Abstract
A metal-resistant engineered Pichia pastoris was developed here to fulfil the metal bioleaching in aqueous conditions. Parent and recombinant yeasts were grown in YPD medium containing different concentrations of ion metals. XRD, electron microscopy and particle size analyser were used for the characterisation and the nanoparticle analyses. The nanoparticle production kinetics were studied by ICP-OES. The cytotoxicity of nanoparticles was assayed against human cell lines. Media colours changed to a range from purplish-brown to grey during early fermentation stages. The maximum biosorption capacities were recorded 81.23 and 493.35 mg/g for gold and palladium in batch conditions, respectively. Various physical investigations proved monodispersed spherical nanoparticles around 100 nm in size. Pure palladium nanoparticles and PdCl2 represented the least cytotoxic potency towards T47D and EPG85.257 cells. The results demonstrated that the genetically modified yeast is a cost-effective, high-throughput, robust, and facile system for metal biosorption.
Introduction
Nanotechnology is a field of science that introduces functional materials in the nanometre scale such as nanowires, nanotriangles, nanorods, nanostars and nanoparticles. On the other hand, gene delivery, drug delivery, cancer therapy, antibacterial tools, biosensors, therapeutic facilities and clinical diagnostics fields have been completely revolutionized by nanoparticle applications in nanomedicine [Citation1,Citation2]. Owing to the extensive applications of nanoparticles in modern technology, the generation of nanoparticles has become an attractive research field in nanotechnology. In the last decade, a variety of methods was employed for their synthesis including physical, chemical and biological routes. The physicochemical methods like lithography, laser ablation, high-energy irradiation, electrochemistry and chemical reduction require the use of hazardous materials and high-energy consumption [Citation3,Citation4]. Environmentalists always warn about hazardous wastes and adverse effects of these chemicals and methods; therefore, the development of safe biological methods or green nanotechnology was inevitable [Citation5]. In green nanotechnology, a variety of biological resources of bacteria, yeasts, algae, fungi, plants, cellular organelles or enzymes are used as eco-friend bioreactors that facilitate the production of nanoparticles [Citation4,Citation6]. The biotransformation/bioleaching systems for the nanometal synthesis eliminate the use and generation of toxic agents [Citation7].
Various microorganisms have the potency to accumulate metallic ions from their surrounding environment. They use different Defence strategies to eliminate metal toxicity. Some cells absorb the ions and produce zero-valent nanoparticles with their inherent biochemical processes in the extracellular or intracellular pathways. Restriction of metal movement into the cells, metal-binding/adsorption to the cellular barriers, metal chelation (using amino acids, organic acids, or metallothioneins ligands), active compartmentalization in organelles/vacuole, cellular sedimentation, efflux transportation, metal–protein interaction with chaperones or heat shock proteins, glutathionylation using glutathione S-transferases, and cytochrome P450-dependent monooxygenases detoxification are amongst the main cellular mechanisms to metal tolerance [Citation8,Citation9].
Various studies have reported biosynthesis of gold (Au) and palladium (Pd) nanoparticles by bacteria, yeast, fungi, plant or their cellular extracts. Gold and palladium are precious metals with various applications in industries, agriculture and medicine. Because of the increasing demand and limited resources for them, recovery of gold and palladium are economically attractive [Citation10]. Gold nanoparticles due to exclusive properties, facile synthesis and surface modification have been extensively used in research fields such as photothermal therapy, chemical and biological sensing, biomedical imaging, microscopy imaging, drug delivery, catalysis, DNA labelling and cancer treatment [Citation11,Citation12]. Palladium widely used in catalysis and electronics. Palladium nanoparticles have unique physical, chemical and optical properties compared to the bulk materials, so they have been received special attention for applications in plasmonic waveguiding, catalysis, fuel cells, anti-bacterial applications, magnetic recordings, chemo-optical transducers, drug delivery and cancer therapy [Citation13–15].
Previously, we demonstrated a high-throughput biological synthesis of silver and selenium nanoparticles with stable, less toxic and highly dispersed features in the genetically modified Pichia pastoris cloned with a resistant reductive enzyme [Citation16]. That was a great motivation to follow the ability of such recombinant Pichia pastoris in the bioreduction of gold and palladium. Here, in the presence of toxic gold and palladium ion concentrations, recombinant Pichia pastoris grew well due to metal biotransformation using resistant cytochrome b5 reductase.
Method and materials
Chemicals and reagents
Media reagents and supplements for Pichia culture were supplied from Invitrogen-Life Technologies (Carlsbad, CA, USA). Human cell culture media and ingredients including RPMI-1640, FBS, penicillin, and streptomycin were purchased from Gibco (Grand Island, NY, USA). Palladium chloride (PdCl2), chloroauric acid trihydrate (HAuCl4 3 H2O), and all other chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, USA).
Bioaccumulation capacity and growth kinetics
A multiple copy recombinant Pichia pastoris that highly overexpress a metal resistant sequence of cytochrome b5 reductase was selected from the previous study with the Mut+ phenotype. The parental yeast specious and the recombinant Cyb5R-clone were cultivated in YPD media (1% w/v Yeast extract, 2% w/v Peptone, 2% w/v Dextrose). YPD media in baffled Erlenmeyer flasks was inoculated with the factory cells with the initial OD600 ≈ 0.01. The flasks covered by a two-folded cheesecloth so that oxygen can be efficiently transferred to the cells. Approximately 0.5% methanol was daily supplemented to the yeast suspension to induce the AOX1 promoter and the expression of heterologous NADH-cytochrome b5 reductase. Cells were cultivated at 30 °C in a shaker incubator with 250 rpm to reach the optical cell density of 0.1. Then, appropriate amounts of HAuCl4 and PdCl2 stock concentrations were added to the culture flasks to reach serial dilutions of either 0, 5, 10, 15, 20 and 25 mM of HAuCl4 or 0, 10, 20, 30, 40, 50 and 60 mM of PdCl2. Flasks were sampled 1 ml four times daily for 3 days. The maximum specific growth rate (μmax) was defined as the slope of the linear regression of natural logarithms of the growth (Y-axis) versus time (independent variant) intervals before reaching the stationary phase [Citation16].
Reduction capacity and nanometal production yield
Genetically engineered Pichia pastoris cell overexpressing CyB5R was inoculated in 500 ml YPD medium containing 0.5% methanol. The culture was then cultivated to the cell density of 1. The culture was supplemented with either HAuCl4 at 10 mM or PdCl2 at 60 mM final. The free-heavy metal medium was considered as negative control. Then, a series of 10 ml aliquots of the cultures were sampled volumetrically at 0-, 6-, 12-, 24-, 36-, 48-, 60- and 72-h post-metal injection. Absorbance values at 600 nm were recorded using a scanning multi-well spectrophotometer. Then, the suspension samples were centrifuged at 5000×g for 10 min, the yeast pellets were carefully washed three times with PBS, and the biomass was recorded after freeze-drying. Five hundred milligrams of dried samples were subjected to a microwave-assisted acid digester according to a modified EPA-3052 protocol. Gold and palladium contents in the samples were measured using ICP-OES (Optima™ 7300-DV, PerkinElmer, Shelton, USA) instrument [Citation17].
Physical characterization of purified nanoparticles
The engineered Pichia cells were cultured in YPD containing 0.5% methanol and maximum tolerated concentrations of HAuCl4 or PdCl2 (10 mM and 60 mM, respectively) and placed in a shaker incubator for 72 h at 30 °C. Then, the cells were centrifuged, washed twice with PBS, resuspended in lysis buffer (0.03% w/v EDTA; 0.6% w/v NaCl, 1% w/v SDS; 0.5% v/v Triton X100; 10 mM Tris pH = 8), and subjected to a mechanical cell rupture (Silent Crusher™, Heidolph, Germany). Afterward, the lysate was clarified three times with Tris/HCl buffer (1.5 M; pH 8.3) containing 1% w/v SDS. Nanometals were purified through a biphasic water/octanol (1:1 v/v) mixture. Nanoparticles tend to gravity settling through both phases and rapidly collect at the bottom of the tube. Sediments were washed with excess chloroform and ethanol, respectively. Purified metals were rinsed with deionized water and finally freeze-dried. The identity and crystalline nature of nanometals were evaluated using an X-ray diffractometer (STOE, Germany) at a wide-angle range of 2θ from 10 to 80 degrees with high resolution. The size and zeta potential of nanoparticle were recorded by a dynamic laser light scattering instrument (Malvern, ZEN3600, UK). An atomic force microscope (AFM, JPK, Deutschland), a transmission electron microscope (TEM, EM10C-100KV, Zeiss, Germany), and a high-resolution scanning electron microscope (FESEM, Zeiss, Germany) were used for imaging the surfaces of Pichia cells during nano-gold production [Citation16].
Nanometal cytotoxicity assay
T47D (human epithelial breast cancer) and EPG85.257 (human gastric carcinoma) cell lines were cultured in 96-well plate in RPMI medium supplemented with 10% (v/v) FBS, two mM L-glutamine, 100 mg/ml streptomycin and 100 IU/ml penicillin. The plates were placed in a humidified atmosphere with constant CO2 and temperature for 24 h. Afterward, the cells were treated with various concentrations of either nanoparticles or metal ions (0–10 mM) for five days. The viable cell fraction was relatively quantified using colorimetric MTT assay. The drug concentration corresponding to a reduced survival rate of 50%, in comparison to the untreated cells was considered as IC50. IC50 values were calculated from the regression equation of the scattering plot of the viable fraction versus drug concentration [Citation18].
Statistical analysis
All experiments were carried out three independent times. Results were reported as the mean values ± SE. p-Values less than 0.05 were considered to be statistically significant using Student's t-test.
Results
Metal ion interferences with Pichia growth kinetics
The metal resistant NADH-cytochrome b5 reductase variant activity in the intracellular fluid was recognized 31 IU/ml from the previous study. The growth rate of the engineered yeast and its parental counterpart was monitored for 5 days. Wild-type strain grew well in a natural logarithmic pattern (Ln(x) = Ln(x0) + 0.51× t) in the absence of the heavy metals and represented a doubling time equal to 81.55 min and reached the plateau phase in the first few days. The letter x indicates yeast dry biomass (mg) and the letter t represents the elapsed time (hours). However, Gold and palladium ions significantly reduced the growth potentials of such parental strains. On the other hand, the metal resistant Pichia persisted more during the initial exponential growth phase and the growth kinetic follow natural logarithmic equations: Ln(x) = 0.42 × t- 8.27 and Ln(x) = 0.40 × t- 8.24 in the presence of 10 μM and 60 μM of gold (III) and palladium (II) ions, respectively ( and Supplementary Figure S1).
Table 1. Growth kinetics of engineered Pichia and the parental counterpart in the presence and absence of chloroauric acid trihydrate (HAuCl4 3 H2O).
Table 2. Growth kinetics of engineered Pichia and the parental counterpart in the presence and absence of palladium chloride (PdCl2).
Bioaccumulation and nanoparticle production kinetics
The recombinant yeast growth and nanoparticle production curve were plotted in the optimum concentration (10 mM of HAuCl4 and 60 mM of PdCl2). The result showed that biosorption of metal ions is not always proportional to the cell growth rate in the recombinant Pichia pastoris ( and Supplementary Figure S2). The maximum absorption efficiency of HAuCl4 and PdCl2 in recombinant yeast was obtained 56.39% and 79.79%, respectively (). The gold biosorption capacity (mg/g) and nano-gold formation yield (%) could be calculated using y = −22.79× Ln(t) + 112.95 and y = 4.12× Ln(t) + 37.52 formulas during the fermentation time, respectively. In contrast, y = 177.78× Ln(t) − 233.23 and y = 6.87× Ln(t) + 49.91 are the best equations represent palladium biosorption capacity (mg/g) and nano-palladium production yield (Supplementary Figures S2 and S3).
Figure 1. Growth kinetics and biosorption capacities. Engineered Pichia pastoris containing an active variant of cytochrome-b5 reductase was treated either with 10 mM HAuCl4 (A) or 60 mM PdCl2 (B). Growth kinetic and biosorption capacity were recorded based on dry cell weight (mg/ml) and ICP-OES data, respectively. The values represent the means of three independent experiments (Mean ± Standard Error).
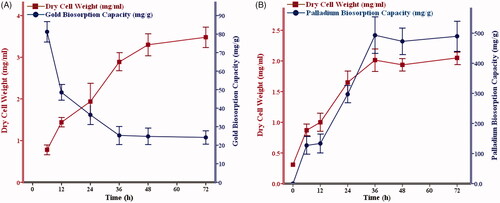
Table 3. Biosorption yield for gold and palladium in the recombinant yeast during the fermentation time.
Physical properties of biosynthetic nanostructures
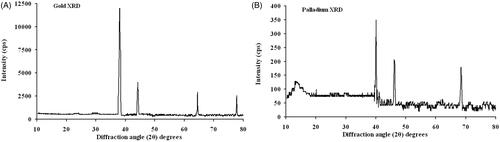
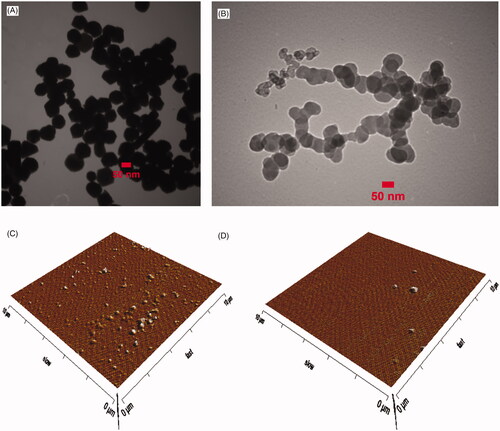
Cellular colours were influenced rapidly during the fermentation process in the presence of both metal ions. Their colours changed to ranges from purplish-brown to grey in the media. The colour provides an early sign of intracellular nanoparticle synthesis since the control batches stably remained yellow during incubation (Supplementary Figure S4). The X-ray diffraction spectrum indicated a special peak in the total spectrum of 2θ values from 10 to 80 for pure Au and Pd nanoparticles. Four distinct reflections at 38.1, 44.3, 64.5 and 77.8 degrees were present for gold and three distinct reflections 40.1, 46.3 and 68.5 degrees were present for palladium that confirmed the production of the corresponding pure nanometals by recombinant yeast (). The average size distribution was determined approximately 100 nm and zeta potential was estimated between −14 to −21 mV using particle size analyzer. AFM and TEM micrographs exhibited spherical forms of the nanoparticles with almost smooth surfaces. Microscopic results and particle size analyzer data are in excellent agreement ( and Supplementary Figures S5, S6).
Cytotoxicity assay
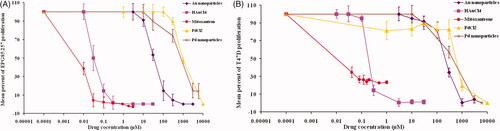
Cytotoxicity analyses of HAuCl4, PdCl2, and their corresponding metallic nanoparticles were conducted on EPG85.257 and T47D lines. Mitoxantrone was consumed as the standard positive cytotoxic agent. Palladium ions and their nanoparticle counterparts represented the least toxicity compared to mitoxantrone (p < .05). Gold (III) salts exhibited significant toxic effects on both cells (p < .001). Cytotoxic data showed that bioreduction of gold salts significantly eliminate the cell toxicity (p < .001). On the other hand, there are no statistical differences between Pd (II) ions and nano-palladiums toxicity ( and Supplementary Table 1).
Figure 4. Cytotoxicity differences between two oxidation states of gold and palladium on EPG85.257 (A) and T47D cell (B). The cells were treated with increasing concentrations of the metal forms (0 to 10,000 μM) for 5 days. Cell viability was considered as the fraction of survived cells to the total cells in untreated chambers using MTT assay. The values indicate the three independent experiments in triplicate (Mean ± Standard Error).
Discussion
The demand for precious metals is rapidly rising due to various industrial and medical applications while high-grade natural resources are being depleted. In contrast, conventional mining processes cause a high-degree of environmental pollution and require extensive Labour, time and energy. Some industrial wastes such as electronic wastes, wastewaters and mining wastes are considered as secondary resources for metal recovery because they sometimes possess high quantities of metals compared to their own ores. Biomolecules such as vitamins, proteins, enzymes, organic acids or polysaccharides play a key role in nanoparticle production by acting as reductants and stabilizing agents. The function of these biomaterials in biological means eliminating the need for toxic substances usage and called green chemistry [Citation19,Citation20].
The previous study represented that engineered Pichia pastoris is an efficient option for the silver and selenium uptake and biotransformation. Cytochrome-b5 reductase arms Pichia to be a metal-resistant cell and to survive in high concentrations of the heavy metal ions. NADH-dependent reductase enzyme mediates the electron transport from NADH to a single FAD group. Then, the prosthetic FAD domain directly catalyzes the stoichiometric transfer of reducing equivalents, electrons, to the small heavy metal ion partners. Such engineered yeast usage for nanoparticle productions gain several advantages over physicochemical methods, immobilized enzyme bioreactors and non-transformed factory cells due ease of use, automation and the cell manipulation, inexpensive growth requirements and investment, simple scaling-up, high biomass yield, time-/cost-effectiveness, cofactors' needless and eco-friendliness. On the other hand, biosynthetic routes could provide good control over the recombinant enzyme production in the cell and consequently size distribution of nanoparticles than some of the physicochemical methods. Such properties introduced the developed microorganism as an excellent tool in nanotechnology science [Citation16,Citation21–24].
and represent that the maximum specific growth of the parental yeasts was greatly influenced in the presence of the metal ions because gold and palladium are nonessential metals. They inhibit physiological metabolism in the cells by interfering with protein functions through triggering oxidative stress, denaturant-induced complexation, occupying the functional enzymes sides, or replacing with essential cofactors in metalloproteins. In contrast, metal-resistant yeasts have tolerated more to the metal ions. Similar articles showed involving of the antioxidant system in heavy metals detoxification in yeast cells. For example, selenium can induce apparent oxidative stress in yeast cells. Antioxidant enzyme activation is the first Defence strategy against the presence of trace amounts of metal ions, but oxidative damage and cellular death are inevitable due to higher concentrations of the ions because the protection system is not sufficient [Citation25]. Our data confirmed that overexpression of a reductive CyB5R enzyme in Pichia cells can reinforce the antioxidant system of yeast to protect the cells from oxidative stress induced by the presence of excessive amounts of toxic ions by one of the additional detoxification processes.
The decline in the growth kinetics in the metal resistant Pichia could be interpreted by the innate toxic effects of the ions in high concentrations, energy consumption for recombinant enzyme production, NADH coenzyme depletion, and high demand for respiratory oxygen for enzymatic bioreduction processes [Citation26]. Interestingly, palladium ions/nanoparticles were less poisonous for both yeast and human cells ( and Supplementary Table 1). Although aurum chloride demonstrated the highest cytotoxicity on both human cells, there are no statistical differences between the two oxidation states of palladium toxicity. The metal states triggered cytotoxicity in a time-, dose- and cell type-dependent manner. For example, palladium was reported to initiate chronic rather than acute toxicity on the cells [Citation27]. Higher treatment concentrations than 60 mM were not possible due to the precipitation of PdCl2 in the neutral fermentation medium.
The maximum gold biosorption capacity in the recombinant Pichia was recorded 81.23 mg/g only after the first stages of fermentation. The gold absorption efficacy reached the summit right after the culture supplementation, probably owing to the high ion availabilities, presence of enough vacant binding sites in the cells and reduced capacity of the enzymes. Although yeast cells continued to grow during later stages, biosorption capacity diminished gradually, that is mainly due to gold uptake arrest via inhibition of endocytosis or activation of efflux pumps [Citation28,Citation29]. On the other hand, palladium biosorption coincided with trophophase during recombinant CyB5R production. These results indicate that palladium is more dependent on CyB5R production and biotransformation. The level of palladium bioaccumulation was 493.35 mg/g in recombinant yeast and is the highest rate biosorption capacity that has been reported [Citation30,Citation31].
Physical investigations of the products confirmed the crystalline nature of the nanometals. The particle size analyzer and TEM images represented highly monodispersed nanoscale spheres. Homogenous particles in size and shape could be produced only in optimum metal ion concentration, fermentation conditions, and media supplementation ( and Supplementary Figure S5). TEM micrographs represent a marked tendency of the nanometals to self-aggregation in water due to negative zeta potentials (Supplementary Figure S6). The zeta potential is surface potential that is influenced by several factors like metal concentration, media ionic strength, pH, innate metallic features, and the surface functionalization state [Citation32]. The industrial revolution transformed world economies but increased heavy metal pollution on the earth. Removing metal ions using the engineered yeasts via bioaccumulation strategy develops a novel era in green chemistry and would provide adequate resources of precious metals in the world shortly.
Author contributions
S. A. Mirzaei designed and coordinated the study and revised the final manuscript. F. Elahian designed bioremediation kinetics, statistical analyses and participated in the manuscript writing and intellectual discussions of the data. R. Heidari, V. Charghan, and E. Asadbeik were the students involved in the experiments as part of their theses.
Supplementary_Data.pdf
Download PDF (375.2 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ahmed S, Ahmad M, Swami BL, et al. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J Adv Res. 2016;7(1):17–28.
- Dobrowolska P, Krajewska A, Gajda-Raczka M, et al. Application of Turkevich method for gold nanoparticles synthesis to fabrication of SiO2@Au and TiO2@Au core-shell nanostructures. Materials. 2015;8(6):2849–2862.
- Wicki A, Witzigmann D, Balasubramanian V, et al. Nanomedicine in cancer therapy: challenges, opportunities, and clinical applications. J Control Release. 2015;200:138–157.
- Shah M, Fawcett D, Sharma S, et al. Green synthesis of metallic nanoparticles via biological entities. Materials. 2015;8(11):7278–7308.
- Singh P, Kim YJ, Zhang D, et al. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 2016;34(7):588–599.
- Mittal AK, Chisti Y, Banerjee UC, Synthesis of metallic nanoparticles using plant extracts. Biotechnol Adv. 2013;31(2):346–356.
- Raveendran P, Fu J, Wallen SL, Completely “green” synthesis and stabilization of metal nanoparticles. J Am Chem Soc. 2003;125(46):13940–13941.
- Hulkoti NI, Taranath TC, Biosynthesis of nanoparticles using microbes- a review. Colloids Surf B Biointerfaces. 2014;121:474–483.
- Olaniran AO, Balgobind A, Pillay B, Bioavailability of heavy metals in soil: impact on microbial biodegradation of organic compounds and possible improvement strategies. IJMS. 2013;14(5):10197–10228.
- Saratale RG, Karuppusamy I, Saratale GD, et al. A comprehensive review on green nanomaterials using biological systems: Recent perception and their future applications. Colloids Surf B Biointerfaces. 2018;170:20–35.
- Thakor AS, Jokerst J, Zavaleta C, et al. Gold nanoparticles: a revival in precious metal administration to patients. Nano Lett. 2011;11(10):4029–4036.
- Khan MS, Vishakante GD, Siddaramaiah H, Gold nanoparticles: a paradigm shift in biomedical applications. Adv Colloid Interface Sci. 2013;199–200:44–58.
- Momeni S, Nabipour I, A simple green synthesis of palladium nanoparticles with sargassum alga and their electrocatalytic activities towards hydrogen peroxide. Appl Biochem Biotechnol. 2015;176(7):1937–1949.
- Cheong S, Watt JD, Tilley RD, Shape control of platinum and palladium nanoparticles for catalysis. Nanoscale. 2010;2(10):2045–2053.
- Kang S, Shin W, Kang K, et al. Revisiting of Pd nanoparticles in cancer treatment: all-round excellence of porous Pd nanoplates in gene-thermo combinational therapy. ACS Appl Mater Interfaces. 2018;10:13819–13828.
- Elahian F, Reiisi S, Shahidi A, et al. High-throughput bioaccumulation, biotransformation, and production of silver and selenium nanoparticles using genetically engineered Pichia pastoris. Nanomedicine. 2017;13(3):853–861.
- Tavakoli L, Yamini Y, Ebrahimzadeh H, et al. Development of cloud point extraction for simultaneous extraction and determination of gold and palladium using ICP-OES. J Hazard Mater. 2008;152(2):737–743.
- Gholamian Dehkordi N, Elahian F, Khosravian P, et al. Intelligent TAT-coupled anti-HER2 immunoliposomes knock downed MDR1 to produce chemosensitize phenotype of multidrug resistant carcinoma. J Cell Physiol. 2019;234(11):20769–20778.
- He J, Kappler A, Recovery of precious metals from waste streams. Microb Biotechnol. 2017;10(5):1194–1198.
- Wang J, Chen C, Biosorbents for heavy metals removal and their future. Biotechnol Adv. 2009;27(2):195–226.
- Hutchison JE, Greener nanoscience: a proactive approach to advancing applications and reducing implications of nanotechnology. ACS Nano. 2008;2(3):395–402.
- Thakkar KN, Mhatre SS, Parikh RY, Biological synthesis of metallic nanoparticles. Nanomedicine. 2010;6(2):257–262.
- Doane TL, Burda C, The unique role of nanoparticles in nanomedicine: imaging, drug delivery and therapy. Chem Soc Rev. 2012;41(7):2885–2911.
- Duan H, Wang D, Li Y, Green chemistry for nanoparticle synthesis. Chem Soc Rev. 2015;44(16):5778–5792.
- Kieliszek M, Błażejak S, Bzducha-Wróbel A, et al. Effect of selenium on growth and antioxidative system of yeast cells. Mol Biol Rep. 2019;46(2):1797–1808.
- Allen CCR, Boudet CJ, Hardacre C, et al. Enhancement of whole cell dioxygenase biotransformations of haloarenes by toxic ionic liquids. Rsc Adv. 2014;4(38):19916–19924.
- Hosseini MJ, Jafarian I, Farahani S, et al. New mechanistic approach of inorganic palladium toxicity: impairment in mitochondrial electron transfer. Metallomics. 2016;8(2):252–259.
- Punjabi K, Yedurkar S, Doshi S, et al. Biosynthesis of silver nanoparticles by Pseudomonas spp. isolated from effluent of an electroplating industry. IET Nanobiotechnol. 2017;11(5):584–590.
- Gunduz N, Ceylan H, Guler MO, et al. Intracellular accumulation of gold nanoparticles leads to inhibition of macropinocytosis to reduce the endoplasmic reticulum stress. Sci Rep. 2017;7(1):40493.
- Sari A, Mendil D, Tuzen M, et al. Biosorption of palladium(II) from aqueous solution by moss (Racomitrium lanuginosum) biomass: equilibrium, kinetic and thermodynamic studies. J Hazard Mater. 2009;162(2–3):874–879.
- Lim JS, Kim SM, Lee SY, et al. Quantitative study of Au(III) and Pd(II) ion biosorption on genetically engineered Tobacco mosaic virus. J Colloid Interface Sci. 2010;342(2):455–461.
- Skoglund S, Hedberg J, Yunda E, et al. Difficulties and flaws in performing accurate determinations of zeta potentials of metal nanoparticles in complex solutions-Four case studies. PLoS One. 2017;12(7):e0181735.