?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
There are several signal pathways involved in bone regeneration that can be triggered by several factors including mechanical, physical and chemical factors. Chemical growth factors are commonly used in differentiation induction to stem cells, while have side effects. In this study, inorganic poly-phosphate (poly-P) as a natural-based molecule was used to induce Wnt/β-catenin signalling in adipose-derived mesenchymal stem cells (AT-MSCs) while cultured on 2D and 3D culture systems. Common osteogenic markers were investigated to detect the influence of Wnt/β-catenin signalling induction on osteogenic differentiation of AT-MSCs and signalling genes up regulation was also evaluated by its related gene expression measurement. Results were shown that Cyclin-D1 and β-catenin gene expression was significantly increased in those cells treated by poly-P. Osteogenic differentiation of those stem cells with higher Cyclin-D1 and β-catenin gene expression was significantly higher than other groups except those stem cells cultured under osteogenic medium. According to the results, inorganic poly-P can trigger osteogenic differentiation in stem cells through Wnt/β-catenin signalling and this potential is almost close to common osteogenic growth factors and this could be used as natural-based molecules in bone regeneration, apart from concerns about the use of chemical factors.
Introduction
Homeostasis of bone tissue in the healthy adults requires a sensitive balance between bone resorption/formation that done, respectively, by osteoclasts and osteoblasts. Therefore, understanding of the related molecular signalling surrounds this two cell type is essential for designing effective bone therapeutics in regeneration medicine protocols. Genetic and signalling studies in human and mice have established a significant relationship between Wnt/β-catenin signalling and bone formation. Recent studies have proven one of the potent bone anabolic signals is Wnt signalling that reprogrammes directly multiple aspects of bone physiology and especially osteoblast metabolism [Citation1–4]. Wnt proteins are a family of secreted glycoproteins that regulate development and homeostasis of embryonic and adult tissues, including those derived from mesenchymal cell lineages [Citation4]. In the absence of Wnt, cytoplasmic β-catenin protein is continuously kept low through interactions with the Axin complex, including scaffolding protein Axin, casein kinase 1 (CK1), glycogen synthase kinase 3 (GSK3), and tumour suppressor adenomatous polyposis coli gene product (APC) [Citation5,Citation6]. The binding of Wnt to Frizzled (Fz) receptor complex results in phosphorylation of the Lrp co-receptors and recruitment and tethering of GSK-3b and Axin to the ligand-receptor complex. This complex is resulting in the stabilization and accumulation of cytoplasmic β-catenin [Citation7,Citation8]. Stabilized β-catenin transmitted into the nucleus of the cell to interact with the several regulators and transcription factors [Citation9]. Albeit several studies have been explored different modes of interaction between BMP and Wnt signalling pathways [Citation10,Citation11], there are a few studies to involve the effect of different biomolecules and biomaterials for the Wnt signalling in the bone regeneration. However, there are several studies, which investigated the Wnt signalling in bone repairing by mesenchymal stem cells (MSCs) [Citation12,Citation13]. Inorganic polyphosphate (PolyP) is one of the molecules with pro-thrombotic and pro-inflammatory properties in the circulatory system [Citation14,Citation15] that recently has been reported as calcification enhancer of osteoblasts culture medium [Citation16]. Our recent study showed that there is a RAGE-dependent poly P-mediated crosstalk between mTOR and the GSK-3/Wnt/β-catenin signalling network in endothelial cells [Citation14], on the other hand, has been reported polyP can be an important role for repairing long bone osteoarticular damages [Citation17].
Therefore, the purpose of this study is to explore promising potential of inorganic poly-P70 in the enhancement of osteogenic differentiation of human adipose-derived mesenchymal stem cells (AT-MSCs) while cultured on a 3-D culture system. Electrospun poly(caprolactone) (PCL) nanofibres were selected in this study as a most well-known appropriate scaffold for bone tissue engineering according to its biocompatibility and biodegradability. To do this, AT-MSCs were seeded on tissue culture polystyrene (TCPS) and PCL scaffolds and their osteogenic differentiation potential were examined under treatment of inorganic poly-P70 (as a Wnt/β-catenin signalling inducer), iCRT3 (as a Wnt/β-catenin signalling inhibitor), osteogenic medium (OM, as a positive control) and basal media (as a negative control). After stem cell isolation and characterization, osteogenic differentiation was analysed through common osteogenic markers such as Alizarin-red staining, alkaline phosphatase (ALP) activity, calcium content assay and osteogenic genes expression. Fold change expression of tow Wnt/β-catenin signalling gene markers were also measured for all groups.
Method and materials
Nanofibres preparation by electrospinning
Poly(caprolactone) solution was prepared and its nanofibres were synthesized using electrospinning method which was reported in our previously published article [Citation18]. In brief, 1.2 g of PCL (PCL, MW = 80,000, Sigma Aldrich, St. Louis, MO) was added to the 10 ml chloroform (7.5 ml) (Merck, Darmstadt, Germany) and dimethylformamide (2.5 ml) (Merck, Darmstadt, Germany) and kept at 35 °C for 4 h on stirrer. A blunted needle content solution was positioned at the distance of 20 cm from a cylindrical collector, under the potential difference of 24 kV, and steady flow rate of 0.5 ml/h.
Plasma surface treatment
To increase hydrophilicity of the obtained random oriented PCL scaffolds, plasma treatment was applied, using a low-frequency generator (40 kHz) with a cylindrical quartz reactor (Diener, Electronics, Ebhausen, Germany).
Nanofibres sterilization
Before starting cell culture, nanofibrous scaffold was punched into circles with 1.5 cm in diameters and sterilized using UV radiation and then with ethanol (70%). To ensure non-microbial contamination, scaffold was incubated in Dulbecco’s Modified Eagle’s Medium (DMEM) (Invitrogen Co, Carlsbad, CA) supplemented by 10% Fetal Bovine Serum (FBS) (Sigma Aldrich, St. Louis, MO), 5% CO2 and 37 °C for overnight and analysed by invert light microscope.
Nanofibres characterization
Fabricated nanofibrous scaffold was characterized morphologically and contact angle measurement was also done before and after plasma treatment. The morphology of the fabricated nanofibres was evaluated by scanning electron microscopy (SEM; KYKY, EM3200, Beijing, China). Hydrophilicity of the nanofibrous scaffold was also studied using contact angle goniometer (Krüss, Hamburg, Germany). A water droplet was dropped on the surface of nanofibres and the contact angle was measured after 10 s.
Mesenchymal stem cells isolation and characterization
Human abdominal fat tissues were obtained by liposuction surgery (Taleghani General Hospital, Tehran, Iran) according to the medical ethics committee guidelines, Ministry of Health IR, Iran. Mesenchymal stem cells (MSCs) were isolated from adipose tissues according to the previously reported protocol [Citation19].
Differentiation potential of isolated MSCs was confirmed by osteogenic, adipogenic and chondrogenic differentiation and also their potential for differentiated stem cells was evaluated by Alizarin-red, Oil-red and Alcian Blue staining methods, respectively, at the passage 3 according to the previously reported for MSCs [Citation20,Citation21].
Cell culture
Isolated and characterized adipose-derived MSCs (AT-MSCs) were seeded on sterilized PCL scaffolds in several groups and conditions. For MTT analysis, Four groups were designed including, AT-MSCs cultured on TCPS as a control group, AT-MSCs cultured on TCPS under iCRT3 (iCRT3 as an inhibitor of Wnt/β-catenin signalling), AT-MSCs cultured on TCPS under Poly-P (Poly-P70 an inducer of Wnt/β-catenin signalling) and AT-MSCs cultured on PCL.
Osteogenic differentiation process was carried out in eight groups including first four groups, which stem cells were cultured on TCPS under basal media, iCRT3, Poly-P70, osteogenic media (OM) and the second four groups, which stem cells were cultured on PCL under basal media, iCRT3, Poly-P70 and OM (OM: DMEM with 10% FBS, Dexamethasone (10 µM) (Peprotech, Rocky Hill, NJ), Ascorbic Acid (50 µg/ml) (Merck, Darmstadt, Germany) and β-glycerophosphate (10 µM) (Sigma Aldrich, St. Louis, MO)). The groups cultured under basal media were considered as 2D (TCPS) and 3D (PCL) control groups. All groups were incubated in a humidified atmosphere of 5% CO2 at 37 °C, and media was exchanged two times per week until the end of period of study.
Biocompatibility evaluation
Scanning electron microscopy
SEM was used for study the morphology of stem cells while seeded on the surface of the fabricated scaffold. All cell-seeded scaffold groups were washed by PBS and then fixed with glutaraldehyde solution (2.5%) for 1 h at room temperature. After removing of the fixator, samples were rewashed by PBS. Then, dehydration was done through alcohol concentration gradients. Finally, all of the scaffold-seeded cells were subjected to a scanning electron microscope (SEM; KYKY, EM3200, Beijing, China) in 5 and 14th days after cell seeding.
MTT assay
MTT assay was used for evaluation of the proliferation rate of seeded AT-MSCs at the present of Wnt/β-catenin’s inhibitor, inducer and PCL nanofibres. AT-MSCs were seeded with an initial cell density of 104 cells per in a 4-well culture plate. 1 and 5 d after cell seeding, 50 μl of MTT solution (5 mg/ml in DMEM) was added to each well (n = 3). All groups were incubated at 37 °C for 3.5 h. After that supernatant was removed, Dimethyl sulfoxide (DMSO) (Sigma Aldrich, St. Louis, MO) was added for dissolution of the dark-blue intracellular formazan, which produced by mitochondrial dehydrogenases of living cells. The optical density of the solutions was read at a wavelength of 570 nm in a micro-plate reader (Bio-Tek Instruments, Winooski, VT).
Alkaline phosphates activity
ALP activity of the differentiated stem cells was measured by ALP Kit according to the manufacturer’s protocol (Pars Azmoon, Tehran, Iran). In brief, the total protein of differentiated cells was extracted using 200 μl of RIPA lysis buffer at days 7 and 14. The lysate was centrifuged at 15,000 RPM at 4 °C for 15 min, and the ALP activity of supernatant was measured at the present of the substrate at 450 nm. Total protein was finally used for normalizing of the enzyme activity level (IU).
Calcium content assay
Amount of deposited calcium by differentiated stem cells, was measured by Calcium Content Kit (Pars Azmoon, Tehran, Iran) at days 7 and 14 after cell seeding according to the manufacturer’s protocol. The samples were washed with PBS and incubated in 0.6 N HCL (Merck, Darmstadt, Germany) on ice followed by shaking for 4 h. Then, optical density of samples was measured at 570 nm at the present of kit reagent via a micro-plate reader (Bio-Tek Instruments, Winooski, VT) and finally the values were normalized a serial dilution of calcium concentrations.
Real-time RT-PCR
The expression change folds of osteogenic and Wnt/β-catenin signalling related gene markers were measured by real-time RT-PCR at days 7 and 14 after cell seeding. Runt-related transcription factor 2 (Runx2), Osteocalcin (OC) and Collagen type 1 (Col1) as osteogenic gene markers and β-catenin and Cyclin-D1 as Wnt/β-catenin signalling related gene markers were considered. Total RNA was extracted by Qiazol reagent (Qiagen, Hilden, Germany) and for reverse transcription (RT), the first-strand cDNA was synthesized by random hexamers (Vivantis, San Diego, CA) using M-MLV Reverse Transcriptase (Vivantis, cat. no. RTPL12; San Diego, CA). For polymerase chain reaction (PCR), denaturation was done at 95 °C for 3 min and followed by 40 cycles at 95 °C for 20 s. Then, annealing and elongation were performed for 30 s at 60 and 72 °C, respectively. The used primer sequences in this study are depicted in . SYBR Premix Ex Taq Master mix (Takara, Kusatsu, Japan) was used for Real-time RT-PCR. The expression of target genes was calculated through relative quantification model in comparison to the endogenous control, β2M. Rotor-Gene 6000 (Corbett, Concorde, NSW and Australia) was used to quantify the gene expression levels.
Table 1. Primers sequences used for real-time RT-PCR analysis.
Statistics
Each experiment was repeated independently at three times in vitro. Acquired data were reported as means ± standard deviation (SD). REST 2009 software was used for analysing real-time RT-PCR results. Data comparison was done through one-way analysis of variance (ANOVA). All analyses were performed by SPSS version 17.0 (Chicago, IL) software. p Values of less than .05 were considered as statistically significant.
Results
Stem cell characterization
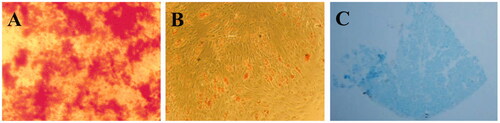
Isolated AT-MSCs were characterized at the passage 3 by osteogenic, adipogenic and chondrogenic differentiation potentials. Alizarin-red, Oil-red and Alcian blue staining were performed two weeks after differentiation induction. As shown in , mineralization due to osteogenic differentiation was detected by alizarin red staining at the end of two weeks, oil drop vesicles were also detected when stem cells cultured under adipogenic induction medium and through oil-red staining (). Chondrogenic potential was also confirmed by alcian-blue staining which ECM’s glycol-amino-glycan (GAG) was stained in differentiated cells ().
Figure 1. Alizarin-red staining of isolated stem cells cultured under osteogenic induction medium after two weeks (A); Oil-Red staining of isolated stem cells cultured under adipogenic induction medium after two weeks (B); Alcian blue staining of isolated stem cells cultured under chondrogenic induction media after two weeks (C).
Scaffold characterization
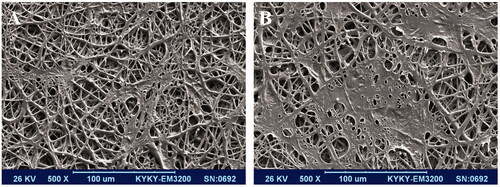
SEM imaging was used for morphological evaluation of fabricated scaffold. As shown in , scaffold was smooth and bead free and in nanometre size 800 ± 300 nm. SEM was also used for biocompatibility assessment (cell attachment, growth and spread) of the scaffold when stem cells cultured on them under osteogenic medium at days 5 () and 14 () and images were demonstrated that the cells were absolute proliferated and spread on the surface of scaffold.
Figure 2. Morphology of the stem cell-seeded PCL nanofibrous scaffolds, stem cells seeded scaffold after 5 d (A) and after 14 d (B).
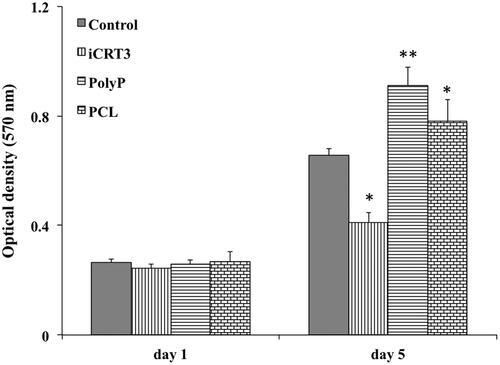
MTT assay was used for viability assessment of stem cells while cultured on the surface of scaffold and TCPS at the present of iCRT3 and Poly-P70 at days 1 and 5. Stem cells demonstrated an increasing proliferation pattern during the period of study (). On day one after cell seeding, differences were not significant between groups, but on day 5, significantly highest proliferation rate was detected for AT-MSCs cultured under poly-P70 treatment and significantly lowest proliferation rate was detected for those stem cells cultured under iCRT3 treatment. In addition, proliferation rate of stem cells cultured on PCL nanofibres were also showed a significant increase in comparison with those cultured on TCPS as control and those cultured under iCRT3 treatment. According to the results, PCL nanofibres do not have any cytotoxicity effect and in the other hands poly-P70 and iCRT3 have proliferative and anti-proliferative effects on stem cells, respectively.
ALP activity and calcium content
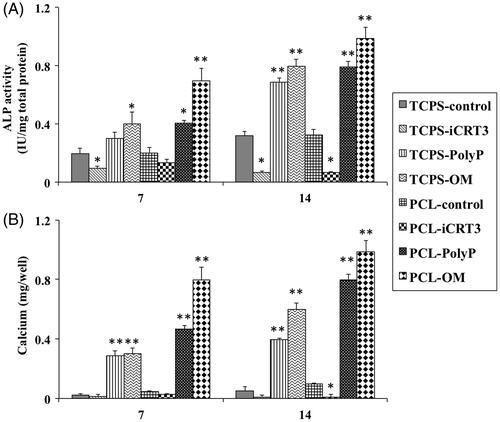
ALP activity was evaluated during osteogenic differentiation of AT-MSCs cultured on all eight groups, such as TCPS-control, TCPS-iCRT3, TCPS-pily-P70, TCPS-OM, PCL-control, PCL-iCRT3, PCL-polyP70 and PCL-OM (). All groups showed an increasing ALP activity’s pattern during the period of study except those stem cells treated with iCRT3 that showed decreasing pattern. Significantly highest ALP activity was detected in PCL-OM groups at days 7 and 14, no difference was observed between PCL-poly-P70 and TCPS-OM groups while ALP activity of both was significantly higher than other five groups. The main important output of this test was iCRT3 causes the decrease of the ALP activity as well as poly-P70 causes the increase of the ALP activity. Calcium content of the TCPS-control, TCPS-iCRT3, PCL-control and PCL-iCRT3 was not changed during the period of study (), while highest mineralization was observed in PCL-OM as well as TCPS-OM group during all days. After that calcium content of PCL-poly-P70 was increased significantly higher than other groups as well as TCPS-poly-P70 group. Overall, in ALP activity and calcium content, the highest increase was detected in groups were treated with OM while the closest amount to this group was for groups that were treated with poly-P70, which was significantly higher than other groups.
Figure 4. The ALP activity (A) and Calcium content (B) assays of isolated stem cells while cultured on eight groups including: TCPS with basal media (TCPS-control), TCPS with iCRT3 (TCPS-iCRT3), TCPS with inorganic polyphosphate (TCPS-poly-P), TCPS with osteogenic medium (TCPS-OM), PCL with basal media (PCL-control), PCL with iCRT3 (PCL-iCRT3), PCL with inorganic polyphosphate (PCL-poly-P) and PCL with osteogenic medium (PCL-OM). The significant differences (p<.05) and (p<.01) between groups are indicated with star sign and two-star sign, respectively.
Gene expression analysis
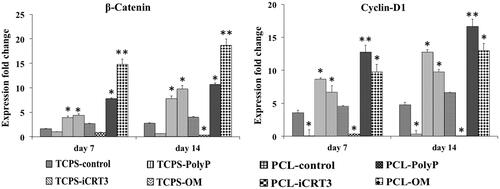
To investigate the effects of poly-P70 on osteogenic differentiation of AT-MSCs through Wnt/β-catenin signalling in 2D and 3D systems, three osteogenic gene markers () and two Wnt/β-catenin signalling gene markers () was evaluated by real-time RT-PCR. Relative expression of Runx-2 in PCL-OM was increased significantly at day 7. Increased expression of this gene in PCL-poly-P70 and TCPS-OM was also significant in comparison with other groups as well as AT-MSCs cultured on TCPS-poly-P70 compared with control groups and groups treated by iCRT3. Expression of Runx-2 at day 14 was also showed the same pattern of day 7, but highest expression was detected at those stem cells cultured under OM. Relative expression of OC in AT-MSCs cultured under poly-P70 and OM showed an increasing pattern during the period of study while its expression not changed significantly in other groups. Although, expression at days 7 and 14 in 3D system was significantly more than those stem cells cultured on 2D system. Expression of Col-1 was increased in all groups except those stem cells treated with iCRT3 during the period of study. This gene was increased significantly in AT-MSCs cultured under poly-P70 or OM while expression of this gene was significantly increased in stem cells cultured on PCL rather than TCPS. To investigate the Wnt/β-catenin signalling gene markers involvement during the osteogenic differentiation process stimulated by poly-P70, expression level of β-catenin and Cyclin-D1 genes was measured at days 7 and 14 during differentiation process. The highest expression level of β-catenin was detected in AT-MSCs during the period of study, which was treated with OM cultured on PCL, although its expression in TCPS-polyP70 and TCPS-OM was not significantly different. It was shown that β-catenin up regulated by poly-P70 and OM as well as down regulated by iCRT3. But for cyclin-D1, those stem cells treated by poly-P70 showed significantly highest increase in comparison with other groups, although its increase in PCL groups was more than TCPS group. In addition, iCRT3 almost completely inhibited expression of cylin-D1 during the period of study.
Figure 5. Expression fold changes of three osteogenic gene markers. Runt-related transcription factor 2 (Runx2), Osteocalcin and Collagen type 1 (Col1) on days 7 and 14 for isolated stem cells while cultured on eight groups including: TCPS with basal media (TCPS-control), TCPS with iCRT3 (TCPS-iCRT3), TCPS with inorganic polyphosphate (TCPS-poly-P), TCPS with osteogenic medium (TCPS-OM), PCL with basal media (PCL-control), PCL with iCRT3 (PCL-iCRT3), PCL with inorganic polyphosphate (PCL-poly-P) and PCL with osteogenic medium (PCL-OM). The significant differences (p<.05) and (p<.01) between groups are indicated with star sign and two-star sign, respectively.
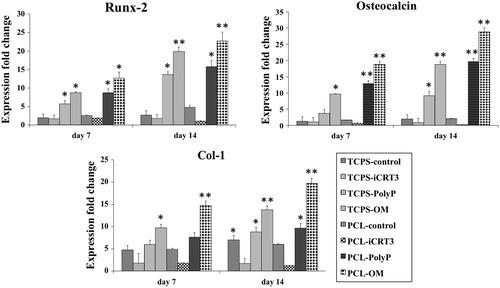
Figure 6. Expression fold changes of two Wnt/β-catenin signalling gene markers. β-catenin and Cyclin-D1 on days 7 and 14 for isolated stem cells while cultured on eight groups including: TCPS with basal media (TCPS-control), TCPS with iCRT3 (TCPS-iCRT3), TCPS with inorganic polyphosphate (TCPS-poly-P), TCPS with osteogenic medium (TCPS-OM), PCL with basal media (PCL-control), PCL with iCRT3 (PCL-iCRT3), PCL with inorganic polyphosphate (PCL-poly-P) and PCL with osteogenic medium (PCL-OM). The significant differences (p<.05) and (p<.01) between groups are indicated with star sign and two-star sign, respectively.
Discussion
Given the side effects of chemical growth factors and the inability to use them in clinical studies, the use of natural molecules as alternatives to launching stem cell differentiation signal pathways alone or in combination with scaffolds can be very useful. As mentioned, one of the most important involved signal pathways during osteogenesis is Wnt/β-catenin signalling. Thorfve et al. observed that a local release of incorporated Li + from PLGA at the bone fracture site shows Li + cannot an enhancer of early bone growth; however, it affects the Wnt signalling pathway [Citation22]. Xiao et al. demonstrated the targeted overexpression of low molecular weight (LMV) fibroblast growth factor-2 (FGF-2) isoform in osteoblast precursors enhanced bone defect healing by increased osteoblast activity along with increased canonical Wnt signalling [Citation23]. Ping et al. demonstrated in two separate studies that melatonin can induce bone regeneration and enhancing osteogenic differentiation at osteolytic sites by activating Wnt/β-catenin signalling pathway [Citation24,Citation25]. In addition, they introduced icariin as inducer osteogenic differentiation of MSCs which promote new bone formation at a titanium-particle-induced osteolytic site through the activated Wnt/β-catenin signalling pathway [Citation25]. Yang et al. confirmed the role of the Wnt-β-catenin pathway in a sandblasted-large-grit-acid etched (SLA)-treated titanium surface for the osteogenic activity of bone marrow-derived stromal cells [Citation26]. Auh et al. investigated the effects of sulfuretin (A flavonoid derived from Rhus verniciflua) on in vitro osteoblastic differentiation of primary osteoblasts isolated from mice calvariae and suggested that sulfuretin acts by the activation of several signalling pathways especially Wnt/β-catenin signalling to promote in vitro osteoblast differentiation and improved in vivo bone regeneration [Citation27]. Dixit et al. demonstrated that Medicarpin, a Natural Pterocarpan, promotes new bone regeneration and healing at the different type of bone fractures in the rat by activating Wnt and Notch signalling pathways [Citation28]. Liu et al. showed acetylsalicylic acid (ASA) is able to significantly improve exfoliated deciduous teeth-derived stem cells (SHED) mediated osteogenic differentiation by upregulation of Wnt/β-catenin cascade and telomerase reverse transcriptase (TERT) [Citation29]. One of the most beneficial molecules that can be extracted from the human body for example from serum and is critical for cellular function and skeletal mineralization is inorganic phosphate. After we showed at our previous study which inorganic poly-P can be caused to increase of cyclin-D1 and followed by increase in cell proliferation via Wnt/β-catenin cascade [Citation14], in this study, we tried to investigate the effect of inorganic poly-P on osteogenic differentiation potential of AT-MSCs while cultured on 3D system, since Wnt/β-catenin signalling has a critical role during osteogenesis. Inorganic poly-P is a linear polymer contains energy-rich phosphor-anhydride bonds, which has been identified in the variety of mammalian cell lines and tissues [Citation30]. Our MTT result was showed that proliferation rate of AT-MSCs cultured under poly-P treatment was significantly increased in comparison to the control groups, it was also significantly more than PCL cultured stem cells that showed its critical role on proliferation. ALP activity, calcium content and gene expression analysis were also showed higher osteogenic markers in stem cells treated with poly-P compared to the other groups expect those cultured under osteogenic medium. Groups with more osteogenic markers expressed more Wnt/β-catenin signalling gene markers, which indicate the involvement of this signalling pathway in the bone differentiation process. Hacchou et al. previously presented the hypothesis of the stimulation effect of poly-P on osteoblasts and facilitating bone formation [Citation31]. Tsutsumi et al. investigated poly-P effect on Murine MC3T3-E1 osteoblastic cells and reported poly-P as an effective substance for bone regeneration [Citation32]. Another study reported that simultaneously using of bFGF and poly-P could provide a better effect on the bone regeneration in the rabbit [Citation33]. There are a few studies that investigate the signalling pathway of poly-P differentiation of osteogenic cells from hAT-MSCs. Ozeki et al. in a study reported that poly-P-induced matrix metalloproteinase (MMP)-3 regulates differentiation of osteogenic cells from human adipose tissue-derived MSCs [Citation34].
Conclusion
According to the results, inorganic poly-P as a natural molecule could be used as a biodegradable osteoinducer to induce bone regeneration. This compound can induce osteogenesis almost close to common three osteogenic factors including dexamethasone, acid ascorbic and β-glycerophosphate without their side effects, and since there is naturally present in the human body can extract from the serum and use in the treatment. In addition, our results were showed that Wnt/β-catenin signalling pathway has a great role in poly-P can be mediated osteogenesis. Finally, further investigations can more help to the use of poly-P in bone regeneration, for example its effects can be more significant when incorporated in nanostructure to in-situ release at the site of the bone defects.
Acknowledgments
The authors thank School of Advanced Technologies in Medicine, Shahid Beheshti University of Medical Sciences for technical support.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Rodda SJ, McMahon AP. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development. 2006;133:3231–3244.
- Maeda K, Takahashi N, Kobayashi Y. Roles of Wnt signals in bone resorption during physiological and pathological states. J Mol Med. 2013;91:15–23.
- Tu X, Joeng KS, Nakayama KI, et al. Noncanonical Wnt signaling through G protein-linked PKCδ activation promotes bone formation. Developmental Cell. 2007;12:113–127.
- Karner CM, Long F. Wnt signaling and cellular metabolism in osteoblasts. Cell Mol Life Sci. 2017;74:1649–1657.
- Kimelman D, Xu W. β-Catenin destruction complex: insights and questions from a structural perspective. Oncogene. 2006;25:7482.
- He X, Semenov M, Tamai K, et al. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development. 2004;131:1663–1677.
- Taelman VF, Dobrowolski R, Plouhinec JL, et al. Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell. 2010;143:1136–1148.
- Hrckulak D, Kolar M, Strnad H, et al. TCF/LEF transcription factors: an update from the internet resources. Cancers. 2016;8:70.
- MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26.
- Rahman MS, Akhtar N, Jamil HM, et al. TGF-β/BMP signaling and other molecular events: regulation of osteoblastogenesis and bone formation. Bone Res. 2015;3:15005.
- Lin GL, Hankenson KD. Integration of BMP, Wnt, and Notch signaling pathways in osteoblast differentiation. J Cell Biochem. 2011;112:3491–3501.
- Li J, Hu C, Han L, et al. MiR-154-5p regulates osteogenic differentiation of adipose-derived mesenchymal stem cells under tensile stress through the Wnt/PCP pathway by targeting Wnt11. Bone. 2015;78:130–141.
- Kong X, Li X, Zhang C, et al. Aqueous fraction of huge formula promotes osteogenic differentiation of bone marrow stromal cells through the BMP and Wnt signaling pathways. Rejuvenation Res. 2016;19:509–520.
- Hassanian SM, Ardeshirylajimi A, Dinarvand P, et al. Inorganic polyphosphate promotes cyclin D1 synthesis through activation of mTOR/Wnt/β‐catenin signaling in endothelial cells. J Thromb Haemost. 2016;14:2261–2273.
- Hassanian SM, Avan A, Ardeshirylajimi A. Inorganic polyphosphate: a key modulator of inflammation. J Thromb Haemost. 2017;15:213–218.
- Kato K, Morita K, Hirata I, et al. Enhancement of calcification by osteoblasts cultured on hydroxyapatite surfaces with adsorbed inorganic polyphosphate. In Vitro Cell Dev Biol Anim. 2018;54:449–457.
- Müller WEG, Neufurth M, Wang S, et al. Amorphous, smart, and bioinspired polyphosphate nano/microparticles: a biomaterial for regeneration and repair of osteo-articular impairments in-situ. Int J Mol Sci. 2018;19:427.
- Arjmand M, Ardeshirylajimi A, Maghsoudi H, et al. Osteogenic differentiation potential of mesenchymal stem cells cultured on nanofibrous scaffold improved in the presence of pulsed electromagnetic field. J Cell Physiol. 2018;233:1061–1070.
- Ardeshirylajimi A, Soleimani M, Hosseinkhani S, et al. A comparative study of osteogenic differentiation human induced pluripotent stem cells and adipose tissue derived mesenchymal stem cells. Int J Stem Cell Res Ther. 2014;1:235–244.
- Ghiaee A, Pournaqi F, Vakilian S, et al. Adapted dexamethasone delivery polyethylene oxide and poly (e-caprolactone) construct promote mesenchymal stem cells chondrogenesis. Artif Cell Nanomed Biotechnol. 2017;45:1640–1648.
- Ardeshirylajimi A, Vakilian S, Salehi M. Renal differentiation of Mesenchymal stem cells seeded on nanofibrous scaffolds improved by Human renal tubular cell lines conditioned medium. ASAIO J. 2016;63:356–363.
- Thorfve A, Bergstrand A, Ekström K, et al. Gene expression profiling of peri-implant healing of PLGA-Li + implants suggests an activated Wnt signaling pathway in vivo. PLoS One. 2014;9:e102597.
- Xiao L, Ueno D, Catros S, et al. Fibroblast growth factor-2 isoform (low molecular weight/18 kDa) overexpression in preosteoblast cells promotes bone regeneration in critical size calvarial defects in male mice. Endocrinology. 2014;155:965–974.
- Ping Z, Hu X, Wang L, et al. Melatonin attenuates titanium particle-induced osteolysis via activation of Wnt/β-catenin signaling pathway. Acta Biomater. 2017;51:513–525.
- Wang J, Tao Y, Ping Z, et al. Icariin attenuates titanium-particle inhibition of bone formation by activating the Wnt/β-catenin signaling pathway in vivo and in vitro. Sci Rep. 2016;6:23827.
- Yang G, Fang W, Liu T, et al. Gene expression profiling of bone marrow-derived stromal cells seeded onto a sandblasted, large-grit, acid-etched-treated titanium implant surface: the role of the Wnt pathway. Arch Oral Biol. 2016;61:71–78.
- Auh QS, Park KR, Yun HM, et al. Sulfuretin promotes osteoblastic differentiation in primary cultured osteoblasts and in vivo bone healing. Oncotarget. 2016;7:78320.
- Dixit M, Raghuvanshi A, Gupta CP, et al. Medicarpin, a natural pterocarpan, heals cortical bone defect by activation of Notch and Wnt canonical signaling pathways. PLoS One. 2015;10:e0144541.
- Liu Y, Chen C, Liu S, et al. Acetylsalicylic acid treatment improves differentiation and immunomodulation of SHED. J Dent Res. 2015;94:209–218.
- Kulaev IS. Biochemistry of inorganic polyphosphates. Reviews of physiology, biochemistry and pharmacology. Vol. 73. Berlin, Germany: Springer; 1975. p. 131–158.
- Hacchou Y, Uematsu T, Ueda O, et al. Inorganic polyphosphate: a possible stimulant of bone formation. J Dent Res. 2007;86:893–897.
- Tsutsumi K, Saito N, Kawazoe Y, et al. Morphogenetic study on the maturation of osteoblastic cell as induced by inorganic polyphosphate. PLoS One. 2014;9:e86834.
- Yuan Q, Kubo T, Doi K, et al. Effect of combined application of bFGF and inorganic polyphosphate on bioactivities of osteoblasts and initial bone regeneration. Acta Biomater. 2009;5:1716–1724.
- Ozeki N, Mogi M, Hase N, et al. Polyphosphate-induced matrix metalloproteinase-13 is required for osteoblast-like cell differentiation in human adipose tissue derived mesenchymal stem cells. Biosci Trends. 2016;10:365–371.