Abstract
Cytoplasmic p27 plays an important role in regulating the cell cycle. Recent studies have revealed p27 protein translocation from the nucleus to the cytoplasm in many tumour cells. The aim of this study was to investigate the role and molecular mechanisms of cytoplasmic p27 in the progression of nasopharyngeal carcinoma (NPC) and to explore its prognostic value. We found increased cytoplasmic p27 expression by immunohistochemistry in NPC tissues, and its expression level was significantly correlated with the T classification and TNM clinical stage of NPC. The survival rate was significantly lower for NPC patients with cytoplasmic p27 immunopositivity than for NPC patients with cytoplasmic p27 immunonegativity, and cytoplasmic p27 was an independent risk factor that affected the prognosis of patients with NPC. Cytoplasmic p27 promoted the proliferation, cell cycle progression, migration, and invasion of NPC cells, increased Bim-1 and Twist1 protein levels, and decreased RhoA-GTP level. Collectively, these findings suggest that cytoplasmic relocalization of p27 is involved in the pathogenesis of NPC and is closely related to the unfavourable prognosis of patients with NPC. Therefore, cytoplasmic p27 might be a useful prognostic factor and potential therapeutic target for patients with NPC.
Introduction
Nasopharyngeal carcinoma (NPC) is a common head and neck malignant tumour. The occurrence of NPC is related to ethnic susceptibility, genetic factors, and Epstein-Barr virus infection. NPC has a high degree of malignancy, is mostly a poorly differentiated squamous cell carcinoma, and is prone to local infiltration and cervical lymph node metastasis. At present, the molecular pathogenesis of NPC metastasis has not been fully elucidated.
The p27 protein, also known as cyclin-dependent kinase inhibitor B (DKNIB), was discovered by Polyak in 1994 and is a member of the Cip/Kip family. The p27 protein plays a regulatory role in cell cycle progression and proliferation [Citation1–3]. Normally located in the nucleus, p27 can inhibit the cyclin E/cyclin A-cyclin-dependent kinase (CDK) 2 complexes, thereby negatively regulating G1–S phase transition. Therefore, the p27 protein is considered a tumour suppressor [Citation4]. A decrease in or loss of p27 expression in the nucleus and cytoplasmic translocation of p27 often occur in many types of tumours. Cytoplasmic p27 is closely related to a poor prognosis in patients with tumour. Therefore, cytoplasmic p27 is considered an oncogenic protein [Citation5–9]. Studies have shown that the serine/threonine protein kinase Akt protein is a key molecule that regulates the cytoplasmic p27 protein level; it can phosphorylate the threonine at position 157 of the p27 protein and restrict the cytoplasmic p27 protein to the nucleus [Citation10]. Thus, subcellular localization of p27 is determined by the phosphorylation of key amino acid residues. Cytoplasmic p27 can bind to the RhoA protein and inhibit the RhoA-ROCK-mediated regulation of actin stability, thereby promoting the migration and infiltration of tumour cells through CDK-independent mechanisms [Citation11]. However, studies on the regulatory functions of cytoplasmic relocalized p27 in the development of NPC and the prognosis of patients with NPC are extremely rare.
As tumour-promoting genes, Bim-1 and Twist1 are involved in the proliferation and invasion of human tumour cells and are both highly expressed in NPC cell lines and NPC tissues. Overexpression of Bim-1 or Twist1 is negatively correlated with the prognosis of patients with NPC, suggesting that Bim-1 and Twist1 play oncogenic roles in NPC development [Citation12,Citation13]. However, whether cytoplasmic p27 participates in the proliferation, migration and invasion processes of NPC cells by regulating the expression of Bim-1 or Twist1 in NPC cells remains unclear.
To clarify the role, molecular mechanism and clinical significance of cytoplasmic p27 in the regulation of proliferation and metastasis of NPC, we applied immunohistochemistry to detect subcellular localization of p27 in NPC tissues and evaluated the relationship between the cytoplasmic p27 expression level and the clinical pathological features and prognosis of patients with NPC. In addition, we performed in vitro experiments to determine the effect of cytoplasmic p27 on the proliferation, migration, and invasion of NPC cells and its underlying molecular mechanisms.
Materials and methods
Sample collection
A total of 113 patients with NPC in Hangzhou First People's Hospital were recruited for this study from December 2006 to January 2016. The diagnoses of all participating patients were confirmed by nasopharyngeal biopsy and histopathology. None of these patients received radiotherapy and/or chemotherapy before recruitment. Clinical staging was performed according to the 7th edition of TNM classification of the American Joint Committee on Cancer Staging System (AJCC) for NPC [Citation14]. The histological differentiation grade was confirmed according to the classification of the World Health Organization (WHO, 2005). Nasopharyngeal mucosal tissue specimens from 28 patients with chronic nasopharyngitis were selected as a control group. This study was approved by the Ethics Committee of Hangzhou First People’s Hospital, and all 141 patients signed informed consent voluntarily.
Clinical data and follow-up
The clinical data for each patient, including sex, age, and lesion range, were recorded in detail. During the follow-up, overall survival (OS) for all patients with NPC was recorded. OS was defined as the time between radiotherapy and death or last follow-up. The last follow-up was 31 December 2016.
Immunohistochemistry
The En Vision method was used for immunohistochemical staining. First, tissue paraffin blocks were cut into 4–5 μm sections. The sections were placed onto glass slides, deparaffinized with xylene, and hydrated using a gradient of alcohol. Antigen retrieval was performed using a sodium citrate buffer solution (pH 6.0). After incubation with 3% hydrogen peroxide for 7 min to block endogenous peroxidase activity, an anti-human p27 antibody (polyclonal rabbit IgG, C-19, sc528, Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a 1:50 dilution was added drop-wise to each section, which was then incubated at room temperature for 1 h. According to the instructions of the En Vision immunohistochemical staining kit (K5007, Dako, Glostrup, Denmark), the sections were incubated with a horseradish peroxidase-conjugated secondary antibody at room temperature for 20 min and finally visualized using the chromogen diaminobenzidine for 3 min.
Immunohistochemistry results were evaluated independently by two pathologists. A light yellow to brownish yellow colour in the cytoplasm and/or nucleus indicated positive p27 staining. p27 immunoreactivity was scored based on the staining intensity and the percentage of positively stained cells [Citation15,Citation16]. The staining intensity was recorded as 0 (no colour reaction), 1 (light yellow, mild reaction), 2 (yellow, moderately reaction), or 3 (brownish yellow, intense reaction). The percentage of positively stained cells was evaluated using a scale (0: none, 1: 1–20%, 2: 21–50%; 3: 51–80%, 4: >80%). The final score for p27 immunoreactivity was obtained by multiplying the scores of the staining intensity and the percentage of positively stained cells and provided an immunoreactive score (IRS) ranging from 0 to 12. Staining with an IRS ≥ 2 was considered positive, while that with IRS < 2 was defined as negative.
Cell cultures
The NPC cell line CNE2 was provided by the Sun Yat-sen University Cancer Centre. CNE2 and 293 T cell lines were cultured in a conventional manner using Dulbecco’s modified Eagle’s medium (DMEM) (Life Technologies, Invitrogen TM) with 10% foetal bovine serum (FBS) (containing 1.5 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin) in a constant temperature incubator at 37 °C with 5% CO2.
Preparation of recombinant lentiviruses overexpressing p27
Upstream and downstream primers (5′-AGGGTTCCAAGCTTAAGCGGCCGCGCCACCATGGTGAGCA-3′ and 5′-ATCAGTAGAGAGTGTCGGATCCCG-3′) of human p27 were synthesized. The primers contained the homologous sequences of NotI and BamHI from the lentiviral vector LV5, which were then mixed with OligoMix, and a subclone of the vector was prepared using PCR. mCherry gene fragments were then prepared by polymerase chain reaction (PCR) amplification of the primers. The targeted DNA fragment was purified by agarose electrophoresis and then ligated with the lentiviral vector LV5, followed by transformation with competent cells and selection of the positive clone vector LV5-mCherry-GFP.
p27-shRNA (primers: 5′-TGAGCCAGCGCAAGTGGAATTTTTCAAGAGAAAATTCCACTTGCGCTGGCTCTTTTTTG-3′ and 5′-GATCCAAAAAAGAGCCAGCGCAAGTGGAATTTTCTCTTGAAAAATTCCACTTGCGCTGGCTCATGCA-3′), p27WT (primers: 5′-AGGGTTCCAAGCTTAAGCGGCCGCGCCACC-3′ and 5′-ATCAGTAGAGAGTGTCGGATCCCGTCATGCATC-3′), and p27Mut (T157A) (primers: 5′-AGGGTTCCAAGCTTAAGCGGCCGCGCCACCATGTCAA-3′; 5′-AGAATCGTCGGCTGCAGGTCGCTTCCTTATTCCT-3′; 5′-AGCGACCTGCAGCCGACGATTCTTCTACTCAAAACAAAAG-3′ and 5′-ATCAGTAGAGAGTGTCGGATCCCGTCATGCATCGCTTAC-3′) DNA fragments were prepared and integrated into the vector LV5-mCherry-GFP; then, they were transformed into competent cells, amplified, and screened for positive clones. The 293 T cells in the logarithmic growth phase were transfected with the above lentivirus vectors using Lipofectamine 2000 and cultured at 37 °C with 5% CO2 for 48 h in a humidified incubator. The supernatant was then collected, concentrated, and stored at −80 °C until use.
Immunofluorescence assay
Cell-seeded glass slides were washed three times with phosphate-buffered saline (PBS). Then, the cells were fixed with 4% paraformaldehyde for 15 min and permeabilized with 0.1% Triton X-100 at room temperature for 20 min. Next, the slides were incubated with a blocking solution (2% goat serum) at room temperature for 30 min. After the blocking solution was decanted, a sufficient amount of rabbit anti-human p27 monoclonal antibody (ab32034, Abcam, Cambridge, MA, USA) at a 1:250 dilution was added to each slide. The slides were placed in a wet box and incubated at 4 °C overnight. After adding a fluorescein isothiocyanate-conjugated secondary antibody at a 1:100 dilution, the slides were incubated in a wet box at 37 °C for 1 h. Cell nuclei were stained with 4′,6-diamidino-2-phenylindole in the dark for 5 min and then washed four times with PBS. The slides were mounted with antifade mounting medium and observed under an Olympus fluorescence microscope.
Cell proliferation assay
Cell proliferation rates were determined using a Cell Counting Kit-8 (CCK-8) (OBIO Technology, Shanghai, China). Cells (2 × 103) were seeded into a 96-well plate, and 10 μl of CCK-8 solution was added to each well at different time points (24, 48, and 72 h) after infection. After incubating at 37 °C for 3 h, the absorbance at 450 nm was measured on a microplate reader.
Cell cycle assay
At 48 h following infection, cells were fixed with 70% precooled ethanol and incubated at 4 °C overnight. Then, the cells were treated with RNase A (KeyGEN) at 47 °C for 30 min. After the cells were stained with PerCP-CY5.5 (BD Biosciences, Franklin Lakes, NJ, USA) in the dark at 4 °C for 30 min, they were detected by flow cytometry (BD Biosciences) for cell-cycle distribution.
Cell scratch-wound assay
Cells (5 × 105) were seeded into a six-well plate. After adherence, the cells were infected with lentivirus for 48 h and a sterile pipette tip was used to make a straight scratch-wound in the middle of the culture dish. The cells were cultured at 37 °C with 5% CO2 and photographed under a microscope at 0, 16, and 24 h, and changes in the width of the scratch-wound were measured.
Cell invasion assay
Diluted BD Matrigel was used to precoat 24-well plates in a transwell chamber (Corning Inc., New York, NY, USA). At 48 h after lentivirus infection, 300 μl of cell suspension (at a cell density of 10 × 105/ml) was added to the 24-well plate in the upper compartment of the chamber, and 500 μl of DMEM containing 10% FBS was added to the 24-well plate in the lower compartment of the chamber. After culturing for 16 and 24 h, the unpenetrated cells in the upper chamber were gently wiped away with a cotton swab. The insert membrane was fixed in 95% ethanol for 15 min and then stained with a 0.1% crystal violet solution for 10 min. The number of stained cells was calculated by selecting five fields randomly under a microscope.
Western blot
At 72 h after lentivirus infection, cells were lysed using a radioimmunoprecipitation assay buffer solution (Sigma-Aldrich, St. Louis, MO, USA), and the proteins were extracted. Equal amounts of supernatants from the lysed cells were analyzed by sodium dodecyl sulphate polyacrylamide gel electrophoresis. The separated proteins were then transferred to a polyvinylidene difluoride membrane. The primary antibodies Bim-1 (monoclonal rabbit IgG, ab126783, Abcam), Twist1 (monoclonal rabbit IgG, AF4009, Affinity, Cincinnati, OH, USA), RhoA (monoclonal rabbit IgG, ab187027, Abcam), RhoA-GTP (ab211168, Abcam), and GAPDH (polyclonal rabbit IgG, ab9485, Abcam) and ECL substrate (Thermo, Rockford, IL, USA) were used for protein detection.
Statistical analysis
Statistical analyses were performed using SPSS software (version 19.0; SPSS, Inc., Chicago, IL, USA). Data were expressed as the mean ± standard error of the mean (SEM). A χ2 test was conducted to determine the difference in cytoplasmic p27 expression between groups and its relationship with clinicopathological features. The Kaplan–Meier method was employed to plot survival curves. A log-rank test was performed to compare the differences in survival between the groups. The Cox proportional hazard model was adopted for multivariate analysis. Differences between multiple groups were analyzed by one-way analysis of variance (ANOVA), followed by post-hoc Bonferroni tests. A p < .05 indicated that the difference was statistically significant.
Results
General clinical information for patients with NPC
Among the 113 patients with NPC who were included in the study, there were 87 males and 26 females. The male to female ratio was 3.34:1, the age range was 10–94 years, the mean age was 50.6 ± 14.3 years, and 59 patients were 50 years or older. The study group contained the following classifications: 70 T1–T2 cases, 43 T3–T4 cases, 41 cases without lymph node metastases, 72 cases with lymph node metastases, and 7 cases with distant metastases. There were 27 cases of stage I, 19 cases of stage II, 39 cases of stage III, and 28 cases of stage IV. There were 2 cases of keratinizing carcinomas and 111 cases of nonkeratinizing carcinomas, including 14 cases of nonkeratinizing differentiated carcinomas and 97 cases of nonkeratinizing undifferentiated carcinomas.
Cytoplasmic p27 expression in NPC tissues
To evaluate the role of cytoplasmic p27 in NPC, we first determined the cytoplasmic p27 expression level in NPC and nasopharyngeal mucosal tissues using immunohistochemical assays. The positive expression rate of cytoplasmic p27 (57.5%, 65/113) was significantly higher in NPC tissues than in nasopharyngeal mucosal tissues (28.6%, 8/28) (p = .006, and ), indicating that cytoplasmic p27 may play an important role in the development of NPC.
Figure 1. Immunohistochemical staining for p27. (A) p27 showing strong cytoplasmic and nuclear immunopositivity in NPC tissues (original magnification: ×400). (B) p27 showing cytoplasmic negativity in nasopharyngeal mucosal tissues (original magnification: ×400).
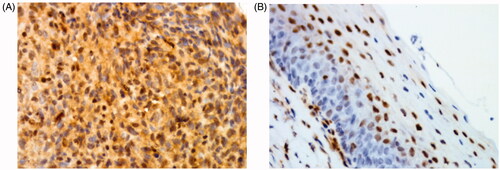
Table 1. The expression level of cytoplasmic p27 in nasopharyngeal carcinoma tissues.
Relationship between cytoplasmic p27 expression and clinicopathological features in patients with NPC
details the relationship between the cytoplasmic p27 expression level in NPC tissues and the patient clinicopathological features. Cytoplasmic p27 expression in NPC tissues was not significantly correlated with sex, age, N classification, M classification, or pathological type but was significantly related to the T classification (T1–T2 vs. T3–T4; p = .034) and TNM clinical stage (I–II vs. III–IV; p = .034). These results suggest that the cytoplasmic p27 protein may be involved in the progression of NPC as a tumour-associated molecule.
Table 2. The relationships between the expression level of cytoplasmic p27 and clinicopathological features in patients with nasopharyngeal carcinoma.
Survival analysis
The 113 patients with NPC in this study had an average follow-up time of 41.3 months (2–108 months), and 9 cases were lost to follow-up; the rate of loss to follow-up was 7.9%. There were 28 cases of death (24.7%, 28/113). Among them, 27 patients died of local recurrence or distant metastasis of NPC, and 1 patient died of other causes. The median survival time was 76.8 months, and the 5-year overall survival rate was 75%.
To investigate the effect of cytoplasmic p27 expression on the prognosis of patients with NPC, we adopted the Kaplan–Meier method and the log-rank test to analyze the relationship between the cytoplasmic p27 expression level in NPC tissues and patient survival. The results demonstrated that the expression level of cytoplasmic p27 had a significant impact on the survival rate of patients with NPC, and the survival rate was significantly lower for patients with positive cytoplasmic p27 expression than for those with negative expression (p = .006, ). For patients aged 50 years or older, the survival rate was significantly lower for patients with positive cytoplasmic p27 expression than for those with negative expression (p = .009, ). In addition, patients with positive cytoplasmic p27 expression had a worse prognosis than those with negative expression in T3–T4 classification (p = .004, ), N0 classification (p = .009, ), M0 classification (p = .026, ) and M1 classification (p = .017, ). Patient clinicopathological features and cytoplasmic p27 expression level were introduced into the Cox proportional hazard model for multivariate regression analysis. These data indicate that age, M classification, TNM clinical stage, and cytoplasmic p27 expression were independent risk factors for the prognosis of patients with NPC ().
Figure 2. The effect of cytoplasmic p27 expression on the survival time of patients with NPC. (A) The expression level of cytoplasmic p27 predicts the overall survival time of patients with NPC; (B–F) The correlation of cytoplasmic p27 expression with survival time in strata analysis in age (≥50 years old) of patients with NPC (B), T3–T4 classification (C), N0 classification (D), M0 classification (E), and M1 classification (F).
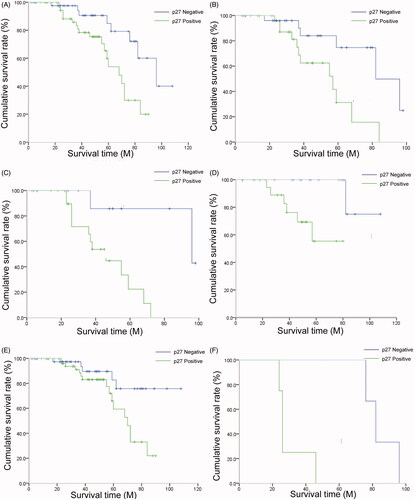
Table 3. Multivariate analysis of overall survival rate of 113 patients with nasopharyngeal carcinoma.
p27 expression level and subcellular localization in NPC cells infected with recombinant lentivirus
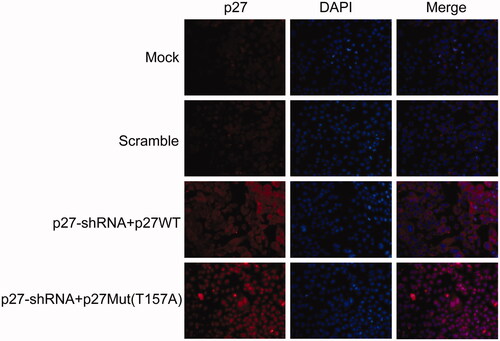
NPC cells (CNE2) were infected with lentiviruses. After 72 h, the fluorescence intensities were significantly higher for cells in the p27-shRNA + p27WT and p27-shRNA + p27Mut (T157A) groups than for those in the scramble group. In addition, the p27 protein in the p27-shRNA + p27WT group was located primarily in the cytoplasm and nucleus, whereas the p27 protein in the p27-shRNA + p27Mut (T157A) group was distributed mainly in the nucleus (). These results demonstrate that the p27-shRNA + p27Mut (T157A) lentivirus can affect the subcellular p27 protein localization in NPC cells, that is by translocating p27 from the cytoplasm to the nucleus.
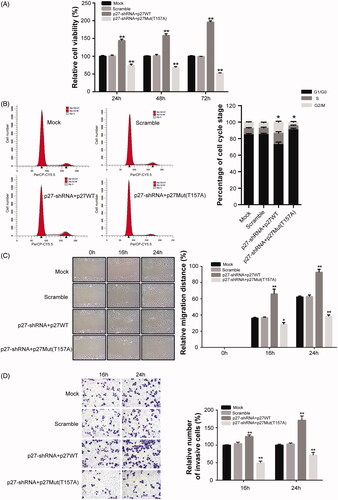
Effect of p27 translocation on the proliferation, cell cycle, migration, and invasion of NPC cells
NPC cells (CNE2) were infected with lentiviruses for 24, 48, and 72 h. At each time point, the cell viability of the p27-shRNA + p27WT group was significantly increased, and the cell viability of the p27-shRNA + p27Mut (T157A) group was significantly reduced compared with that of the scramble group (p < .01, ). Furthermore, at 48 h after lentivirus infection, the percentages of cells in the S and G2/M phases were notably higher in the p27-shRNA + p27WT group than in the scramble group (p < .05, ). As shown in , at 16 and 24 h after infection, compared with those of the scramble group, the migration and invasion abilities of the cells in the p27-shRNA + p27WT group were significantly increased, while those of the p27-shRNA + p27Mut (T157A) group were significantly decreased (p < .05). Our results indicate that the upregulation of cytoplasmic p27 expression can promote the proliferation, cell cycle progression, migration, and invasion of NPC cells, while the nuclear p27 protein can act as a tumour suppressor to inhibit cell growth.
Figure 4. The effects of mislocalized p27 on the proliferation, migration, and invasion of NPC cells. (A) Cell viability of NPC cells was determined by CCK-8 assay. (B) The cell-cycle distribution was examined using flow cytometry. (C) Cell scratch-wound assay was conducted to analyze the migration ability of NPC cells. (D) Invasion ability of NPC cells was detected by Transwell assay. *p < .05, **p < .01 versus scramble group. All data are presented as mean ± SEM from three independent experiments.
Effect of p27 translocation on the protein levels of bim-1, Twist1, RhoA, and RhoA-GTP in NPC cells
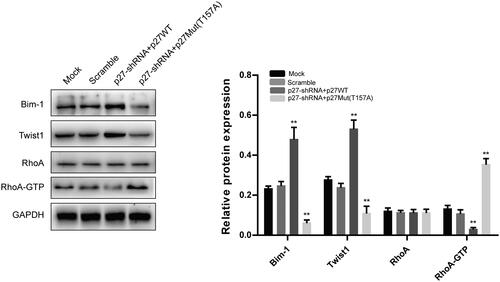
NPC cells (CNE2) were infected with lentiviruses for 72 h. Compared with the scramble group, the p27-shRNA + p27WT group had significantly increased Bim-1 and Twist1 protein levels and significantly decreased RhoA-GTP protein levels, while the p27-shRNA + p27Mut (T157A) group had significantly decreased Bim-1 and Twist1 protein levels and significantly increased RhoA-GTP protein levels. There were no significant differences in RhoA protein levels among the groups. These results suggest that p27 translocation can regulate Bim-1, Twist1 and RhoA-GTP protein levels in NPC cells ().
Discussion
Abnormal NPC cell proliferation promotes the progression of NPC. The cell cycle is regulated and controlled by the orderly polymerization and activation of a series of regulatory factors. The main molecules that are involved in cell cycle regulation include cyclin, cyclin-dependent kinases (CDKs), and cyclin-dependent kinase inhibitors (CDKIs) [Citation17]. CDKIs inhibit CDK activity by binding to cyclin, CDK, or a cyclin-CDK complex, resulting in cell cycle arrest and inhibition of cell proliferation. Based on their structure and function, CDKIs are divided into two broad classes of inhibitors of CDK4 (Ink4) and CDK-interacting protein/kinase inhibition protein (Cip/Kip). Ink4, including p16, p15, p18, and p19, specifically inhibit the activity of cyclin D-dependent kinases CDK4/CDK6. Cip/Kips, including p21, p27, and p57, exert inhibitory activities by acting on a wide variety of cyclins. As a CDKI, p27 expression is significantly decreased in the tissues of many head and neck tumours, such as oral cancer, laryngeal cancer, and NPC [Citation6,Citation18–22]. In-depth studies have demonstrated that many tumour cells exhibit significantly increased cytoplasmic p27 protein levels due to p27 translocation from the nucleus to the cytoplasm [Citation23,Citation24]. The immunohistochemical analyses we conducted confirmed that the cytoplasmic p27 expression levels were significantly higher in NPC tissues than in nasopharyngeal mucosal tissues.
The p27 gene is involved in regulating the cell cycle. Abnormal p27 expression in the cytoplasm or nucleus is closely related to the clinicopathological features and prognosis of cancer patients. In the study on the relationships between p27 expression and the clinicopathological features and prognosis of patients with head and neck squamous cell carcinoma, Shimada et al. [Citation25] found that the p27 expression level was negatively correlated with tumour size, pathologic stage, and degree of differentiation; moreover, the cumulative survival rate was significantly higher for patients with a high p27 expression level than for patients with a low p27 expression level, probably due to p27protein degradation by the ubiquitin ligase Pirh2. Vallonthaiel et al. [Citation9] found that positive cytoplasmic p27expression in tumour cells was associated with a low TNM clinical stage (stages I and II) in patients with oral squamous cell carcinoma. In addition, among oral squamous cell carcinoma patients with associated lymph node metastases, the disease-free survival was significantly lower for patients with positive cytoplasmic p27 expression than for those with negative expression. Our study also reached a similar conclusion: the expression level of cytoplasmic p27 in NPC tissues was significantly correlated with the T classification and TNM clinical stage of patients with NPC. The survival rate was significantly lower for patients with positive cytoplasmic p27 expression than for patients with negative expression, and the cytoplasmic p27 level was an independent risk factor affecting the prognosis of patients with NPC. Therefore, cytoplasmic p27 can be used as an important biomarker for predicting the prognosis of many cancer patients.
In contrast to the nuclear p27 protein, the cytoplasmic p27 protein can exert tumour-promoting effects in human tumour cells through a non-CDK-dependent mechanism [Citation5,Citation7]. It has also been shown that in chronic myeloid leukaemia progenitor cells and breast cancer cells, the Akt protein can mediate p27 protein phosphorylation at T157, block p27 protein entry into the nucleus, and restrict the p27 protein to the cytoplasm so that in can exert its function in regulating the cell cycle of the progenitor cell and promoting cell proliferation [Citation24,Citation26]. Roy et al. [Citation23] further discovered that the cytoplasmic p27 protein level was significantly increased in CD34+ stem/progenitor cells during the progression of chronic myeloid leukaemia, which could decrease the RhoA-GTP level and inhibit cell death by increasing SAPK/JNK phosphorylation. In vitro and in vivo animal experiments of breast cancer also found that cytoplasmic p27 could exert oncogenic functions by regulating Akt stability, cell survival, and tumorigenicity [Citation27]. FOXO3a phosphorylation in the anaplastic large-cell lymphoma cell line (Ba/F3 cells) resulted in nuclear exclusion of this transcriptional regulator, and downregulation of p27 and Bim-1 expression, which could regulate cell survival and proliferation [Citation28]. Moreover, cytoplasmic p27 knockdown in metastatic PI3K-activated cancer cells can reduce STAT3 binding to the Twist1 promoter, Twist1 promoter activity and Twist1 expression, revert epithelial-mesenchymal transition (EMT) and impair metastasis, whereas activated STAT3 can rescue p27 knockdown [Citation29]. Therefore, the oncogenic effect of cytoplasmic p27 can be mediated at least in part by regulating the expression levels of RhoA-GTP, Bim-1 and/or Twist1, which may depend on SAPK/JNK phosphorylation, FOXO3a phosphorylation, and/or STAT3 activation. Our study has also demonstrated that elevated cytoplasmic p27 level can promote the proliferation, cell cycle progression, migration, and invasion of NPC cells, upregulate the expression of Bim-1 and Twist1, and inhibit RhoA-GTP protein level. Conversely, a mutation in the p27 protein at T157 could increase the nuclear p27 protein level and exert the tumour-suppression effect of p27. It can be concluded that p27 in the cytoplasm and nucleus of NPC cells can display tumour-promoting and tumour-suppression effects, respectively, possibly through non-CDK-dependent and CDK-dependent mechanisms.
In conclusion, cytoplasmic p27, as an oncogenic protein, is highly expressed in NPC. It can promote the proliferation, migration, and invasion of NPC cells possibly through non-CDK-dependent mechanisms. Furthermore, the expression level of cytoplasmic p27 is closely related to the prognosis of patients with NPC. Therefore, cytoplasmic p27 might conduct as a biomarker for prognosis prediction of patients with NPC and a potential therapeutic target for NPC.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13(12):1501–1512.
- Currier AW, Kolb EA, Gorlick RG, et al. p27/Kip1 functions as a tumor suppressor and oncoprotein in osteosarcoma. Sci Rep. 2019;9(1):6161.
- Fiedler M, Renner P, Schubert J, et al. Predictive value of FHIT, p27, and pERK1/ERK2 in salivary gland carcinomas: a retrospective study. Clin Oral Invest. 2019;23(10):3801.
- Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994;78(1):67–74.
- Denicourt C, Saenz CC, Datnow B, et al. Relocalized p27Kip1 tumor suppressor functions as a cytoplasmic metastatic oncogene in melanoma. Cancer Res. 2007;67(19):9238–9243.
- Fillies T, Woltering M, Brandt B, et al. Cell cycle regulating proteins p21 and p27 in prognosis of oral squamous cell carcinomas. Oncol Rep. 2007; 17:355.
- Ahn J, Hong SA, Lee SE, et al. Cytoplasmic localization of Jab1 and p27 Kip1 might be associated with invasiveness of papillary thyroid carcinoma. Endocr J. 2009;56(5):707–713.
- Wander SA, Zhao D, Slingerland JM. p27: a barometer of signaling deregulation and potential predictor of response to targeted therapies. Clin Cancer Res. 2011;17(1):12–18.
- Vallonthaiel AG, Singh MK, Dinda AK, et al. Prognostic significance of cytoplasmic p27 in oral squamous cell carcinoma. J Oral Pathol Med. 2016;45(7):475–480.
- Shin I, Yakes FM, Rojo F, et al. PKB/Akt mediates cell-cycle progression by phosphorylation of p27(Kip1) at threonine 157 and modulation of its cellular localization. Nat Med. 2002;8(10):1145–1152.
- Besson A, Gurian-West M, Schmidt A, et al. p27Kip1 modulates cell migration through the regulation of RhoA activation. Genes Dev. 2004;18(8):862–876.
- Song LB, Zeng MS, Liao WT, et al. Bmi-1 is a novel molecular marker of nasopharyngeal carcinoma progression and immortalizes primary human nasopharyngeal epithelial cells. Cancer Res. 2006;66(12):6225–6232.
- Song LB, Liao WT, Mai HQ, et al. The clinical significance of twist expression in nasopharyngeal carcinoma. Cancer Lett. 2006;242(2):258–265.
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474.
- Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe. 1987;8(3):138–140.
- Ihnen M, Kress K, Kersten JF, et al. Relevance of activated leukocyte cell adhesion molecule (ALCAM) in tumor tissue and sera of cervical cancer patients. BMC Cancer. 2012;12(1):140.
- Lim S, Kaldis P. Cdks, cyclins and CKIs: roles beyond cell cycle regulation. Development. 2013;140(15):3079–3093.
- Baba Y, Tsukuda M, Mochimatsu I, et al. Reduced expression of p16 and p27 proteins in nasopharyngeal carcinoma. Cancer Detect Prev. 2001;25(5):414–419.
- Hwang CF, Su CY, Huang SC, et al. Low expression levels of p27 correlate with loco-regional recurrence in nasopharyngeal carcinoma. Cancer Lett. 2003;189(2):231–236.
- Salerno G, Di Vizio D, Staibano S, et al. Prognostic value of p27Kip1 expression in basaloid squamous cell carcinoma of the larynx. BMC Cancer. 2006;6(1):146.
- Moreno-Galindo C, Hermsen M, García-Pedrero JM, et al. p27 and BCL2 expression predicts response to chemotherapy in head and neck squamous cell carcinomas. Oral Oncology. 2014;50(2):128–134.
- Gao L, Gu W, Zheng J, et al. Clinicopathological and prognostic significance of p27 expression in oral squamous cell carcinoma: a meta-analysis. Int J Biol Markers. 2013;28(4):329–335.
- Roy A, Lahiry L, Banerjee D, et al. Increased cytoplasmic localization of p27kip1 and its modulation of RhoA activity during progression of chronic myeloid leukemia. PLoS One. 2013;8(10):e76527.
- Viglietto G, Motti ML, Bruni P, et al. Cytoplasmic relocalization and inhibition of the cyclin-dependent kinase inhibitor p27(Kip1) by PKB/Akt-mediated phosphorylation in breast cancer. Nat Med. 2002;8(10):1136–1144.
- Shimada M, Kitagawa K, Dobashi Y, et al. High expression of Pirh2, an E3 ligase for p27, is associated with low expression of p27 and poor prognosis in head and neck cancers. Cancer Sci. 2009;100(5):866–872.
- Chu S, McDonald T, Bhatia R. Role of BCR-ABL-Y177-mediated p27kip1 phosphorylation and cytoplasmic localization in enhanced proliferation of chronic myeloid leukemia progenitors. Leukemia. 2010;24(4):779–787.
- Wu FY, Wang SE, Sanders ME, et al. Reduction of cytosolic p27Kip1 inhibits cancer cell motility, survival, and tumorigenicity. Cancer Res. 2006;66(4):2162–2172.
- Gu TL, Tothova Z, Scheijen B, et al. NPM-ALK fusion kinase of anaplastic large-cell lymphoma regulates survival and proliferative signaling through modulation of FOXO3a. Blood. 2004;103(12):4622–4629.
- Zhao D, Besser AH, Wander SA, et al. Cytoplasmic p27 promotes epithelial-mesenchymal transition and tumor metastasis via STAT3-mediated Twist1 upregulation. Oncogene. 2015;34(43):5447–5459.