-1/PlGF ratio) in different ethnic groups. Odds ratios (ORs) with 95% confidence intervals (CI) were calculated to measure microscopic maternal characteristics. We used p < .05 as the cutoff for statistical significance. Receiver operating characteristics (ROC) curves and tabulations of ROC results were performed for determinations of area under the curve (AUC), and three predictive values were calculated at selected cut-off values with determination of OR, specificity and sensitivity. Sub-analyses on early-onset and late-onset preeclampsia in different ethnic populations were also analysed in the same time.
Results
Description and clinical features of preeclampsia patients and healthy controls
presents the demographic and clinical characteristics of the study population. The characteristics of the preeclampsia group include maternal age, pre-gestation BMI, gestational age, systolic blood pressure and diastolic blood pressure. All are significantly higher in the preeclampsia group than in the control group. Deeper comparison of the potential covariates between 136 cases and 350 controls was provided in . Women who had a maternal age of 25–29 (OR 1.21, 95%CI 0.63–2.33) and 30–34 (OR 1.37, 95%CI 0.66–2.85) had lower odds of preeclampsia. A low BMI (<18.5) (OR 0.517, 95%CI 0.235–1.138) was not a risk factor for preeclampsia. In addition, maternal age of ≥35 (OR 1.37, 95%CI 0.66–2.85), overweight (BMI 25–30) (OR 3.69, 95%CI 2.36–6.63) and obese (BMI ≥30) (OR 3.299, 95%CI 1.12–9.70) were all associated with higher odds of preeclampsia. Finally, women who are non-Han Chinese (OR 2.766, 95%CI 1.84–4.17), had a medical history (OR 2.55, 95%CI 1.28–5.06) and those with prior children (OR 1.74, 95%CI 1.10–2.77) were more likely to get preeclampsia.
Table 2. Microscopic characteristics of the maternal characteristics in women with preeclampsia.
The distribution of preeclampsia in different nationalities is shown in . The incidence of preeclampsia in Uygur (49.4%), Kazak (53.3%) and other minorities (55.56%) was significantly higher than that in the Han nationality (20.69%). In our data, there were 40 mild cases of preeclampsia and 96 severe cases when stratifying by clinical severity. Of all the patients with severe preeclampsia, the proportion in non-Han Chinese populations was larger than in Han Chinese populations (13.17% vs. 32.34%, respectively).
Table 3. Distribution of preeclampsia and severe-preeclampsia in different nationalities.
Association between sFlt-1, PlGF and sFlt-1/PlGF levels and preeclampsia in different nationalities
The serum levels of sFlt-1 and PlGF are shown in . sFlt-1 levels were significantly higher in preeclamptic women compared to healthy controls (median (range)): 7303.81 (724.2–39,911) pg/ml vs. 2508.69 (232.70–20090.00) pg/ml, respectively. Similarly, the ratio of sFlt-1/PlGF was significantly higher in preeclamptic women compared to healthy controls: (241.68 (1.04–7903.51) vs. 14.29 (0.64–633.55)), respectively. There was a significant decrease in the levels of PIGF: 189.39 (4.84–1100) pg/ml vs. 429.87 (31.71–2472.00) pg/ml, respectively. The three serum values varied greatly among the nationalities. In all the statistically significant comparisons between groups in the table, the ratio of preeclampsia, severe-preeclampsia and the control group in sFlt-1/PlGF of non-Han Chinese (21.76, 27.53) is higher than Han-Chinese people (6.06, 13.85). Further, the Uygur is markedly higher in sFlt-1/PlGF ratio than other nationalities. Non-Han Chinese populations are more likely to suffer from severe preeclampsia than Han Chinese populations, and the difference between serum values was greatest between the severe preeclampsia patients and the healthy controls.
Table 4. Detection results of serum values.
Diagnostic results of three groups of serum values are shown in . The AUC of sFlt-1/PlGF (0.802, 95%CI 0.753–0.851) is higher than sFlt-1 (0.761, 95%CI 0.707–0.815) and PlGF (0.790, 95%CI 0.743–0.837). The cut-off values for diagnosing preeclampsia in Han Chinese and non-Han Chinese are presented in .
Figure 1. Predictive performance of the sFlt-1, PlGF and sFlt-1/PlGF ratio in women with preeclampsia and control subjects.
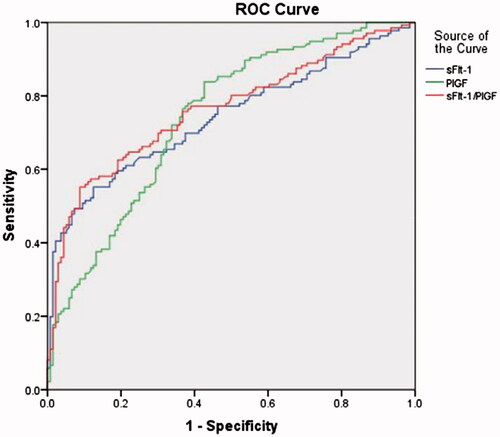
Table 5. Cut-off values of sFlt-1, PIGF and sFlt-1/PIGF for preeclampsia.
Discussion
To the best of our knowledge, this is the first study to compare the sFlt-1/PlGF ratio-test across different nationalities of the Xinjiang Uygur Autonomous Region in China for the prediction of preeclampsia-related adverse outcomes during pregnancy. We generated the data using commercially available and fully automated immunoassays to profile women with preeclampsia and compared their data to that for healthy controls. These tests were targeted for the prediction of preeclampsia in the short-term, which eliminates interference from human factors and ensures the reliability and validity of these data. As noted in previous studies, some patients who did not meet the classical diagnostic criteria for preeclampsia experienced comparable incidences of adverse outcomes. This indicates that the clinical characterization of preeclampsia is still in development [Citation24]. Previous measures of blood pressure and proteinuria report a positive predictive value of only 20% in detecting preeclampsia-related adverse outcomes [Citation25], indicating that a more comprehensive approach integrated with clinical characterization to predict the onset of preeclampsia in a short period of time is greatly needed.
Our study demonstrates that across all participants, a maternal age ≥35 and a pre-gestation BMI ≥25 are at higher risk to develop preeclampsia, in agreement with previous studies [Citation26]. Additionally, prior diagnosis of preeclampsia and prior children appear to be associated with the development of preeclampsia. Regarding ethnicity, non-Han Chinese populations are more likely to develop preeclampsia, and disease rates were significantly higher in the Uygur, Kazak and Hui populations than in Han Chinese populations.
The complete etiology of preeclampsia is still unknown, but our data show that the imbalance between angiogenic factors, such as sFlt-1 and PlGF, closely relates to its pathogenesis. Our results demonstrate that the Roche Elecsys immunoassay system is an effective tool for the prediction and diagnosis of preeclampsia, and that increased levels of sFlt-1 and/or decreased levels of PlGF are associated with adverse maternal and neonatal outcomes. Consistent with previous studies [Citation17,Citation27], we found that the optimum sFlt-1/PlGF ratio cut-off is >38.83 to identify preeclampsia, with an ROC AUC of 0.802 (57.4% sensitivity/95.1% specificity, 95%CI 0.753–0.851), compared with the sFlt-1 cut-off levels of 4549 pg/ml with an ROC AUC of 0.761 (55.1% sensitivity/91.1% specificity, 95%CI 0.707–0.815), and the PIGF cut-off levels of 125 pg/ml with an ROC AUC of 0.790 (92.1% sensitivity/57% specificity, 95%CI 0.743–0.837). Therefore, sFlt-1/PlGF ratio is demonstrated to be a better diagnostic tool than the either as single biomarkers, and the specificity of the detection of the sFlt-1/PlGF ratio is higher than other methods, including traditional tests.
The Xinjiang Uygur Autonomous Region is located in the northwest area of China and is a multi-ethnic community. Previous studies showed that preeclampsia is associated with race and ethnicity, and Roberts and Cooper [Citation28] believed that racial characteristics are directly linked to the onset of severe preeclampsia, with genetic polymorphisms in different ethnic backgrounds as a possible cause. Pegoraro and Ranjith [Citation29] and El Sahly et al. [Citation30] believed that genetic polymorphisms result in predispositions among individuals of different ethnicities which could accelerate the rate at which preeclampsia develops. Data analysis showed that maternal ethnicity could predict differences in the development of preeclampsia. In this study, we found that ethnic differences do exist in preeclampsia rates, with a higher occurrence at greater severity in pregnant woman from ethnic minorities in China. Women from Uygur and Kazak populations progressed rapidly from normal pregnancy to severe preeclampsia, and the patients from these populations were more likely to develop severe preeclampsia.
Due to differences in customs and eating habits across different ethnicities, there may be variation in predictive indicators for preeclampsia. Therefore, clinical attention should be paid to the high-risk ethnic minority populations via dynamic monitoring, which is conducive to the early prevention, diagnosis, and treatment for preeclampsia, and there needs to be further research of the predictive indicators of preeclampsia in the Uygur and Kazak populations to better address their needs.
There are some limitations to this study. First, the ethnic sample size is relatively small and may result in statistical bias resulting in the need for the further verification. Second, the predictive value of the sFlt-1/PlGF ratio has not been specifically examined for multiple pregnancies and early onset preeclampsia, which may impact our results. Third, the predictive value of the sFlt-1/PlGF ratio was examined only once, and continuous detection is needed for more accurate results. Finally, collaborations with multi-centre observations and verification are needed to determine if the cut-off value of 38.83 for sFlt-1/PlGF ratios will be clinically actionable for high-preeclampsia-risk pregnancies.
In conclusion, this study shows that the measurements of sFlt-1/PlGF ratios during pregnancy may have predictive value for women who are at risk for preeclampsia in the Xinjiang Uygur Autonomous Region and represent a clinically useful biomarker to diagnose preeclampsia. This may identify susceptible populations, especially those from minority nationalities in Xinjiang, to facilitate early prevention and improved outcomes for both the mother and the child.
Supplementary_File.docx
Download MS Word (15.9 KB)Acknowledgements
Thanks are due to Y. Yang for assistance with the experiments and to C. Chen for valuable discussion. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure statement
We have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company.
References
- Chen CW, Jaffe IZ, Karumanchi SA. Pre-eclampsia and cardiovascular disease. Cardiovasc Res. 2014;101:579–586.
- Roberge S, Villa P, Nicolaides K, et al. Early administration of low-dose aspirin for the prevention of preterm and term preeclampsia: a systematic review and meta-analysis. Fetal Diagn Ther. 2012;31:141–146.
- Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–799.
- Hod T, Cerdeira AS, Karumanchi SA. Molecular mechanisms of preeclampsia. Cold Spring Harb Perspect Med. 2015;5:a023473.
- Khan KS, Wojdyla D, Say L, et al. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066–1074.
- Osungbade KO, Ige OK. Public health perspectives of preeclampsia in developing countries: implication for health system strengthening. J Pregnancy. 2011;2011:481095.
- Grill S, Rusterholz C, Zanetti-Dallenbach R, et al. Potential markers of preeclampsia – a review. Reprod Biol Endocrinol. 2009;7:70.
- Wu P, van den Berg C, Alfirevic Z, et al. Early pregnancy biomarkers in pre-eclampsia: a systematic review and meta-analysis. IJMS. 2015;16:23035–23056.
- Schiettecatte J, Russcher H, Anckaert E, et al. Multicenter evaluation of the first automated Elecsys sFlt-1 and PlGF assays in normal pregnancies and preeclampsia. Clin Biochem. 2010;43:768–770.
- Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683.
- Staff AC, Braekke K, Harsem NK, et al. Circulating concentrations of sFlt1 (soluble fms-like tyrosine kinase 1) in fetal and maternal serum during pre-eclampsia. Eur J Obstetr Gynecol Reprod Biol. 2005;122:33–39.
- Hertig A, Berkane N, Lefevre G, et al. Maternal serum sFlt1 concentration is an early and reliable predictive marker of preeclampsia. Clin Chem. 2004;50:1702–1703.
- Wikstrom AK, Larsson A, Eriksson UJ, et al. Placental growth factor and soluble FMS-like tyrosine kinase-1 in early-onset and late-onset preeclampsia. Obstetr Gynecol. 2007;109:1368–1374.
- Kita N, Mitsushita J. A possible placental factor for preeclampsia: sFlt-1. Curr Med Chem. 2008;15:711–715.
- Tarasevičienė V, Grybauskienė R, Mačiulevičienė R. sFlt-1, PlGF, sFlt-1/PlGF ratio and uterine artery Doppler for preeclampsia diagnostics. Medicina. 2016;52:349–353.
- Venkatesha S, Toporsian M, Lam C, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Yearbook of pathology & laboratory. Nat Med. 2006;12:642.
- Zeisler H, Llurba E, Chantraine F, et al. Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N Engl J Med. 2016;374:13–22.
- Rana S, Powe CE, Salahuddin S, et al. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation. 2012;125:911–919.
- Verlohren S, Herraiz I, Lapaire O, et al. The sFlt-1/PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am J Obstetr Gynecol. 2012;206:58.e1–58.
- Verlohren S, Herraiz I, Lapaire O, et al. New gestational phase-specific cutoff values for the use of the soluble fms-like tyrosine kinase-1/placental growth factor ratio as a diagnostic test for preeclampsia. Hypertension. 2014;63:346–352.
- De OL, Peraçoli JC, Peraçoli MT, et al. sFlt-1/PlGF ratio as a prognostic marker of adverse outcomes in women with early-onset preeclampsia. Pregnancy Hypertension. 2013;3:191–195.
- Gilstrap L, Ramin S. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia; 2002;77:67–75.
- Simhan H, Caritis S. Prevention of preterm delivery. N Engl J Med. 2007;357:477–1980.
- Saleh L, Verdonk K, Jan Danser AH, et al. The sFlt-1/PlGF ratio associates with prolongation and adverse outcome of pregnancy in women with (suspected) preeclampsia: analysis of a high-risk cohort. Eur J Obstetr Gynecol Reprod Biol. 2016;199:121–126.
- Zhang J, Klebanoff MA, Roberts JM. Prediction of adverse outcomes by common definitions of hypertension in pregnancy. Obstetr Gynecol. 2001;97:261–267.
- Makowsky K, Schücking BA. Management of women with obesity in pregnancy. Praev Gesundheitsf. 2012;7:87–94.
- Perales A, Delgado JL, De La Calle M, et al. sFlt-1/PlGF for early-onset pre-eclampsia prediction: STEPS (study of early pre-eclampsia in Spain). Ultrasound Obstetr Gynecol. 2016;50:373–382.
- Roberts JM, Cooper DW. Pathogenesis and genetics of pre-eclampsia. Lancet. 2001;357:53–56.
- Pegoraro RJ, Ranjith N. Plasminogen activator inhibitor type 1 (PAI-1) and platelet glycoprotein IIIa (PGIIIa) polymorphisms in young Asian Indians with acute myocardial infarction. Cardiovasc J S Afr. 2005;16:266–270.
- El Sahly HM, Reich RA, Dou SJ, et al. The effect of mannose binding lectin gene polymorphisms on susceptibility to tuberculosis in different ethnic groups. Infect Dis. 2004;36:106–108.
