Abstract
Decellularized extracellular matrix (dECM) has been considered as a promising scaffold in xenotransplantation, yet natural tissue dECM is often mechanically weak and rapidly degraded, compromising the outcomes. How to restore the mechanical strength and optimise the in vivo degradation, but maintain the microstructure and maximumly suppress the immune rejection, remains challenging. For this aim, we prepared and characterised various crosslinked decellularized rabbit uterus matrix (dUECM) and evaluated in vivo performance after uterus xenotransplantation from rabbit to rat. Naturally derived genipin (GP) and procyanidins (PC) were chosen to crosslink the dUECM, producing significant mechanical enhanced crosslinked-dUECM along with prolonged enzymatic degradation rate. Xenogeneic subcutaneous graft studies revealed that PC- and GP-crosslinked dUECM experienced significant cell infiltration and caused low immune reactions, indicating the desired biocompatibility. In vivo transplantation of GP- and PC-crosslinked dUECM to a uterus circular excised rat yielded excellent recellularization ability and promoted uterus regeneration after 90 days. While the reconstruction efficacy of crosslinked dUECM is highly depended on the crosslinking degree, crosslinking condition must be carefully evaluated to balance the role of crosslinked dECM in mechanical and biological support for tissue regeneration promotion.
Introduction
Though many medical advances have been achieved in women infertility treatment, there are still some women suffering from absolute uterine factor infertility which so far is untreatable. Also, endometrial cancer, endometriosis and severe intrauterine adhesion might often lead to uterus removal or dysfunctions and female infertility [Citation1]. Uterus transplantation has been studied in different animal models for decades and showed great potential in infertility treatment, becoming a practical option for women with these uteri caused infertility to get pregnant and have babies [Citation2,Citation3]. Recently, a case study in Brazil firstly reported that a baby was born using transplanted uterus from a deceased donor [Citation3], which demonstrated the uterus transplantation is a potential avenue. However, the clinical donor is severely deficient, limiting the application of homotransplantation. Tissue xenotransplantation that transplant cells, tissues, and organs from one species to another provides an alternative option for women who urgently need uterus transplantation treatment [Citation4]. However, host immune rejections are still common after transplantation, and the recipients are subjected to intake immunosuppressors for life-long time [Citation5].
Decellularized extracellular matrix (dECM) derived from xenogeneic tissues has great potential for use as a mechanical and biological support in tissue regeneration [Citation6,Citation7]. Decellularization can remove nuclei, non-structural protein, and other cellular antigens to reduce the immune response after implantation, even in a xenogeneic model. These decellularized tissues are composed of extracellular matrix proteins that are conserved among different species, and that can serve as scaffolds for cell attachment, migration, and proliferation to promote host tissue regeneration. In addition to inherent cell compatibility, dECM possess the desired shape and the strength of the scaffold where the materials derived. However, the decellularized tissue matrix is mechanically weak and rapidly degraded in vivo, leading to a compromised transplant efficiency. The mechanical support role of dECM is essential for tissue regeneration and construction; thus, further pre-treatment strategies were applied to optimise the mechanical properties and structural stability [Citation8–10].
Crosslinking methods aim to stabilise collagen-based biomaterials to prevent tissue degradation in vivo, to minimise the immune response, and to stabilise the tissue before implantation [Citation7,Citation11]. Although the crosslinking of xenograft matrix successfully reduces immunogenicity, many challenges remain to be overcome before large-scale use in tissue engineering and clinical treatments. The poor biocompatibility, decreased degradation, and the bioactivities of crosslinkers are considered as the current primary challenges for the crosslinked dECM in xenotransplantation [Citation12–14]. Thus, how to find the optimal matrix crosslinking method has been studied to endorse the decellularized matrix with superior mechanical strength and biocompatibility. Glutaraldehyde (GA) is the most commonly used crosslinker in natural tissue treatment [Citation15]. It is essential to find the suitable GA crosslinking concentration without toxicity. Meanwhile, some natural derived crosslinkers also showed improved characteristics. For example, procranidins (PC) and genipin (GP) crosslinking improved anti-calcification and biocompability while reduced immunogenicity and cytotoxicity [Citation16,Citation17]. Crosslinked decellularized tissue derived scaffold might suitably improve the mechanical properties and biocompatibility, and reduce immune reaction to promote organ regeneration. However, currently, there are only very few studies evaluated the degreadation and biocompability of crosslinked dECM in vivo [Citation18].
The objectives of our present study include (i) to prepare and characterise the rabbit decellularized uterine matrix (dUECM), (ii) to determinate and compare the crosslinking condition on the mechanical properties, degradation, and biocompatibility of crosslinked-dUECM in vitro and in vivo, and (iii) to evaluate the in vivo uterine regeneration and construction after xenotransplantation of crosslinked dUECM. Thus, in this study, we prepared rabbit dUECM via the chemical/physical method and crosslinked with PC and GP at low, middle and high concentration. We then analysed the three-dimensional (3D) microstructure, remaining ECM components, mechanical strength, enzymatic degradation, compatibility, and investigated the potential of the crosslinked rabbit dUECM for xenogeneic uterus transplantation use.
Results
Characterisation of decellularized rabbit uterus matrix
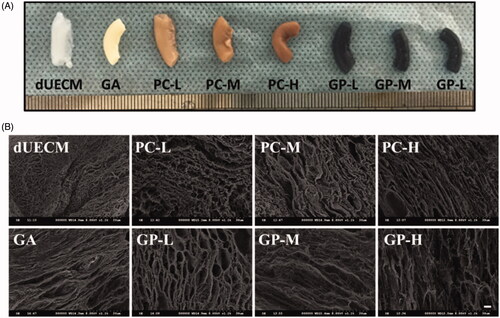
reports the characteristics of decellularized rabbit uterus. After a combined physical and chemical decellularization, an apparent colour change from reddish brown to white can be observed in . The size and shape of the acquired dUECM was similar to the native rabbit uterus, but the texture was less tight. During the decellularization process, nucleus was fully removed to minimise the host immune rejection. It was shown that DAPI staining in fluorescence () and brown signal indicating nucleus in H&E () were almost invisible and over 95% of DNA was removed (), demonstrating the success of decellularization.
Figure 1. Characterisation of decellularized rabbit uterus matrix (dUECM). (A) Macroscopic inspection of the uterus before (native) and after (dUECM) decellularization. DAPI (B) and haematoxylin and eosin (H&E) staining (C) show that most of the nuclei were removed. (D) Masson staining and immunofluorescent micrographs of rabbit uterus thin sections before and after decellularization (native and dUECM, respectively) using antibodies against extracellular matrix components. Quantification of residual DNA (E), total protein (F) and soluble collagen (G). MT, Masson staining (fibrin), Col IV, collagen IV; LN, laminin. The scale bar represents 100 μm. ***, p < .001.
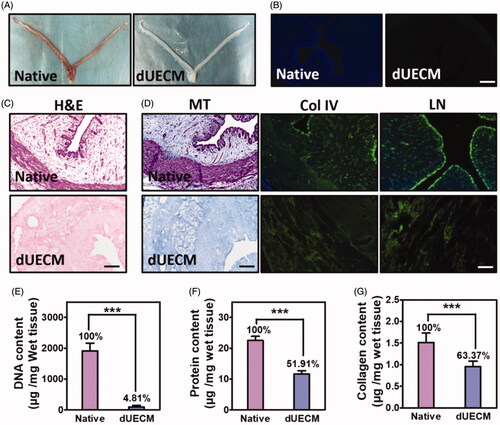
The decellularized tissues have preserved uterus vascular conduits and endometrial glands in the basement membrane, indicating the maximum preservation of extracellular structure (). Also, 51.91% of the total proteins in the dUECM was preserved. Immunofluorescent micrographs () confirm the consideration reservation of major extracellular matrix composition, including collagen fibres, collagen IV and laminin, and they displayed in a physiological normal distribution. The immunostaining revealed that both the structural and basement membrane components of the uterus matrix were retained. Quantitative results showed that 0.9544 μg collagens were remained in per mg wet tissues after decellularization, also confirming that the majority collagens (∼63.37%) was retained in the dUECM ().
Characterisation of crosslinked dUECM
We selected two naturally derived crosslinkers at various concentrations to study the effect of the crosslinking process on the properties of dUECM. We referred to the previously published papers [Citation7–9] and selected low, middle, and high concentration of PC (0.05%, 0.1%, and 0.2%) and GP (0.25%, 0.625%, and 1%) to prepare PC-L, PC-M, PC-H, GP-L, GP-M, GP-H crosslinked dUECM respectively. The common chemical crosslinker, GA (0.625%), was used as a reference. After crosslinking, the colour of dUECM changed from white to brown (PC-crosslinking) or dark blue (GP-crosslinking). As the crosslinker concentration increased, this trend became more obvious (). Compared to the uncrosslinked dUECM, crosslinked dUECM had a stiffer texture and the size got slightly smaller. However, the uterine conduit remained intact. Microstructures were observed by SEM revealed that 3 D network microstructure was well preserved in dUECM after crosslinking (). A more obvious and regular porous structure was observed in PC and GP crosslinked dUECM compared to that of non-crosslinked counterparts. As the crosslinker concentration increased, more dense structure and gradually decreased fibre gap were found in PC- and GP-crosslinking groups.
Mechanical properties of crosslinked dUECM
All decelluarized methods will affect the mechanical properties of the samples. Here, uniaxial tensile tests were used to evaluate the mechanical properties of fresh native rabbit uteri, dUECM and crosslinked dUECM (Supplementary Figure S1). The size of tested samples kept similar to study the effect of the proposed crosslinking condition on the mechanical response of dUECM samples [Citation19]. The tensile strength results (Supplementary Figure S1(A,B)) showed that dUECM samples required significant lower forces (110.1 KPa) to break compared to the fresh native uterus (225 KPa), or compared to GA crosslinked dUECM (385 Kpa). In general, the breaking tensile strength of the crosslinked dUECM was greater than those of the uncrosslinked group (Supplementary Figure S1(A,B)); however, the PC-crosslinking and GP-crosslinking showed opposite trends as the crosslinking increased. The breaking tensile strength of PC-crosslinking increased as tested crosslinker concentration increased, while that of GP-crosslinking decreased. Similar trends were found in the elongation at break (Supplementary Figure S1(C,D)) and elastic modulus (Supplementary Figure S1(E,F)). In elongation study, the extensibility exhibited no obvious change after decellularization and GA-crosslinking and PC-crosslinking (Supplementary Figure S1E), and only a slight increase in GP-crosslinked samples (Supplementary Figure S1F). Modulus strength was used to describe the force required to deform the tested samples, and the results were similar to the tensile strength. Decellularization treatment lowers the strength to deform the uterus (189 KPa vs. 383 KPa for dUECM and native uterus), and the crosslinking strategy could offset this and restore the mechanical properties. The GA-crosslinking dUECM had the highest modulus strength result (607 KPa). It is interesting that PC-M crosslinked samples showed no significant difference in breaking tensile strength, elongation at break and elastic modulus compared to those of the fresh native uterus.
In vitro degradation
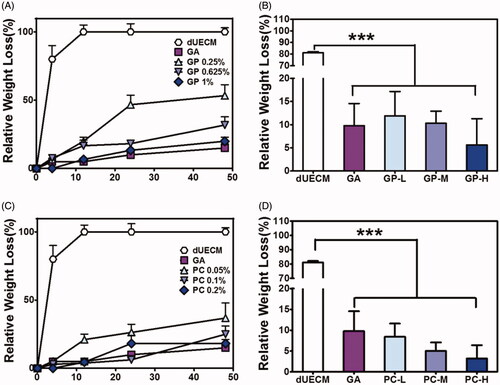
Collagen is one of the major components in ECM, thus here collagenase is used to mimic the degradation profile of PC- and GP-crosslinked dUECM (). Uncrosslinked dUECM was fully digested by collagenase within 12 h, while most crosslinked dUECM had over 80% mass reserved at the same time point, indicated the improved enzymatic resistance (). In short time collagenase incubation (4 h), the relative weight loss of crosslinked dUECM was much less than that of uncrosslinked dUECM (). Generally, PC- and GP-crosslinked dUECM were more durable to the collagenase at a higher crosslinking degree. GA-crosslinked dUECM induced minimum weight loss (∼15%) after 48 digestion among all the crosslinked groups.
In vitro biocompatibility
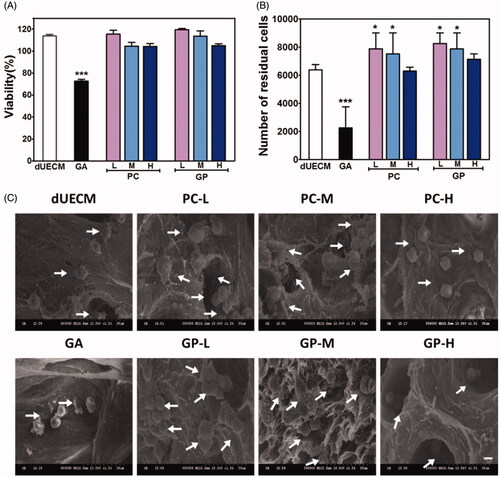
Cytotoxicity and cell adhesion of crosslinked dUECM and their lixivium were studied to evaluate the biosafety of crosslinking strategy. As shown in , the lixivium of uncrosslinked dUECM promoted cell proliferation compared to the control, and similar results were also found in PC-L, PC-M, and GP-L crosslinked dUECM. The lixivium of high PC- and GP-crosslinked dUECM did not affect the cell proliferation. Conversely, lixivium of GA-crosslinked dUECM exhibited an obvious cytotoxic effect on the proliferation of HUVECs. We also conducted the direct co-incubation of healthy cells and the crosslinked dUECM (). After incubation, the number of residual cells slightly increased in low PC- and GP-crosslinked groups, just similar to the lixivium viability results. GA-crosslinked groups experienced dramatic cell decrease. The microscope images also confirmed the residual cell counting results (Supplementary Figure S2). The cell density and morphology of HUVEC cells incubated with various dUECM for 48 h did not exhibit significant change except GA-crosslinking groups. Cell seeding results in showed that cell could successfully adhere to co-incubated decellularized matrix among all groups, but the cell morphology and numbers differed. The cells seeded on uncrosslinked and GA-crosslinking dUECM seems to shrink and deformed with limited cellular score. On the other hand, those on PC- and GP- crosslinked scaffolds showed the regular shape and had a greater cell number in the visual area compared to the former mentioned groups.
Figure 4. In vitro biocompatibility of crosslinked dUECM. (A) The viability of HUVECs in the lixivia of different scaffolds based on the MTT assay. (B) The number of residual cells on non-, GA- PC- and GP-crosslinked dUECM. (C) SEM micrographs of HUVECs cells incubated with different concentrations of non-, GA-, PC- and GP-crosslinked dUECM for 48 h. The arrows point to the cells that adhere to the immersed dUECM. The scale bar represents 30 μm. *, p < .05; ***, p < .001.
In vivo biocompatibility and host immunogenicity of xenogeneic implants
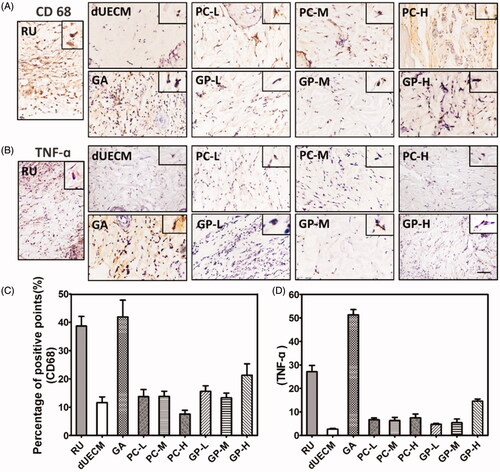
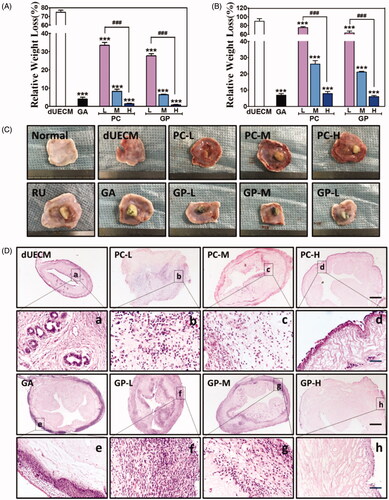
Here, the prepared dUECM samples derived from rabbit subcutaneously implanted into SD rat to evaluate the biocompatibility of dUECM in the xenogeneic model and the host immune reactions. As shown in , embedded dUECM showed significant degradation during xenogeneic implantation for 7 days and 14 days (). Noticeably, dUECM exhibited significant weight loss, with 74.80% for 7 days and 85% for 14 days, indicating its in vivo instability. In general, the crosslinking strategy could enhance the degradation resistance of xenogeneic implants (). The degradation behaviours of crosslinked dUECM was negatively correlated to the crosslinking degree. For PC-crosslinking groups, weight loss of implanted samples went from 75.68% to 1.40% when the PC concentration increased from 0.05%(PC-L) to 0.20%(PC-H) at 14 days, indicating an increasing resistance to xenogeneic enzymatic digestion. Similar trend was also found in GP-crosslinking groups. The H&E staining results (Supplementary Figure S3 and ) showed that the dUECM groups had the highest cell infiltration rate among all groups and the characteristic of multiple endometrial glands was also found over 14 days implantation. The cell infiltration was negatively correlated to the crosslinking degrees (). In PC-H and GP-H crosslinked groups, cells only infiltrated into the very peripheral area with limited numbers. It is also worth mentioning that in the GA-crosslinking groups, great numbers of neutrophil and lymphocyte infiltrated into the surface area. The macrophage marker CD68 and proinflammatory factor marker TNF-α were evaluated to study the host reactions to the xenogeneic implants after 7 days (Supplementary Figure S4) and 14 days (). The decellularized uterus decreased the expression of CD68 and TNF-α compared with the native rabbit uterus. Nearly all crosslinked groups showed the increased infiltration of neutrophils/macrophages and overexpressed TNF-α, while uncrosslinked matrix received the lowest immunogenicity results. Naturally derived crosslinker (PC & GP) groups presented a relatively mild degree of neutrophil and lymphocyte infiltration after 7 days and 14 days. Moreover, the inflammatory reactions of dUECM with PC- and GP-crosslinking at 14 days were significantly reduced compared to those at 7 days. However, GA-crosslinking groups exhibited the highest proinflammatory factor accumulation among all decellularized samples.
Figure 5. Characterisation of non- and crosslinked dUECM after subcutaneous xenogeneic implants experiment. The relative weight loss after subcutaneously embedding of dUECM samples after 7 days (A) and 14 days (B). Morphological results (C) and H&E staining after 14 days of subcutaneous embedding experiment (D). The black scale bars represent 100 μm and the blue scale bars represent 10 μm. ***, p < .001.
In vivo xenotransplantation for uterus regeneration
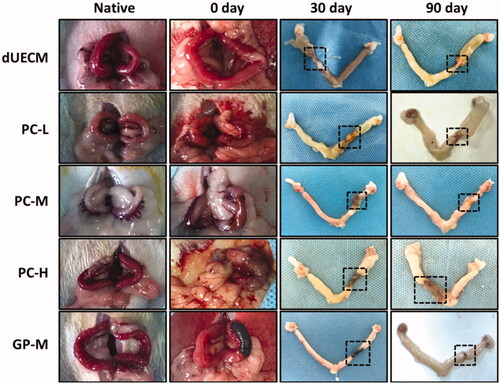
The in vivo effect of crosslinked rabbit dUECM xenotransplantation for rat uterus regeneration was evaluated in rat uterus excision/regeneration models (). Briefly, the rats underwent segmental circular uterine excision and following replaced by same length of dUECM, PC-L, PC-M, PC-H and GP-M crosslinked dUECM in the partially defective uterine. 30 and 90 days after xenotransplantation, those surgically treated rats were sacrificed for further analysis. 30 days after surgery, dUECM without crosslinking treatment were entirely degraded and the rat uterus only connected by the outer adhesive mucosa (). The crosslinked dUECM remained at the implanted site but significantly decreased in size as shown in images. It was surprising that the Y-shape uterus remained symmetrical, indicating the uterus regeneration at the junction between native uterus and transplanted dUECM. After 90 days, PC-M-crosslinking group exhibited best regeneration results with limited scaffold remained and apparent uterus regeneration as evidenced by newborn uterus tissues near the implanted area. While PC-L crosslinked dUECM at 90 days exhibited similar results as dUECM at 30 days, fully degraded before the uterus reconstruction and induced uterus broken and failed uterus repair. Besides that, high crosslinker concentration leaded to a slow degradation and the scaffold was remained in the excised site with no obvious uterus regeneration promotion even after 90 days.
Figure 7. In vivo evaluation of crosslinked dECM for uterus regeneration and construction in a xenogeneic rat model. Dotted lines indicate repair sites.
H&E staining and immunochemistry studies were performed to characterise the extent of uterus regeneration (). An obvious recellularization was observed in all groups after 30 days (), and the cell distribution in PC-L groups appeared to be even in the whole uterus matrix section. After 90 days, a resembled normal uterus endometrial structure and cell distribution was shown in the transplanted dUECM in all remaining groups (). Comparatively, PC-M- and GP-M-crosslinked dUECM induced a clear uterus regeneration with intact endometrial structure and numerous secretory glands, while PC-H group showed limited regeneration signs.
Figure 8. H&E and immunohistochemical staining of CK, Ki67, ɑ-SMA, TNF-ɑ, and CD68 after transplantation at 30 days (A) and 90 days (B). The scale bar represents 100 μm. ***, p < .001.
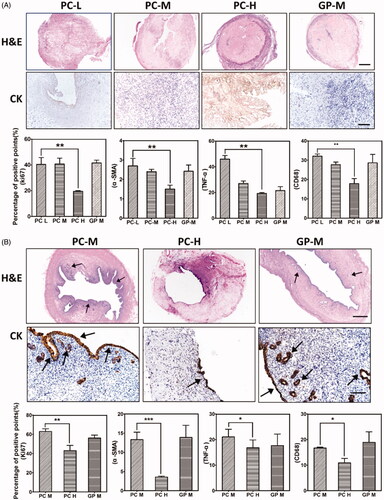
Immunohistochemistry studies were conducted to determine the cytokeratin (CK), Ki67, ɑ-SMA expressions for the evaluation of cell proliferation, endometrial regeneration and uterus construction ( and Supplementary Figure S5). As shown in , after 90 days, the uterus secretion glands and lumen were well delineated with CK-positive epithelium in PC-M and GP-M groups at the excision/replacement segment. Comparably, the PC-H group only showed partial epithelial regeneration at 90 days, and no secretory glands were observed. After xenotransplantation, Ki67-positive cells represented about 30%, 45%, 20%, 40% for PC-L, PC-M, PC-H, and GP-M groups, respectively () at 30 days, and about 65%, 40% and 60% for PC-M, PC-H, and GP-M groups, respectively () at 90 days. The cell proliferation pattern was in accordance with the visual figures of transplanted dUECM samples (). Apparently, as shown in Supplementary Figure S5, ɑ-SMA positive muscle bundles could be detected at 30 days after surgical for all tested groups, and after 90 days, more ɑ-SMA positive muscle bundles formed and a clear organised separate muscle layer was generated. Compared to the PC-H group, PC-M-crosslinked dUECMs had more ɑ-SMA positive muscle bundles and clear boundary underneath the endometrial layer. As shown in groups of PC-M and GP-M, the muscle layer was relatively thin at 30 days, but the degree of repair was evidenced by the significant overexpression of Ki67, CK and ɑ-SMA. After 90 days, PC-M- and GP-M-crosslinking groups had developed full endometrium structure and considerate secretory glands, while PC-H group had limited cell infiltration and cytokeratin proteins expression.
Host immunogenicity after xenotransplantation was evaluated by immunochemistry analysis (Supplementary Figure S5), and the semi-quantitative results were displayed in . Compared to that of 30 days, the CD68 and TNF-ɑ positive ratio were both decreased significantly at 90 days. And the CD68 and TNF-ɑ positive signal decreased as the crosslinking degree increased at 30 days, but these trends became inconspicuous after 90 days. Overall, the immune rejections were acceptable with few inflammation occurrences, low microphage infiltration, and negligible pre-inflammatory factor accumulation for xenotransplantation.
Discussions
To our best knowledge, the present study is first to describe and compare the characteristics of crosslinked decellularized rabbit uterus, which can become beneficial for transplanting the decellularized matrix from another species to promote host uterus regeneration. We compared different crosslinking conditions and provided a detailed qualitative and quantitative description of the mechanical properties, enzymatic degradation, biocompatibility, cell infiltration/recellularization, and host immunogenicity after the crosslinking treatment.
The decellularization process is aiming to remove almost all of the cellular components that might induce immunogenicity but preserving the structural and biochemical properties. Recently reported methods to make decellularize tissues are more likely to combine the physical, chemical or enzymatic strategies to achieve higher quality dECM scaffold/biomaterials [Citation11,Citation20–26]. We have previously prepared decellularized brain matrix by agitation in the following decellularization baths and completely removed the cellular components with entire brain structure [Citation27,Citation28]. Here, a similar method was adopted to decellularize the rabbit uterus. The obtained dUECM had minimum DNA content, but considerably maintain the bioactive compositions, including fibronectin, collagen IV and laminin (). However, after decellularization, obtained tissues were visually less tight and could not even maintain the tube-like structure of uterus. The tested tensile strength and elastic modulus dramatically decreased, as well as the enzymatic resistance to degradation both in vitro and in vivo. Similar results published in other papers have shown that the decellularized tissue preserves its microstructure but not the mechanical properties and stability [Citation29,Citation30]. The compromised mechanical properties and the rapid enzymatic degradation led to a failed mechanical support during tissue regeneration. After one month, the stitched dUECM has been already totally degraded while the uterus was visually disconnected at the surgical site (). Tissue xenotransplantation usually requires the scaffold to be durable to support the attachment, growth, proliferation and differentiate of the cells. If the transplanted tissues are mechanically weak, the tissue will be deformed and failed to maintain the macrostructure, especially for the elaborate tissue structure. The fast degradation rates of decellularized scaffold also hamper the tissue regeneration and reconstruction, leaving the endometrial cells no place to seed and expand. Thus, it will be extremely valuable to search for the solutions to optimise the mechanical properties and the biodegradable properties for the decellularized matrix application in tissue regeneration and reconstruction.
Extracellular matrix is an ideal natural scaffold in tissue regeneration due to their unique architecture, and major compositions are critical to direct the cell attachment and create a favourable bio-environment for cell proliferation, and differentiation. Many other scientists and we have reported that the mixture of decellularized matrix degradation/smash promotes cell proliferation, migration and tissue regeneration [Citation31–33]. In our study, the bioactivity of the decellularized matrix was confirmed in the biocompatibility study, and the lixivium of dUECM medium could promote the HUVEC cells proliferation compared to negative control (). Besides, dUECM showed the best cell filtration (), scaffold recellularization/differentiation, and the formation of characteristic endometrial secretory glands, indicating the tissue regeneration promotion effect of the decellularized matrix compositions. It is pointed out that ECM preservation is critical for the function of acellular matrix to promote cell proliferation [Citation34]. In our study, the major ECM compositions, including collagen IV, laminin, and fibrin, were well retained, which was also beneficial for cell seeding and expanding after xenotransplantation. Also, to fully explore the regeneration power of the decellularized matrix, proper degradation that leads to the sustained release of collagens, fibrins and other components is required.
The crosslinking strategy has emerged as a potential treatment to improve the mechanical weakness and optimise the in vivo stability of decellularized matrix. The crosslinking between reactive sites among collagens is one popular method to produce mechanically reinforced extracellular matrix scaffold for tissue engineering application [Citation35]. However, traditional crosslinking usually come with the biocompatible issues due to the undesirable crosslinker properties. For examples, water insoluble crosslinker required the use of organic solvents that might cause collagen denaturation and the poor control of the crosslinking degree, therefore resulting in an exacerbated immune reaction. Currently, one commonly applied crosslinker is glutaraldehyde (GA), and the obtained crosslinked decellularized matrix has been applied in multiple organ/tissue regeneration, e.g. heart, bone, brain, and lung [Citation36]. The naturally derived crosslinker, including PC and GP, have been extensively studied due to the fine crosslinking efficiency and excellent biocompatibility as previously introduced. We have systemically compared the scaffold properties of dUECM crosslinked with different crosslinker (GA, PC, and GP). All three crosslinkers reinforce the mechanical strength and slower the degradation rate, but the biocompatible properties differ significantly. GA-crosslinked dUECM caused obvious cell death and host immune reactions with multiple pro-inflammatory factors accumulation. Interestingly, PC- and GP-crosslinked dUECM showed excellent biocompatibility both in vitro and in vivo, even promoting the cell proliferation at a low crosslinking degree. Some papers have reported that crosslinking could mask the target antigens and decrease the host immunogenic rejections [Citation35]. However, in our study, the uncrosslinked matrix received the lowest immunogenicity results in xenogeneic subcutaneous/in-situ transplantation, while all crosslinked groups showed the infiltration of neutrophils/macrophages and the overexpressed TNF-α. It is worth mentioning that, consistent with previously studies, PC- and GP-crosslinked dUECM decreased the immunogenic reactions compared to GA group, exhibited a higher biocompatibility [Citation18,Citation35].
Many crosslinking methods have been exploited, but a suitable crosslinking strategy with balanced mechanical strength and bioactivity is still rare. The degraded extracellular matrix could promote the cell proliferation by providing the essential nutritional ingredients [Citation34], but it is also crucial to provide extended mechanical support at the defected/excised area for cell seeding, proliferation and differentiation within sufficient time to regenerate defect tissue. Here, we prepared PC- and GP-crosslinking dUECM at low, middle, and high crosslinking degree each and evaluated how the different crosslinking degrees affect the physicochemical properties of dUECM and the following in vivo performance. The shape and microstructure were preserved after decellularization, and crosslinked dUECM showed more self-consistent microstructure and better durability compared with non-crosslinked dUECM. The fibre gap gradually decreased as the concentration of PC or GP increased which might affect the cell adhesion and getting into the dUECM (), also evidenced by SEM results. The first step for the tissue regeneration is the recellularization of transplanted scaffold [Citation37]. Thus, it is crucial to create a cell preferable environment with proper fibre gap. It is expectable to see that the overall mechanical strength enhanced after crosslinking (Supplementary Figure S1). The selected GP concentration range is much higher than that of PC, which might cause a higher crosslinking degree of GP-L crosslinking than that of the PC-L crosslinking. As long as the mechanical properties of the decellularized matrix were restored (crosslinked dUECM vs. native tissues), it was sufficient to provide a microstructure if not degraded.
Scaffold stability and its resistance to enzyme digestion are more crucial for the durability of the transplants as the solid mechanical support [Citation38]. As the crosslinker concentration increased, the PC and GP groups exhibited a prolonged degradation period. In the partial uterus xenotransplantation study, the low crosslinked groups (PC-L) and (GP-L, data not show) was quickly degraded and failed to support the uterus reconstruction (). We can see that, as the crosslinking concentration increased, scaffold degradation rate was significantly declined. Therefore, modified with crosslinkers could effectively improve the stability of dUECM, which could support cellular adhesion, proliferation, and new ECM deposition during extended repair process before complete recovery. After 3 months, the high crosslinked groups (PC-H) still connected the excised rat uterus, but with minimal regeneration sign, as evidenced by the low expression of cytokeratin, Ki67, and α-SMA. Even though PC-L and GP-L that quickly degraded in the collagenase solution might be considered undesirable, but they induced more cell infiltration within the xenogenic embedded uterus matrix. A proper concentration could optimise the balance between the degradation and regeneration promotion of decellularized matrix [Citation39]. In our study, PC-M had a similar mechanical strength to the native uterus, and the degraded ECM was replaced by the newly generated tissues overtimes (). After 90 days, the rat uterus was fully regenerated while the decellularized rabbit uterus matrix was mostly degraded. The importance of crosslinking condition is standing out, but how to find the optimal crosslinker concentration needs further investigation. If we could mimic the in vivo degradation and tissue regeneration in vitro, the once-neglected optimised crosslinking condition could be obtained without the high cost and time-consuming factors.
Conclusions
The ultimate goal of our study was to develop a crosslinked decellularized rabbit uterus for xenotransplantation to achieve rat uterus regeneration and reconstruction. The effect of crosslinking treatment on the characteristics of decellularized uterus both in vitro and in vivo were tested and compared. In this study, we proved there was no significant influence on the microstructure of the uterus matrix after crosslinking treatment. Compared to the GA crosslinking dUECM, PC and GP crosslinking groups exhibited excellent cytocompatibility and minimum host immune reactions in a xenogeneic model. In general, crosslinking enhances the mechanical properties and enzymatic resistance both in vitro and in vivo. However, the effect differed in crosslinker type and crosslinking degree. Overall, in all tested crosslinked groups, PC-M treatment produced a crosslinked dUECM with similar mechanical properties to the native uterus, reasonable enzymatic degradation rate, excellent biocompatibility, minimum xenogeneic host immune rejections, and best uterus regeneration and construction after xenotransplantation. Crosslinking condition has to be carefully evaluated to balance the role of crosslinked decellularized tissue in both mechanical/biological support and tissue regeneration promotion.
11202019_Supporting_inforamtion_clear_version.docx
Download MS Word (9.7 MB)Disclosure statement
The authors declare that they have no conflicts of interest to disclose.
Additional information
Funding
References
- Gan L, Duan H, Xu Q, et al. Human amniotic mesenchymal stromal cell transplantation improves endometrial regeneration in rodent models of intrauterine adhesions. Cytotherapy. 2017;19(5):603–616.
- Wall A, Testa G. Living donation, listing, and prioritization in uterus transplantation. Am J Bioethics. 2018;18(7):20–22.
- Brännström M. Current status and future direction of uterus transplantation. Cur Opin Organ Transplant. 2018;23(5):592–597.
- Fernández-Ruiz I. Breakthrough in heart xenotransplantation. Nat Rev Cardiol. 2019;16(2):69.
- Nellore A, Fishman JA. Donor-derived infections and infectious risk in xenotransplantation and allotransplantation. Xenotransplantation. 2018;25(4):e12423.
- Kou l, Sun R, Bhutia YD, et al. Emerging advances in P-glycoprotein inhibitory nanomaterials for drug delivery AU – Kou, Longfa. Expert Opin Drug Delivery. 2018;15(9):869–879.
- Taylor DA, Sampaio LC, Ferdous Z, et al. Decellularized matrices in regenerative medicine. Acta Biomaterialia. 2018;74:74–89.
- Xi C, Haoran F, Haoxuan W, et al. Targeting autophagy enhances apatinib-induced apoptosis via endoplasmic reticulum stress for human colorectal cancer. Cancer Lett. 2018;431:105–114.
- Sukhorukova IV, Sheveyko AN, Firestein KL, et al. Mechanical properties of decellularized extracellular matrix coated with TiCaPCON film. Biomed Mater. 2017;12(3):035014.
- Jang J, Kim TG, Kim BS, et al. Tailoring mechanical properties of decellularized extracellular matrix bioink by vitamin B2-induced photo-crosslinking. Acta Biomater. 2016;33:88–95.
- Hussey GS, Dziki JL, Badylak SF. Extracellular matrix-based materials for regenerative medicine. Nat Rev Mater. 2018;3(7):159–173.
- Yao Q, Kou L, Tu Y, et al. MMP-Responsive “Smart” drug delivery and tumor targeting. Trends Pharmacolog Sci. 2018;39(8):766–781.
- Kou L, Bhutia YD, Yao Q, et al. Transporter-guided delivery of nanoparticles to improve drug permeation across cellular barriers and drug exposure to selective cell types. Front Pharmacol. 2018;9:27.
- Pinheiro A, Cooley A, Liao J, et al. Comparison of natural crosslinking agents for the stabilization of xenogenic articular cartilage. J Orthop Res. 2016;34(6):1037–1046.
- Ma B, Wang X, Wu C, et al. Crosslinking strategies for preparation of extracellular matrix-derived cardiovascular scaffolds. Regen Biomat. 2014;1(1):81–89.
- Wang X, Chang J, Tian T, et al. Preparation of calcium silicate/decellularized porcine myocardial matrix crosslinked by procyanidins for cardiac tissue engineering. Rsc Adv. 2016;6(41):35091–35101.
- Poursamar SA, Lehner AN, Azami M, et al. The effects of crosslinkers on physical, mechanical, and cytotoxic properties of gelatin sponge prepared via in-situ gas foaming method as a tissue engineering scaffold. Mat Sci Eng C. 2016;63:1–9.
- Gao M, Wang Y, He Y, et al. Comparative evaluation of decellularized porcine liver matrices crosslinked with different chemical and natural crosslinking agents. Xenotransplantation. 2019;26(1):e12470.
- Johannes H, Silvia B, Philipp J, et al. Biomechanical and angiogenic properties of tissue-engineered rat trachea using genipin cross-linked decellularized tissue. Biomaterials. 2012;33(3):780–789.
- Roth SP, Glauche SM, Plenge A, et al. Automated freeze-thaw cycles for decellularization of tendon tissue – a pilot study. Bmc Biotechnol. 2017;17(1):13.
- Yamanaka H, Yamaoka T, Mahara A, et al. Tissue-engineered submillimeter-diameter vascular grafts for free flap survival in rat model. Biomaterials. 2018;179:156–163.
- Murab S, Ghosh S. Impact of osmoregulatory agents on the recovery of collagen conformation in decellularized corneas. Biomed Mater. 2016;11(6):065005.
- Negishi J, Funamoto S, Kimura T, et al. Porcine radial artery decellularization by high hydrostatic pressure. J Tissue Eng Regen Med. 2015;9(11):E144–E151.
- Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32(12):3233–3243.
- Xing S, Liu C, Xu B, et al. Effects of various decellularization methods on histological and biomechanical properties of rabbit tendons. Exp Ther Med. 2014;8(2):628–634.
- Rashtbar M, Hadjati J, Ai J, et al. Characterization of decellularized ovine small intestine submucosal layer as extracellular matrix-based scaffold for tissue engineering. J Biomed Mater Res. 2018;106(3):933–944.
- Xu H-L, Mao K-L, Lu C-T, et al. An injectable acellular matrix scaffold with absorbable permeable nanoparticles improves the therapeutic effects of docetaxel on glioblastoma. Biomaterials. 2016;107:44–60.
- Lin Q, Wong HL, Tian FR, et al. Enhanced neuroprotection with decellularized brain extracellular matrix containing bFGF after intracerebral transplantation in Parkinson’s disease rat model. Int J Pharm. 2017;517(1–2):383.
- Lakes EH, Matuska AM, McFetridge PS, et al. Mechanical integrity of a decellularized and laser drilled medial meniscus. J BiomecEng. 2016;138(3):031006–031006-12.
- Purpura V, Bondioli E, Cunningham EJ, et al. The development of a decellularized extracellular matrix-based biomaterial scaffold derived from human foreskin for the purpose of foreskin reconstruction in circumcised males. J Tissue Eng. 2018;9:204173141881261–2041731418812613.
- Yao Q, Gutierrez DC, Hoang NH, et al. Efficient codelivery of paclitaxel and curcumin by novel bottlebrush copolymer-based micelles. Mol Pharmaceutics. 2017; 14(7):2378–2389.
- Xu HL, Tian FR, Xiao J, et al. Sustained-release of FGF-2 from a hybrid hydrogel of heparin-poloxamer and decellular matrix promotes the neuroprotective effects of proteins after spinal injury. Int J Nanomedicine . 2018;13:681–694.
- Chen WCW, Wang Z, Missinato MA, et al. Decellularized zebrafish cardiac extracellular matrix induces mammalian heart regeneration. Sci Adv. 2016;2(11):e1600844.
- Schneider KH, Enayati M, Grasl C, et al. Acellular vascular matrix grafts from human placenta chorion: Impact of ECM preservation on graft characteristics, protein composition and in vivo performance. Biomaterials. 2018;177:14–26.
- Wang Y, Bao J, Wu X, et al. Genipin crosslinking reduced the immunogenicity of xenogeneic decellularized porcine whole-liver matrices through regulation of immune cell proliferation and polarization. Sci Rep. 2016;6(1):24779.
- Yang H, Qiang T, Zhao H. Progress in various crosslinking modification for acellular matrix. Chin Med J. 2014;127(17):3156.
- Chi Ting Au-Yeung G, Sarig U, Sarig H, et al. Restoring the biophysical properties of decellularized patches through recellularization [10.1039/C7BM00208D]. Biomater Sci. 2017;5(6):1183–1194.
- Sarumathi G, Siti SZ, Siti FA, et al. Decellularization and genipin crosslinking of amniotic membrane suitable for tissue engineering applications. J Biomater Sci Polym Ed. 2018;29(17):2051–2067.
- Yao Q, Zheng Y-W, Lan Q-H, et al. Recent development and biomedical applications of decellularized extracellular matrix biomaterials. Mat Sci Eng C. 2019;104:109942.