Abstract
EYA2 is the developmental transcription factor and phosphatase, playing an important role in numerous species in regulating cell death and differentiation. Recent studies showed that EYA2 is dysregulated and involved in the progression of various cancers. However, the expression and role of EYA2 in osteosarcoma remains unclear. Here, we found that EYA2 expression was evidently upregulated osteosarcoma (OS) tissue and cell lines. Next, we predicted EYA2-targeting miRNAs, which was further evaluated using a dual luciferase reporter assay. We found that miR-219a-5p significantly repressed EYA2 expression via binding to the 3′-UTR of EYA2. Furthermore, overexpressed miR-219a-5p significantly repressed OS cell invasion and migration, which was reversed by overexpressed EYA2. While silenced miR-219a-5p induced OS cell invasion and migration, which was reversed by silenced EYA2. In conclusion, our study revealed that miR-219a-5p function as tumour suppressor regulates OS cell invasiveness by repressing EYA2 expression.
Introduction
Osteosarcoma (OS) is the most prevalent primary bone malignancy that mostly occurs in children and adolescents with 10–16 years old [Citation1]. Despite the improvement of the conventional chemotherapy, surgery and radiotherapy, the clinical outcomes for OS patients with metastatic or recurrent OS remains poor and the overall 5-year survival rate remains at approximately 20% [Citation2]. Hence, identification of novel molecular that involved in OS progression and development the effective therapeutic approaches is urgent for improving the survival and prognosis of OS.
Eyes absent homolog 2 (EYA2) is one member of EYA family [Citation3], which function as tyrosine phosphatases or transcriptional coactivator and is involved in various biological functions including cellular death and differentiation, DNA damage, innate immunity and so on [Citation4–6]. Recent studies showed that EYA2 is dysregulated various tumours and involved the tumorigenesis. For example, EYA2, function as a tumour suppressor, is downregulated in pancreatic cancers tissues and cells, and epigenetic expression of EYA2 repressed the growth and metastases of pancreatic adenocarcinoma [Citation7]. But many more studies described EYA2 as an oncogene in breast cancer, lung adenocarcinoma, astrocytoma, leukemogenesis and cervical carcinogenesis [Citation8–13]. However, the expression and role of EYA2 in OS remain unclear.
MiRNA (miRNAs), a class of small non-coding RNAs with ∼22 nucleotides in length, post-transcriptionally regulate gene expression by binding to the 3′ UTR of the mRNA of their target gene [Citation14,Citation15]. Increasing evidence showed that miRNA, function as tumour suppressor genes or oncogenes, participates in the progression of various tumours including OS [Citation16–19]. For example, Wu et al. revealed that MiRNA-145-3p was reported as a tumour suppressor, inhibiting proliferation and induces apoptosis and autophagy of OS cell [Citation20]. miR-506-3p is a tumour suppressor by repressing the proliferation and metastasis of OS cell [Citation21]. miR-223-3p was reported to suppress OS metastasis by targeting CDH6 [19]. However, whether miR-219a-5p involves the progression of OS has not been clarified.
In this study, we detected the EYA2 expression in OS and analyzed the potential mechanism of EYA2 in OS progression. We found that EYA2 was significantly upregulated in tissue and cell lines. In addition, dual luciferase reporter assay showed that EYA2 is the direct target of miR-219a-5p. Finally, the biological function demonstrated that miR-219a-5p significantly repressed OS cell invasion and migration by suppressing EYA2 expression. In conclusion, this study provides a new understanding the miR-219a-5p/EYA2 in the development and progression of OS.
Materials and methods
Tissue samples
OS tumour tissues and adjacent normal tissues were obtained from 48 OS patients at the First Affiliated Hospital of Fujian Medical University (Fujian, China) from 2012 to 2016. Patient characteristics are summarized in . The study was approved by the First Affiliated Hospital of Fujian Medical University. All patients provided written informed consent. All tissues were immediately frozen and stored in liquid nitrogen until RNA or protein extraction.
Table 1. Associations between EYA2 expression and clinicopathological characteristics.
Cell culture and transfection
OS cell lines (MG-63, U2OS, Saos-2 and HOS) and the normal osteoblast cell line (OB3) were purchased from American Type Culture Collection (Manassas, VA). The cells were cultured in Dulbecco’s modified Eagle medium (DMEM; Thermo Fisher Scientific, Rockford, IL) with 10% of fetal bovine serum (FBS; Life Technologies, Grand Island, NY).
miR-219a-5p mimic, miRNA negative control (mimics NC), miR-219a-5p inhibitors and inhibitors control (inhibitors NC) were purchase from GenePharma (Shanghai, China). The EYA2 plasma and control plasma, EYA2 siRNA and negative control (siRNA-NC) were purchase from GenePharma (Shanghai, China). The miRNA, siRNA and plasma were transfected into cells using lipofectamine 2000 (Invitrogen, Waltham, MA) according to the manufacturer’s protocols.
Quantitative reverse-transcriptase PCR (qRT-PCR)
Total RNA from cell and tissue were extracted using Trizol reagent (Thermo Fisher Scientific). One microgram of RNA was used to reverse transcribed into cDNA using an RNeasy Mini kit (QIAGEN, Valencia, CA). The expression of miR-219a-5p and EYA2 was detected using SYBR Green real-time kit (TaKRa) in the ABI 7300 real-time PCR system (Life Technologies). GAPDH and U6 were used as internal controls. Primers for EYA2 were Ford: 5′-GGACAATGAGATTGAGCGTGT-3′ and Rev: 5′-ATGTCCCCGTGAGTAAGGAGT-3′.
Wound-healing assay
The MG-63 cells were transfection with miR-219a-5p mimic, miR-219a-5p inhibitors and miRNA negative control (miR-NC), the EYA2 plasma and control plasma, EYA2 siRNA and negative control(siRNA-NC) and then seeded into a 12-well plate (Corning Co, Corning) with about 5 × 105 cell and cultured at 37 °C in 5% CO2 for 24 h. A 100-µL pipette tip was used to scribe a wound in the well. A microscope (Olympus Corp) was used to record the wound area.
Cell invasion assay
The cell invasion ability was described using matrigel invasion assay as previous described [Citation19]. The MG-63 cells transfection with miR-219a-5p mimic, miR-219a-5p inhibitors and miRNA negative control (miR-NC), the EYA2 plasma and control plasma, EYA2 siRNA and negative control(siRNA-NC) were harvested and seeded into the upper chambers of transwell inserts (8 μm pore size; Corning). The lower compartments were filled with DMEM medium supplemented with 5% FBS. The cells in the upper chamber were removed with a swab after incubation for 12 h in the migration assay or 24 h in the invasion assay.
Dual-luciferase reporter assay
The 3′ UTR of the EYA2 mRNA was cloned into pGLO vector as pGLO-EYA2. The mutated sequence of the EYA2 3′ UTR with the target site of miR-219a-5p mutated was also cloned into pGLO vector as pGLO-EYA2-Mut. For luciferase reporter assay, 20 nM miR-219a-5p was con-transfected with 200 ng pGLO-EYA2 or pGLO-EYA2-Mut in MG-63 cells for 24 h. Relative luciferase activities were evaluated 48 h later using the Dual-Luciferase Reporter Assay Kit (Promega Corp., Madison, WI).
Western blot analysis
Cells were lyzed and then clarified by centrifugation at 12,000 rpm for 30 min at 4 °C. Twenty microgram of total protein was separated by 12% SDS-PAGE using 120 V for 30 min and then 80 V for 2 h. The proteins were transfer to a polyvinyl difluoride membrane (PVDF, Millipore, MA) 900 mA for 90 min. The PVDF membrane was blocked with 5% bovine serum albumin for 60 min at room temperature, and then the membranes were incubated with primary antibodies at 4 °C overnight. The following primary antibodies were used: anti-EYA2 antibody (Abcam, Cambridge, MA); anti-GAPDH antibody (Abcam). The next day, the corresponding horseradish peroxidase conjugate secondary antibody was incubated for 1 h at room temperature. All bands were detected using an ECL kit (Amersham Biosciences, Piscataway, NJ).
Statistical analysis
All statistical analyses were performed using SPSS software, version 17.0. Data are presented as the mean ± standard deviation. Two groups were compared using Student’s t-test (two-tailed) and one-way ANOVA. Multiple comparisons were made using one-way ANOVA. The relationship between EYA2 expression and clinicopathological patient characteristics was analyzed using Student’s t-test and the chi-square test. All data were presented as the mean ± standard error. Statistical significance was defined as p < .05. All experiments were repeated three times.
Results
EYA2 expression was evidently upregulated in OS tissue and cell lines
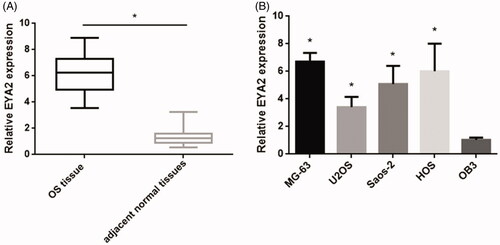
To investigate the potential role of EYA2 in OS, we performed qPCR to examine the expression levels of EYA2 mRNA in 48 pair of OS samples and the adjacent noncancerous tissues (). We found that EYA2 expression level in OS tumour tissue was much higher than that in adjacent normal tissues. The clinical parameters of EYA2 in OS patients and the correlation between clinicopathological characteristics are observed in . The results showed that the data revealed that EYA2 expression was closely associated with TNM stage, metastasis and tumour size. And then we analyzed the expression levels of EYA2 in OS cell lines (MG-63, U2OS, Saos-2, and HOS) and the normal osteoblast cell line (OB3). As shown in , EYA2 expression was much higher in OS cell line than that in the normal osteoblast cell line (OB3). Taken together, these results indicated the important role of EYA2 in OS progression.
Figure 1. EYA2 expression in OS tissue and cell lines. (A) qPCR analysis examined the expression levels of EYA2 in 48 pair of OS samples and the adjacent noncancerous tissues. (B) The expression levels of EYA2 in OS cell lines (MG-63, U2OS, Saos-2 and HOS) and the normal osteoblast cell line (OB3). Values are expressed as mean ± SD, n = 4; *p < .01.
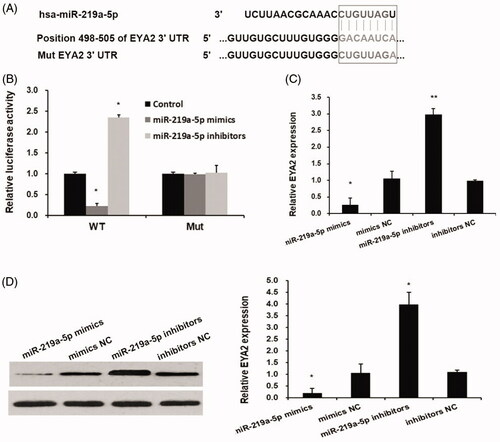
miR-219a-5p regulated EYA2 expression by binding 3′-UTR of EYA2
miRNAs was reported to involve tumour progression by regulating oncogene or tumour suppressor gene expression by binding to the 3′ UTR of their mRNA. In this study, we examined whether EYA2 is regulated by miRNAs. The bioinformatics analyses predicted that EYA2 has a binding site of miR-219a-5p at its 3′ UTR of EYA2 (). Dual luciferase reporter assay showed that miR-219a-5p mimics significantly inhibited the luciferase activity of pGLO-EYA2 but fail to affect the luciferase activity of pGLO-EYA2-Mut (). To determine whether miR-219a-5p regulates EYA2 expression in OS cells, miR-219a-5p mimic and miRNA negative control (mimics NC), miR-219a-5p inhibitors and inhibitors control (inhibitors NC) were transfected into MG63 cell and the expression levels of EYA2 were detected by qPCR and western blot. We found that miR-219a-5p mimics significantly downregulated EYA2 expression and miR-219a-5p inhibitors significantly upregulated EYA2 expression ().
Figure 2. miR-219a-5p regulated EYA2 expression by binding 3′-UTR of EYA2. (A) The bioinformatics analyses predicted that EYA2 has a binding site of miR-219a-5p at its 3′ UTR of EYA2. (B) Dual luciferase reporter assay. Values are expressed as mean ± SD, n = 3; *p < .01 compared with control group. (C) The effects of miR-219a-5p mimics and inhibitors on EYA2 mRNA expression. Values are expressed as mean ± SD, n = 3; *p < .01 compared with mimics NC; **p < .01 compared with inhibitors NC. (D) The effects of miR-219a-5p mimics and inhibitors on EYA2 protein expression. Values are expressed as mean ± SD, n = 3; *p < .01 compared with mimics NC; **p < .01 compared with inhibitors NC.
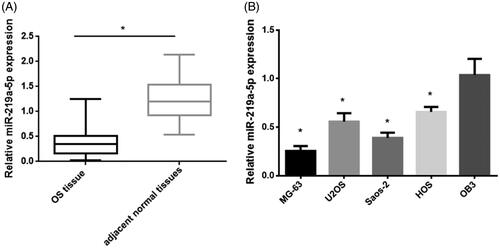
miR-219a-5p expression was downregulated OS tissue and cell lines
We next determined the expression of miR-219a-5p in OS tissue and cell lines. The qPCR results showed that miR-219a-5p expression level in OS tumour tissue was much lower than that in adjacent normal tissues (). Consistent with this result, miR-219a-5p expression was much lower in OS cell line (MG-63, U2OS, Saos-2 and HOS) than that in the normal osteoblast cell line (OB3) (). Taken together, these data indicated the miR-219a-5p may function as tumour suppressor in OS progression.
Figure 3. miR-219a-5p expression in OS tissue and cell lines. (A) qPCR analysis examined the expression levels of EYA2 in 48 pair of OS samples and the adjacent noncancerous tissues. (B) The expression levels of miR-219a-5p in OS cell lines (MG-63, U2OS, Saos-2 and HOS) and the normal osteoblast cell line (OB3). Values are expressed as mean ± SD, n = 4; *p < .01.
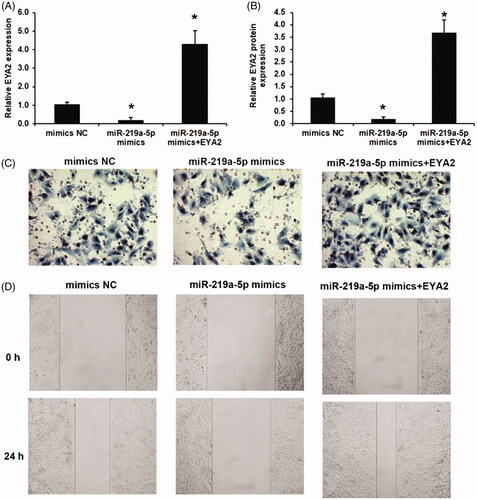
Overexpressed miR-219a-5p repressed OS cell invasion and migration via suppressing EYA2 expression
The effects of miR-219a-5p and EYA2 on OS cell invasion and migration were investigated. As shown in (), miR-219a-5p significantly repressed EYA2 expression, which was reversed by con-transfected with EYA2 plasma in OS cell. The similar results also observed in EYA2 protein expression levels as shown in . We found that the cell invasion ability and cell migration ability were significantly downregulated in miR-219a-5p mimics group compared with that in NC group using a transwell invasion assay () and a scratch wound healing assay (). To ascertain whether miR-219a-5p regulated OS cell function by repressing EYA2, we transfected the EYA2 plasma to OS cells with miR-219a-5p overexpressed to reverse EYA2 expression. We found that overexpressed EYA2 significantly recused the effects of miR-219a-5p on OS cell invasion and migration ().
Figure 4. Overexpressed miR-219a-5p repressed OS cell invasion and migration via suppressing EYA2 expression. (A) Effects of miR-219a-5p mimics and EYA2 plasma on EYA2 mRNA expression. (B) Effects of miR-219a-5p mimics and EYA2 plasma on EYA2 protein expression. (C) Effects of miR-219a-5p mimics and EYA2 plasma on OS cell invasion. (D) Effects of miR-219a-5p mimics and EYA2 plasma on OS cell migration. Values are expressed as mean ± SD, n = 4; *p < .01 vs. mimics NC group.
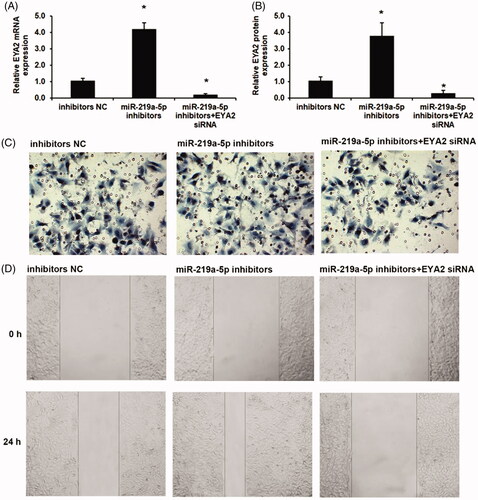
Silenced miR-219a-5p induced OS cell invasion and migration via EYA2 augmentation
Next, the effects of miR-219a-5p inhibitors on cell invasion and migration were then investigated. As shown in , miR-219a-5p inhibitors significantly induced EYA2 expression, which was reversed by con-transfected with EYA2 siRNA in OS cell. The similar results also observed in EYA2 protein expression levels as shown in . We found that silenced miR-219a-5p in OS cells significantly induced cell invasion and migration using a transwell invasion assay and a scratch wound healing assay (). We next used the EYA2 siRNA to knockdown the expression of EYA2. We found that downregulation of EYA2 reversed the effects of miR-219a-5p inhibitors on cell invasion and migration ().
Figure 5. Silenced miR-219a-5p induced OS cell invasion and migration via EYA2 augmentation. (A) Effects of miR-219a-5p inhibitors and EYA2 siRNA on EYA2 mRNA expression. (B) Effects of miR-219a-5p inhibitors and EYA2 siRNA on EYA2 protein expression. (C) Effects of miR-219a-5p inhibitors and EYA2 siRNA on OS cell invasion. (D) Effects of miR-219a-5p inhibitors and EYA2 siRNA on OS cell migration. Values are expressed as mean ± SD, n = 4; *p < .01 vs. mimics NC group.
Discussion
EYA2, function as tyrosine phosphatases or transcriptional coactivator, was reported to be an oncogene in various cancers [Citation8,Citation22]. However, the expression and role of EYA2 in OS remains unknown. In this study, we have shown that EYA2 expression level in OS tumour tissue and OS cells. Moreover, the EYA2 expression was closely associated with the clinicopathological characteristics including TNM stage, metastasis and tumour size. In this study, we report the discovery of a role for EYA2 in OS.
miRNAs involve the biological function via post-transcriptionally regulate gene expression by binding to the 3′ UTR of the mRNA of their target gene. Study showed that miR-30a has been shown to regulate the cell proliferation and migration by targeting EYA2 in breast cancer [Citation22]. miR-338-3p also reported to inhibit EYA2 expression by binding to the 3'-UTR of EYA2 in lung cancer [Citation10]. However, the regulation of EYA2 by miRNAs was not reported in OS. In the present study, bioinformatics analyses prediction and dual luciferase reporter assay showed that miR-219a-5p could bind to EYA2 at its 3′ UTR. miR-219a-5p locates on chromosome 6 (MI0000296) and was reported to involved the neural dysfunction in Alzheimer’s disease brain [Citation23]. Study also showed that it plays important role on normal oligodendrocyte differentiation and myelination [Citation24,Citation25]. miR-219a-5p been shown to mediate various tumour progression, including breast cancer, glioblastoma and papillary thyroid carcinoma [Citation26–28]. For example, Zhuang et al. [Citation27] revealed that miR-219a-5p involves cell migration and EMT of breast cancer. Rao et al. [Citation28] showed that miR-219-5p repressed glioblastoma cell proliferation and migration. Diverse lines of evidence support the hypothesis that miR-219a-5p involved the progression of OS by targeting EYA2. miR-219a-5p expression was downregulated OS tissue and cell lines in this study given the role of miR-219a-5p and EYA2 on cell invasion and migration of breast cancer [Citation22,Citation27] and astrocytoma [Citation11], we described the role of miR-219a-5p and EYA2 on OS invasion and migration. We observed that miR-219a-5p repressed OS cell invasion and migration via suppressing EYA2 expression.
Conclusion
In summary, EYA2 expression was upregulated in OS, which is associated with TNM stage, metastasis and tumour size. EYA2 involves tumour invasion and migration and regulated by miR-219a-5p. In conclusion, the current study sheds light on miR-219a-5p/EYA2 as a crucial factor that enhances OS cell invasiveness and provides a promising therapeutic target for OS treatment.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Fittall MW, Mifsud W, Pillay N. Recurrent rearrangements of FOS and FOSB define osteoblastoma. Nat Commun. 2018;9:2150.
- Wang Y. Long noncoding RNA DANCR, working as a competitive endogenous RNA, promotes ROCK1-mediated proliferation and metastasis via decoying of miR-335-5p and miR-1972 in osteosarcoma. J Pineal Res. 2018;17:89.
- Grifone R, Demignon J, Giordani J, et al. Eya1 and Eya2 proteins are required for hypaxial somitic myogenesis in the mouse embryo. Develop Biol. 2007;302:602–616.
- Jemc J, Rebay I. The eyes absent family of phosphotyrosine phosphatases: properties and roles in developmental regulation of transcription. Annu Rev Biochem. 2007;76:513–538.
- Cook PJ, Ju BG, Telese F, et al. Tyrosine dephosphorylation of H2AX modulates apoptosis and survival decisions. Nature. 2009;458:591–596.
- Krishnan N, Jeong DG, Jung SK, et al. Dephosphorylation of the C-terminal tyrosyl residue of the DNA damage-related histone H2A.X is mediated by the protein phosphatase eyes absent. J Biol Chem. 2009;284:16066–16070.
- Vincent A, Hong SM, Hu C, et al. Epigenetic silencing of EYA2 in pancreatic adenocarcinomas promotes tumor growth. Oncotarget. 2014;5:2575–2587.
- Li Z, Qiu R, Qiu X, et al. EYA2 promotes lung cancer cell proliferation by downregulating the expression of PTEN. Oncotarget. 2017;8:110837–110848.
- Yuan Y, Zheng S, Li Q, et al. Overexpression of miR-30a in lung adenocarcinoma A549 cell line inhibits migration and invasion via targeting EYA2. Acta Biochim Biophys Sin (Shanghai). 2016;48:220–228.
- Liang Y, Xu X, Wang T, et al. The EGFR/miR-338-3p/EYA2 axis controls breast tumor growth and lung metastasis. Cell Death Dis. 2017;8:e2928.
- Wen Z, Liang C, Pan Q, et al. Eya2 overexpression promotes the invasion of human astrocytoma through the regulation of ERK/MMP9 signaling. Int J Mol Med. 2017;40:1315–1322.
- Ono R, Masuya M, Ishii S, et al. Eya2, a target activated by PLZF, is critical for PLZF-RARA-induced leukemogenesis. Mol Cell Biol. 2017;37:e00585-16.
- Bierkens M, Krijgsman O, Wilting SM, et al. Focal aberrations indicate EYA2 and hsa-miR-375 as oncogene and tumor suppressor in cervical carcinogenesis. Genes Chromosom Cancer. 2013;52:56–68.
- Zhang Y, Ren S, Yuan F, et al. miR-135 promotes proliferation and stemness of oesophageal squamous cell carcinoma by targeting RERG. Artif Cells Nanomed Biotechnol. 2018;2:1–10.
- Wu LP, Wu J, Shang A, et al. miR-124 inhibits progression of hepatocarcinoma by targeting KLF4 and promises a novel diagnostic marker. Artif Cells Nanomed Biotechnol. 2017;18:1–9.
- Wang B, Li D, Kovalchuk I, et al. miR-34a directly targets tRNAi(Met) precursors and affects cellular proliferation, cell cycle, and apoptosis. Proc Natl Acad Sci USA. 2018;115:7392–7397.
- Mari L, Hoefnagel SJM, Zito D, et al. microRNA 125a regulates MHC-I expression on esophageal adenocarcinoma cells, associated with suppression of anti-tumor immune response and poor outcomes of patients. Gastroenterology. 2018;34640–34647: S0016-5085.
- Hersi HM, Raulf N, Gaken J, et al. MicroRNA-9 inhibits growth and invasion of head and neck cancer cells and is a predictive biomarker of response to plerixafor, an inhibitor of its target CXCR4. Mol Oncol. 2018 doi:10.1002/1878-0261.12352
- Ji Q, Xu X, Song Q, et al. miR-223-3p inhibits human osteosarcoma metastasis and progression by directly targeting CDH6. Mol Ther. 2018;26:1299–1312.
- Wu G, Yu W, Zhang M, et al. MicroRNA-145-3p suppresses proliferation and promotes apotosis and autophagy of osteosarcoma cell by targeting HDAC4. Artif Cells Nanomed Biotechnol. 2018;1:8.
- Jiashi W, Chuang Q, Zhenjun Z, et al. MicroRNA-506-3p inhibits osteosarcoma cell proliferation and metastasis by suppressing RAB3D expression. Cell Death Dis. 2018;10:1294–1305.
- Fu J, Xu X, Kang L, et al. miR-30a suppresses breast cancer cell proliferation and migration by targeting Eya2. Biochem Biophys Res Commun. 2014;445:314–319.
- Lukiw WJ. Micro-RNA speciation in fetal, adult and Alzheimer's disease hippocampus. Neuroreport. 2007;18:297–300.
- Dugas JC, Cuellar TL, Scholze A, et al. Dicer1 and miR-219 are required for normal oligodendrocyte differentiation and myelination. Neuron. 2010;65:597–611.
- Zhao X, He X, Han X, et al. MicroRNA-mediated control of oligodendrocyte differentiation. Neuron. 2010;65:612–626.
- Shi JA, Lu DL, Huang X, et al. miR-219 inhibits the proliferation, migration and invasion of medulloblastoma cells by targeting CD164. Int J Mol Med. 2014;34:237–243.
- Zhuang C, Yuan Y, Song T, et al. miR-219a-5p inhibits breast cancer cell migration and epithelial-mesenchymal transition by targeting myocardin-related transcription factor A. Acta Biochim Biophys Sin. 2017;49:1112–1121.
- Rao SA, Arimappamagan A, Pandey P, et al. miR-219-5p inhibits receptor tyrosine kinase pathway by targeting EGFR in glioblastoma. PloS One. 2013;8:e63164.
