?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Traditional haemostatic materials generally have slow hemostasis rate and poor biocompatibility. This paper reports on the haemostatic properties and mechanism of silk fibroin (SF). SF-PEG sponge that could be solubilised and changed to gel form by blood was fabricated through mixing SF and polyethylene glycol (PEG, 1500 Da) followed by lyophilisation of the mixed solution. SF-PEG sponge, together with control samples of SF sponge (no PEG) and a commercially available haemostatic material, gelatine sponge, were subjected to the hemostasis tests using a liver trauma model of rabbit. The results showed that SF was superior to gelatine sponge in hemostasis time (136.17 ± 62.27 s and 249.83 ± 29.18 s) and blood loss (2.16 ± 1.27 g vs. 4.97 ± 1.44 g). Furthermore, in vitro experiments indicated SF-PEG sol-gel transition promoted platelet adhesion and aggregation, as well as platelet-fibrinogen interaction. Therefore, except for the physical blocking of bleeding port due to PEG-induced SF fast gelation, SF might also have an impact on blood coagulation process, a phenomenon that has not been reported before. In conclusion, SF is a new type of haemostatic material that might be able to meet the requirements of speed, efficiency and biosafety in a variety of clinical applications.
Introduction
Excessive uncontrolled bleeding resulting from trauma is one of the leading causes of death. Effective control of blood loss can significantly reduce the mortality rate [Citation1]. Effective hemostasis and haemostatic materials are especially critical to blood loss control. Haemostatic materials should have good cytocompatibility, minimal inflammatory reactivity, short treatment time, no adverse effects on tissue healing, and no increase in infection probability. They should also be non-toxic, non-irritating, non-antigenic, soft, breathable, and permeable. Haemostatic materials should be painless, have no apparent foreign body sensation, and have little influence on the wound appearance.
With the rapid development of science and technology, research on medical haemostatic materials has made great progress in recent years [Citation2–6]. Zeolite, chitosan, and gelatine are commonly used as medical hemostasis materials. However, some of the side effects of zeolite include fever and burns [Citation7]. Extraction of chitosan is additionally difficult, and the material is expensive [Citation8]. Gelatine readily causes allergic reactions, and can increase wound infection rates and inflammatory responses [Citation9]. Novel haemostatic materials are needed. The development of a material with good biocompatibility, abundant sources, and excellent haemostatic properties would have considerable clinical significance.
Silk fibroin (SF) is a natural polymeric fibrin extracted from silk and composed of 18 amino acids [Citation10,Citation11]. SF has excellent mechanical properties, good biocompatibility, and biodegradable property [Citation12]. Thanks to these traits, the applications of SF in biomedicine have been widely reported in recent years [Citation13–15]. The haemostatic agent composed of SF (SF), hyaluronic acid (HA), gelatine (gel), and polyvinyl alcohol (PVA) has been reported to reduce nasal bleeding caused by the use of nasal mucosa sponge [Citation16]. Many studies have shown that soluble SF sponge mixed with blood can make blood coagulate rapidly [Citation17,Citation18]. Most existing haemostatic materials that contain silk proteins, however, are combined with other haemostatic materials such as gelatine and chitosan. Thus, it is difficult to highlight the characteristics of SF itself. Also, the complexity of the composition will also complicate the production process and increase the cost. Therefore, the haemostatic material with SF as a main body is still facing challenges.
Polyethylene glycol (PEG) is used in the pharmaceutical industry as an excipient to regulate viscosity and the melting point of the drug formulation. PEG is soluble in water, ethanol and many other organic solvents and has low vapour pressure and good thermal stability. It does not react with most chemicals, does not hydrolyse, nor does it deteriorate. It is non-toxic, and it is a good material for surface coating, sponge and capsule fabrication, and plasticization [Citation19].
Physiological blood coagulation can be divided into three steps: the prothrombin activator formation, thrombin formation, and fibrin formation. Notably, thrombus formed by platelets plays a key role in hemostasis besides fibrin. Normal vascular endothelial cells have an antithrombotic effect. When the vascular endothelium is damaged and the endothelial components exposed, platelets are activated. A haemostatic plug will be formed by platelet adhesion and aggregation, ultimately achieving the initial purpose of hemostasis [Citation20].
According to our previous research [Citation21–23], SF mixed with PEG can rapidly form a gel composed principally of a β-sheet structure. The gel has good biocompatibility, mechanical properties, and adhesive properties. The experimental material examined here is a SF-PEG sponge that is formed by mixing SF with polyethylene glycol with a molecular weight of 1500 Da (PEG1500) and freeze-drying. The sponge is fully soluble once immersed in the solution. Depending on the SF concentration, the solution turns into gel state. Water-insoluble, porous SF sponges have been used as cell-attaching scaffolds for tissue engineering applications. The sponges have water absorption properties, and can maintain their shapes in water for a long period of time. In contrast, the soluble sponge examined in the present study quickly shrinks when it gets wet. The moisture-triggered shrinking and sol-gel transition property make the SF-PEG sponge a promising haemostatic agent for wound treatment. To our knowledge, this has not been reported before. The haemostatic effect of the SF-PEG sponge was examined using an animal model and the haemostatic mechanism was elucidated by a series of in vitro experiments.
Experimental materials and methods
Experimental materials
Lyophilised SF (0.3 g per vial) were provided by Suzhou Simatch Inc. (Suzhou, China). ADP (CAT.A5285-100MG) were purchased from SIGMA-ALDRICH (St. Louis, MO). The QIAamp MinElute Media Kit (CAT.57414) was purchased from QIAGEN (Dusseldorf, Germany). A human HBeAg ELISA Kit (CAT. HG21022) and human hepatitis B virus surface antigen (HBsAg) ELISA Kit (CAT. HG033635) were purchased from TSZ Corporation (Shanghai, China). Anti-Fibrinogen alpha chain antibody [UC45] (CAT. ab19079) and goat Anti -Mouse IgM mu chain (DyLight® 488) (CAT. ab97007) were purchased from Abcam (Cambridge, UK). New Zealand rabbits and Wistar rats were provided by the experimental animal centre of the Second Affiliated Hospital of Harbin Medical University.
Preparation of SF solution and haemostatic sponges
Silk solution with a concentration of 3% w/v was obtained by injecting 10 mL of ultrapure water into the glass vial containing 0.3 g of lyophilised SF using a thin needle. The vial was slightly shaken at room temperature until SF was completely dissolved. The solution obtained was stored at 4 °C. Before use, the silk solution was further diluted to desired concentrations with ultrapure water.
To prepare a haemostatic sponge, SF solution at 3% was divided into two groups. For SF-PEG sponge, the SF solution was mixed with an equal volume of PEG 1500 (3% w/v) solution, so that the mass ratio of SF/PEG was 1/1. The mixed solution with a volume of 15 mL was poured into a 9.5 cm diameter circular dish, frozen at −20 °C for 12 h, and vacuum dried for 48 h in a lyophilizer (LMB-YD-0.5, Shinva Medical Instrument Co., Ltd, China). For SF sponge, ultrapure water was used instead of a PEG solution, and the steps were the same as above.
Fourier transform infrared spectroscopy (FTIR)
SF sponges obtained by lyophilisation were cut with a blade, and a slice (1-2 mm) was randomly taken for compression. The slice was subjected to a surface total reflection mode test using a Nicolet 5700 FT-IR type Fourier transform infra-red spectrometer, with the wave number range between 400 and 4000 cm−1.
Scanning electron microscope (SEM)
SF sponges obtained by lyophilisation were cut into thin slices with a square shape and an area of 3 mm × 3 mm each with a blade. A conductive adhesive was attached to a truncated cone, and the square samples were then placed on the conductive adhesive. A gap of 1–2 mm was left between each sample, then spray gold for 90 s at 20 mA over the samples. Images of porous SF materials were obtained at 3 kV using S-4800 scanning electron microscopy.
Gelation time
The prepared SF and SF-PEG sponges were weighed and dissolved in ultrapure water to obtain a solution with a silk fibroin concentration of 30% w/v. The silk fibroin solution was further diluted with ultrapure water to 5%, 10%, 15%, 20%, and 2 mL of the diluted solution was pipetted to a glass vial. Three vials for each sample were used for testing. The glass vials were sealed and placed in an incubator with a constant temperature (37 °C) and humidity (60%). Gelation was checked within 24 h by inverting the vial and observing the flowability of the solution.
Liver wound treatment using animal models
The animal protocol was approved by the Institution Animal Ethics Committee of Harbin Medical University, China.36 healthy New Zealand rabbits (weighing 1600–2100 g, either sex) were randomly divided into 4 experimental groups: (1) SF-PEG sponge; (2) SF sponge; (3) gelatine sponge; and, (4) blank. Rabbits were anaesthetised by intraperitoneal injection using 3% sodium pentobarbital (40 mg/kg) and fixed for supine conventional on the dissecting table. A midline incision through the skin and abdominal muscles was made to expose the middle liver lobe; the peritoneal fluid around the middle hepatic was absorbed with sterile gauze. The weighed sterile gauze was placed under the distal end of the middle lobe of the liver. A 1.5 cm × 1.0 cm × 0.2 cm wound of the liver was cut with a surgical blade at the distal end of the exposed organ. The blood was immediately wiped off, and 300 mg of different haemostatic materials were used to cover on the wound surface in the SF-PEG sponge group and gelatine sponge group; no haemostatic materials were used in the blank treatment group. The bleeding of the wound was observed, and the haemostatic time and blood loss were recorded.
In vitro hemostasis tests
Observation of platelet aggregation
Five healthy Wistar rats were selected (weighing 250–300g, male or female) to prepare PRP from the blood sample, and were centrifuged for 15 min at 1500 r/min with refrigerated centrifuge (Thermo, SORVALL ST8/8R, shanghai, China). The number of platelets in PRP was counted, and the PRP was diluted to 5 × 105 cells/mL with PBS, pH 7.4. One mL of the PRP suspension was mixed with the following solutions to reach desired concentrations: ADP (20 μM), PEG solution (200 mg/mL). One mL of the PRP suspension was mixed with the SF sponge (200 mg), and SF-PEG sponge (200 mg) respectively, and then diluted to reach desired concentrations with PBS. After being incubated at room temperature for 15 min, the mixtures were dropped into a glass slide, covered with a cover glass, and photographed under a phase-contrast microscope (CKX41FS, OLYMPUS, shanghai, China) to observe changes in platelet morphology.
Platelet adhesion test
There are SF sponge group, PEG solution (50% w/v) group, SF-PEG sponge group, and gelatine sponge (aseptic) group. Five parallel samples were tested in each group, and 10 mg of each sample was placed into the 24-well plate. Five healthy Wistar rats were selected (weighing 250–300g, either sex), and PRP was prepared as outlined in 2.7.1. One mL of diluted PRP solution was pipetted into each well of the 24-well plate, incubated for 3 h at 37 °C, and the number of platelets in each well was recorded. Three replicate samples were checked for each blood sample. The adhesion rate was calculated as follows:
The number of platelets added per well is N1, and the number of platelets in the solution after adhesion is N2.
Flow cytometry to detect the activation of fluorescent
To determine the impact of SF on platelet aggregation and further blood clotting cascade, the interaction between platelet and fibrinogen upon exposure to various haemostatic materials was examined by flow cytometry. Five healthy Wistar rats were selected (weighing 250 g–300g, either sex), and PRP was prepared as outlined in 2.7.1. One mL of the PRP suspension was mixed with the following solutions to reach desired concentrations: ADP (20 μM), PEG solution (200 mg/mL). One mL of the PRP suspension was mixed with the SF sponge (200 mg), and SF-PEG sponge (200 mg) respectively, and then diluted to reach desired concentrations with PBS. The mixtures were incubated in the dark at 37 °C for 15 min, and paraformaldehyde (final concentration 1%) was then added. After 1 mL of the platelet was washed with PBS by centrifugation (5 min at 1500 r/min, SORVALL ST8/8R, Thermo Scientific), 1 μg anti-fibrinogen alpha chain (Abcam, ab19079) was added, incubated at 4 °C for 30 min, re-washed with PBS, pH 7.4, and then 10 μg goat Anti-Mouse IgM mu chain (DyLight® 488) (Abcam, ab97007) was added, incubated at 4 °C for 30 min, re-washed with PBS, pH 7.4. The platelet suspension obtained (1 mL) was subjected to flow cytometry using a flow cytometer (FACSCalibur, BD), with the fluorescence detection setting for Alexa 488.
Detection of fibrinogen expression by ELISA
Five healthy Wistar rats were selected (weighing 250—300 g, male or female), and PRP was prepared as outlined in 2.7.1. One mL of the PRP suspension was mixed with the following solutions to reach desired concentrations: ADP (20 μM), PEG solution (200 mg/mL). One mL of the PRP suspension was mixed with the SF sponge (200 mg), and SF-PEG sponge (200 mg) respectively, and then diluted to reach desired concentrations with PBS, and the mixed solution was incubated for 15 min before being subjected to ELISA assay. Fibrinogen expression levels were then detected with an ELISA kit at room temperature, following the vendor’s instruction.
Statistical analysis
All data were processed by SPSS 21.0 statistical software, two-sided test, where α = 0.05 was the test level. p < .05 indicates that the difference was statistically significant, and p < .01 indicates the difference was very significant.
Results
SF secondary structure
From literature, the absorption peak between 1616 cm−1 and 1637 cm−1 in the amide I band is assigned to β-sheet structure (corresponding to Silk II crystal structure), and the absorption peak located at 1650–1655 cm−1 in random coil structure (corresponding to Silk I structure) [Citation24]. shows the secondary structure of SF-PEG sponge before and after hydration, as well as the lyophilised SF sponge (control). There was a distinct characteristic peak at 1651 cm−1 for SF sponge and SF-PEG sponge, indicating that they were mainly a random coil structure.
Figure 1. The secondary structure of various SF materials determined by FTIR. (A) FTIR spectra of various samples. (B) a β-sheet content analysis. *p < .05.
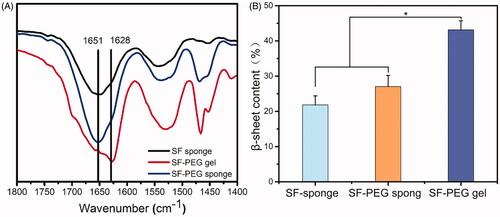
The SF-PEG sponge after hydration and lyophilised (SF-PEG gel) had characteristic peaks at 1628 cm−1 and 1652 cm−1, respectively, and the peak at 1628 cm−1 was weak, suggesting that both random coils and β-sheets dominated the secondary structure of SF-PEG gel. Based on the deconvolution of the characteristic amide I peak, the content of β-sheet structure among all four secondary structures was 22 ± 2.6% for SF sponge, 27 ± 3.2% for SF-PEG sponge, and 43 ± 2.3% for SF-PEG gel (). Apparently, a small amount of β-sheet structure in the SF-PEG sponge did not significantly affect the solubility of SF. When the sponge was hydrated with water, it quickly shrunk and dissolved, yielding a high concentration of SF/PEG mixed solution. The presence of PEG induced the structure formation of SF β-sheet and thereby SF gelation ().
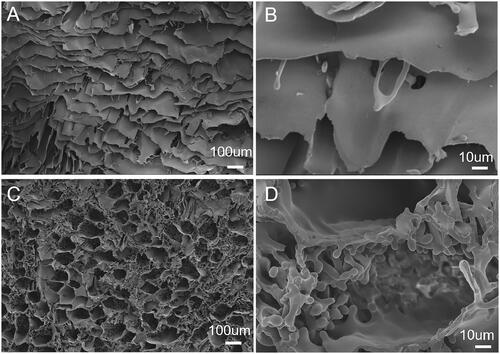
Morphologies of SF and SF-PEG sponges
SF sponge had a smooth surface and a large pore size (). Within the SF-PEG sponges, the structure is relatively rough and loose (). The loose porous structure facilitated water absorption and accelerated the dissolution of sponge. A large number of micro- and nano-sized particles were observed on the surface of SF-PEG gel sample (), likely due to PEG-induced SF nanoparticle and microparticle formation as reported in our previous study [Citation25]. The presence of nano- and microparticles might have a beneficial effect on the mechanical properties of the SF-PEG gel, as these particles might help link the SF gel fibres due to large surface areas. On the other hand, these particles might have a higher content of β-sheet structure compared to the SF gel fibres surrounding them, acting as mechanically and thermodynamically stable cores among the gel network. High content of β-sheet structure (∼40%) in the PEG-induced SF nano- and microparticles have been demonstrated in our previous studies [Citation25,Citation26].
Gelation time
The higher the concentration of the SF-PEG mixed solution, the shorter the gelation time. The pure SF sponge is difficult to gel in a short time. The gelation time of 5% (w/v) SF concentration exceeds one day with or without PEG. When the SF concentration in the SF/PEG mixed solution reached 20% (w/v), the solution gelled within 2 min, but the solution hydrated from the SF-sponge still could not gel within one day (). Therefore, the addition of an appropriate amount of PEG can greatly accelerate SF gelation; in addition, PEG can also facilitate hydration of the SF sponge. These features promote effect of SF-PEG sponge on hemostasis.
Table 1. Gelation time of different silk materials at different silk concentration.
Hemostasis of SF-PEG sponge on liver wound
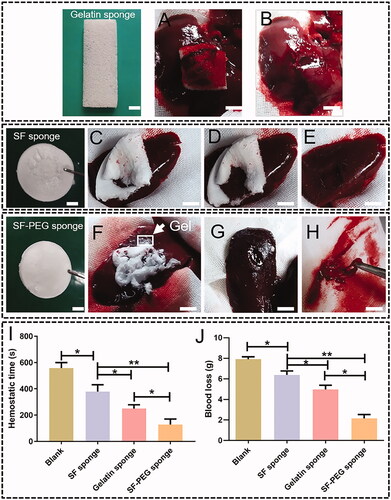
showed samples of SF-PEG, SF sponge and gelatine sponge (control) and the process of liver wound hemostasis. As shown in , SF-PEG sponge and gelatine sponge had blood loss of 2.16 ± 1.27 g and 4.97 ± 1.44 g, significantly less than the blank group (7.92 ± 0.8 g) and SF sponge (6.38 ± 1.24 g). The blood loss of SF sponge was significantly less than the blank group. The difference between SF-PEG sponge and gelatine sponge was statistically significant (p < .05). The haemostatic time of the SF-PEG sponge group and the gelatine sponge group was 136.17 ± 62.27 s and 249.83 ± 29.18 s, significantly shorter than that of the blank group (557.75 ± 42.38) and SF sponge (378.63 ± 50.22 g) (p < .05). The difference between SF sponge and the blank group was statistically significant (p < .05). The haemostatic time of SF-PEG sponge was significantly shorter than that of the gelatine sponge (p < .05, ).
Figure 3. Pictures showing the haemostatic effect of SF-PEG and gelatine sponges on liver wound of rabbits. Upper panel: hemostasis by gelatine sponge. (A) before hemostasis. (B) after hemostasis. Middle panel: hemostasis by SF-PEG sponge. (C) before hemostasis; (D) in hemostasis; (E) after hemostasis; (F) before hemostasis; (G) after hemostasis; (H) SF-PEG gel formed after hemostasis. Lowest panel: blood loss and haemostatic time determined in liver wound model. (I) blood loss. (J) haemostatic time (scale bars are 1 cm). *p < .05; **p < .01.
In vitro assessments of SF haemostatic properties
As indicated in the section “Materials and Methods”, SF-PEG sponge, PEG solution, SF sponge, and a positive control (ADP, adenosine diphosphate) for inducing platelet aggregation were mixed with PRP, and the process of SF-induced platelet aggregation, release, and adhesion was observed under a microscope. There was no obvious platelet aggregation in the blank group and only a slight aggregation of platelets was observed in the PEG group. More platelet aggregation was observed in the SF group than blank and PEG group, and SF-PEG group showed more platelet aggregation than the SF group. The SF-PEG group was similar to the ADP-induced platelet aggregation (). The results indicated that SF sponge and SF-PEG sponge had a remarkable effect on promoting platelet aggregation.
Figure 4. Phase contrast microscopy of platelet morphological changes after exposure to ADP and haemostatic materials. (A) blank group. (B) PEG group. (C) SF group. (D) SF-PEG group. (E) ADP group (scale bars are 100 μm). (B) Platelet adhesion rates determined in the presence of different haemostatic materials. (C) Flow cytometry on rat platelets that interacted with Alexa 488-fibrinogen. From left to right: blank group (dark green), PEG group (light green), SF group (orange), SF-PEG group (red), and ADP (blue) group. (D) detection of fibrinogen by ELISA *p < .05; **p < .01.
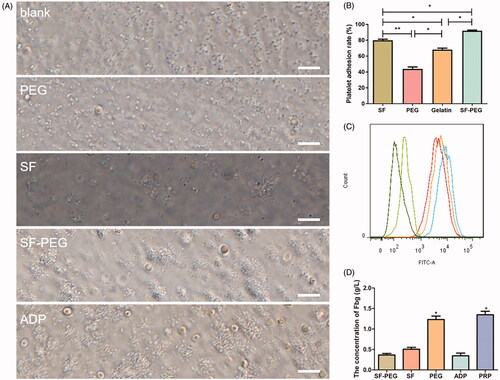
To examine the effect of haemostatic materials on platelet adhesion, we selected gelatine sponge as the control group and used SF-PEG sponge, SF-sponge, and PEG solution in the experiment. The experimental data showed that the SF-PEG sponge had the highest platelet adhesion rate (84 ± 0.7%) among all the samples tested, while the platelet adhesion rate of the PEG group (42 ± 1.3%) was the lowest. The platelet adhesion rate of SF-PEG sponge was significantly higher than that of gelatine sponge (63 ± 1.5%) (p < .05, .
To study the role of SF in inducing platelet aggregation, flow cytometry was used to detect the population of rat platelets that interacted with Alexa 488-fibrinogen. The fluorescence peaks from samples of SF, SF-PEG, and ADP all moved slightly to the right compared with that of the blank group and the PEG group (. This shift indicated that SF enhanced the interaction of platelets and Alexa 488-fibrinogen.
The process of physiological coagulation can be divided into three steps: the formation of prothrombin activator, the formation of thrombin, and the formation of fibrin. To prove that SF can promote physiological coagulation, we examined whether fibrinogen in fibroin-stimulated platelets were converted to fibrin by ELISA [Citation27]. The fibrinogen (Fbg) expression level in SF-PEG sponge, SF sponge and ADP group was significantly lower than that of the other two groups (p < .05), indicating fibrinogen was likely converted to fibrin. There was no statistical difference between these three groups, and between the PRP and PEG groups ().
Discussion
A newly developed haemostatic material mainly composed of silk fibroin protein (SF) was examined in this study. Previous studies have shown that various cells could attach, proliferate and differentiate on SF materials, and the cell compatibility can be improved by changing the physical and chemical properties of SF through cross-linking, blending, grafting, etc. [Citation28–30]. Due to its excellent performance, the reported use of SF in biomedicine has increased; however, its application in hemostasis has not yet been described. In the present study, to better simulate the visceral haemorrhage of the human body, the hepatic haemorrhage model of New Zealand white rabbits were used to evaluate the haemostatic effect of SF materials. The hemostasis time and blood loss were mainly used to evaluate the ability of haemostatic materials to promote blood coagulation. The shorter the clotting time and the less the blood loss, the better the haemostatic effect of the material [Citation31].
In liver wound models, SF-PEG sponge had a better effect on hemostasis than gelatine sponge. The porous gelatine sponge is the most widely used haemostatic material in surgeries. It can absorb a large amount of blood, thereby activating platelets, promoting platelets aggregation, and forming blood clots to block blood vessels or wounds [Citation32,Citation33]. The process requires a large number of platelets and coagulation factors. In cases where the patients' platelets and coagulation factors are reduced, the haemostatic effect of gelatine sponges is greatly weakened. In addition, the adhesion of gelatine sponges to the wound surface is poor and easy to fall off, resulting in secondary bleeding. On the other hand, since gelatine is extracted from type I collagen from animal tissues, the gelatine sponge can readily lead to allergic reactions, as often seen by increased infiltration of local inflammatory cells, especially in the contaminated wound. In this study, blood coagulated after being adsorbed in the gelatine sponge, but the sponge could hardly adhere to the wound, resulting in increased hemostasis time and bleeding volume. In contrast, after the SF-PEG sponge was applied to cover the wound, the sponge absorbed blood and shrunk into gel, which tightly adhered to the wound. The bleeding was quickly stopped as a result.
SF is a natural protein extracted from silkworm cocoons. The gelation process of SF is usually triggered by certain external factors, such as temperature, humidity, pH change, solvent treatment, metal ion action and shear stretching. The secondary structure of SF changed from random coil to β-sheet during gelation, and physical cross-linking occured due to the intermolecular interaction at the β-sheet structure regions [Citation34,Citation35]. In addition to the β-sheet stacks formed, hydrophobic interactions and hydrogen bonding between molecules also contribute to the formation of SF gels [Citation36]. Since β-sheet structure is thermodynamically stable, the aqueous solution of SF gradually transforms into a gel even during storage. However, this process can take weeks to months without the influence of external factors [Citation37]. Protein concentration, temperature, pH values, non-polar solvents, and various ions are important factors influencing the gelation of SF [Citation35,Citation38,Citation39]. Higher protein concentrations, heating, lower pH or the addition of calcium ions, and alcohols can speed up the rate of solution-gel transition. In addition, the presence of shear forces can also promote the gelation of silk proteins [Citation40,Citation41]. Cells can be encapsulated and proliferate in SF gels, and the biocompatibility and biodegradation of SF gels have been demonstrated by in vivo experiments [Citation42]. For the SF-PEG sponge being tested in the study, SF provided structural and mechanical properties, while the highly hydrophilic PEG facilitated water absorption. SF and PEG molecules compete for water after the sponge was hydrated, and SF structural transition from random coils to β-sheets occurred due to the loss of bound water on SF molecules. Such structural transition and gelation took place fast, making SF-PEG sponge capable of physically blocking the bleeding port.
Except for the physical blockage mechanism, we also wanted to know if there were biological reasons behind SF-PEG sponge-promoted hemostasis. After vascular injury, platelets flowing through this blood vessel are activated by the surface of the subendothelial tissue and immediately adhere to the collagen fibres exposed at the lesion. Adhesion is mainly a surface phenomenon, and once adhesion occurs, the aggregation process of platelets occurs immediately. In this study, it was likely that the SF fibres generated in the hydrogel and resembled collagen fibres in the body promoted adhesion and aggregation of the platelets, therefore having impact on blood coagulation. To prove this hypothesis, we conducted in vitro experiments to determine the influence of various materials, i.e. SF-PEG sponge, SF-sponge, PEG alone and gelatine sponge (control), on platelet adhesion and aggregation. It has been shown in the literature that endogenous platelet agonists such as ADP, TxA2, and thrombin can promote platelet adhesion, aggregation and the subsequent blood coagulation process. During this process, intracellular signalling was initiated through binding of fibrinogen to platelets, resulting in platelet aggregation [Citation43]. As shown in , compared to the control samples, SF-PEG sponge induced more platelet adhesion and aggregation, and significantly induced platelet-fibrinogen interaction. The promoting effect of SF-PEG sponge was similar to that of ADP. As to why the SF-PEG sponge group is better than the SF-sponge group in platelet adhesion, it may be caused by the accelerated gel formation after the SF-PEG sponge was hydrated. Semi-solid gel composed of SF nano- and microfibers might be more favourable for platelets to adhere and aggregate and induce blood coagulation. The detailed mechanism will need to be elucidated in the future studies.
Conclusion
The haemostatic properties and hemostasis mechanism of SF-PEG sponge was studied in this paper. Liver wound models using rabbits revealed the positive haemostatic effects of SF-PEG sponge, and the performance was even better than that of clinically used gelatine sponges. SF played a critical role in hemostasis from two aspects: first, SF gel formed in the presence of PEG physically blocked the bleeding port; second, SF might also have an impact on biological process of blood coagulation. It was found that SF could significantly activate platelets in vitro, cause platelet aggregation and adhesion, maintain platelet activity, and promote blood coagulation. The study of the new haemostatic material of SF-PEG sponge is still at infant stage. More comprehensive studies on the release of coagulation factors, and in-depth analyses of the effects of physical, chemical and biological hemostasis pathways will be needed in the future.
Disclosure statement
The authors declare that they have no conflicts of interest to disclose.
Additional information
Funding
Reference
- Dai C, Yuan Y, Liu C, et al. Degradable, antibacterial silver exchanged mesoporous silica spheres for hemorrhage control. Biomaterials. 2009;30(29):5364–5375.
- Alam HB, Chen Z, Jaskille A, et al. Application of a zeolite hemostatic agent achieves 100% survival in a lethal model of complex groin injury in swine. J Traum. 2004;56(5):974–983.
- Pusateri AE, Delgado AV, Dick EJ, et al. Application of a granular mineral-based hemostatic agent (QuikClot) to reduce blood loss after Grade V liver injury in swine. J Traum. 2004;57(3):555–562.
- Pusateri AE, McCarthy SJ, Gregory KW, et al. Effect of a chitosan-based hemostatic dressing on blood loss and survival in a model of severe venous hemorrhage and hepatic injury in swine. J Traum. 2003;54(1):177–182.
- Wedmore I, McManus JG, Pusateri AE, et al. A special report on the chitosan-based hemostatic dressing: experience in current combat operations. J Traum. 2006;60(3):655–658.
- Alpaslan C, Alpaslan GH, Oygur T. Tissue reaction to three subcutaneously implanted local hemostatic agents. Brit J Oral Max Surg. 1997;35(2):129–132.
- Radosevich M, Goubran H, Burnouf T. Fibrin sealant: scientific rationale, production methods, properties, and current clinical use. Vox Sanguinis. 1997;72(3):133–143.
- Brandenberg G, Leibrock LG, Shuman R, et al. Chitosan: a new topical hemostatic agent for diffuse capillary bleeding in brain tissue. Neurosurgery. 1984;15(1):9–13.
- Fukui T, Ii M, Shoji T, et al. Therapeutic effect of local administration of low-dose simvastatin-conjugated gelatin hydrogel for fracture healing. J Bone Miner Res. 2012;27(5):1118–1131.
- Hardy JG, Römer LM, Scheibel TR. Polymeric materials based on silk proteins. Polymer. 2008;49(20):4309–4327.
- Vepari C, Kaplan DL. Silk as a biomaterial. Prog Polym Sci. 2007;32(8–9):991–1007.
- Rockwood DN, Preda RC, Yucel T, et al. Materials fabrication from Bombyx mori silk fibroin. Nat Protoc. 2011;6(10):1612–1631.
- Furuzono T, Taguchi T, Kishida A, et al. Preparation and characterization of apatite deposited on silk fabric using an alternate soaking process. J Biomed Mater Res. 2000;50(3):344–352.
- Zhang K, Mo X, Huang C, et al. Electrospun scaffolds from silk fibroin and their cellular compatibility. J Biomed Mater Res Part A. 2010;93(3):976–983.
- Zhao J, Zhang Z, Wang S, et al. Apatite-coated silk fibroin scaffolds to healing mandibular border defects in canines. Bone. 2009;45(3):517–527.
- Moon BM, Ju HW, Park HJ, et al. The pilot study for the development of the hemostatic agent using silk fibroin for epistaxis. Tissue Eng Regen Med. 2013;10:41–47.
- Lu Q, Zhang S, Hu K, et al. Cytocompatibility and blood compatibility of multifunctional fibroin/collagen/heparin scaffolds. Biomaterials. 2007;28(14):2306–2313.
- Tamada Y. Sulfation of silk fibroin by chlorosulfonic acid and the anticoagulant activity. Biomaterials. 2004;25(3):377–383.
- Strickley RG. Solubilizing excipients in oral and injectable formulations. Pharm Res. 2004;21(2):201–230.
- Yun-Choi HS, Park KM, Pyo MK. Epinephrine induced platelet aggregation in rat platelet-rich plasma. Thrombosis Research. 2000;100(6):511–518.
- Wang X, Partlow B, Liu J, et al. Injectable silk-polyethylene glycol hydrogels. Acta Biomaterialia. 2015;12:51–61.
- Zheng Z, Wu J, Liu M, et al. 3D bioprinting of Self-standing Silk-based bioink. Adv Healthcare Mater. 2018;7(6):1701026.
- Wang Y, Liang M, Zheng Z, et al. Adhesion prevention after laminectomy using silk-polyethylene glycol hydrogels. Adv Healthcare Mater. 2015;4(14):2120–2127.
- Hu X, Shmelev K, Sun L, et al. Regulation of silk material structure by temperature-controlled water vapor annealing. Biomacromolecules. 2011;12(5):1686–1696.
- Wu J, Zheng Z, Li G, et al. Control of silk microsphere formation using polyethylene glycol (PEG). Acta Biomaterialia. 2016;39:156–168.
- Wu J, Wang J, Zhang J, et al. Oral delivery of curcumin using silk nano- and microparticles. ACS Biomater Sci Eng. 2018;4(11):3885–3894.
- Dunn CJ, Goa KL. Fibrin sealant - A review of its use in surgery and endoscopy [Review]. Drugs. 1999;58(5):863–886.
- Wang J, Yi H, Wei Y. Preliminary biocompatibility evaluation of regenerated Antheraea yamamai Silk Fibroin in vitro. J Wuhan Univ Technol-Mat Sci Edit. 2011;26(6):1044–1048.
- Gotoh Y, Tsukada M, Minoura N, et al. Synthesis of poly(ethylene glycol)-silk fibroin conjugates and surface interaction between L-929 cells and the conjugates. Biomaterials. 1997;18(3):267–271.
- Inouye K, Kurokawa M, Nishikawa S, et al. Use of Bombyx mori silk fibroin as a substratum for cultivation of animal cells. J Biochem Bioph Meth. 1998;37(3):159–164.
- Shih MF, Shau MD, Chang MY, et al. Platelet adsorption and hemolytic properties of liquid crystal/composite polymers. Int J Pharm. 2006;327(1–2):117–125.
- Ishihara M, Nakanishi K, Ono K, et al. Photocrosslinkable chitosan as a dressing for wound occlusion and accelerator in healing process. Biomaterials. 2002;23(3):833–840.
- Gustafson SB, Fulkerson P, Bildfell R, et al. Chitosan dressing provides hemostasis in swine femoral arterial injury model. Prehosp Emerg Care. 2007;11(2):172–178.
- Ayub ZH, Arai M, Hirabayashi K. Mechanism of the gelation of fibroin solution. Biosci Biotech Bioch. 1993;57(11):1910–1912.
- Matsumoto A, Chen J, Collette AL, et al. Mechanisms of silk fibroin sol-gel transitions. J Phys Chem B. 2006;110(43):21630–21638.
- Jin H-J, Kaplan DL. Mechanism of silk processing in insects and spiders. Nature. 2003;424(6952):1057.
- Zainuddin Le TT, Park Y, et al. The behavior of aged regenerated Bombyx mori silk fibroin solutions studied by (1)H NMR and rheology. Biomaterials. 2008;29(32):4268–4274.
- Kim U-J, Park J, Li C, et al. Structure and properties of silk hydrogels. Biomacromolecules. 2004;5(3):786–792.
- Hanawa T, Watanabe A, Tsuchiya T, et al. New oral dosage form for elderly patients: preparation and characterization of silk fibroin gel. Chem Pharm Bull. 1995;43(2):284–288.
- Yucel T, Cebe P, Kaplan DL. Vortex-induced injectable silk fibroin hydrogels. Biophys J. 2009;97(7):2044–2050.
- Wang X, Kluge JA, Leisk GG, et al. Sonication-induced gelation of silk fibroin for cell encapsulation. Biomaterials. 2008;29(8):1054–1064.
- Slaughter BV, Khurshid SS, Fisher OZ, et al. Hydrogels in regenerative medicine. Adv Mater. 2009;21(32–33):3307–3329.
- Jill R, Crittenden WB, Zhang Y, et al. CalDAG-GEFI integrates signaling for platelet aggregation and thrombus formation. Nat Med. 2004;10(9):982–986.