?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Organogels are excellent drug carrier for controlled release. Organogels based on amino acid derivatives has been widely used in the area of drug delivery. In this study, a series of the organogel system based on amino acid derivatives gelators was designed and prepared to investigate the structure-property correlation in organogels. To investigate the factors that influence the property of drug release, we varied the formulation in the organogels: gelator structure, gelator concentration, volume of antigelation solvent, and drug loading. Through the Box–Behnken tests, the optimum organogel formulation in vitro was obtained. The self-healing properties of the organogel have been utilised for injection of a model lipophilic risperidone in situ, and sustained release of the drug has been studied over about one week in vivo. In conclusion, the gelation ability of gelators could be adjusted by the gelator structure. Gel property is related with the whole composition of the formulation. As drug carrier, the drug release property of organogels is affected by multiple factors. Our investigation of the gel release property will play a theoretical guiding role in the application in the in situ drug delivery system.
Introduction
Of recent decades, organogels have received much attention for their potential applications in the areas of drug delivery, oil recovery, catalysis and cosmetic [Citation1–3]. As the basic component of the organogel, gelators are usually some low-molecular-mass compounds, such as lecithin, sorbitan derivatives, fatty acid-derivatives, bis-urea compounds, amino acid derivatives, and so on [Citation4–8]. A variety of organic solvents can be immobilised at extremely low concentration of these substances by the forces of non-covalent intermolecular interactions, such as H-bonds, π-π stacking, electrostatic interactions, metal coordination and London dispersion forces established between themselves, which could lead to various entangled structures, like wrinkle, lamellar and fibres [Citation9–12].
Great interests in amino acid derivatives have been raised because of their inherent advantages in preparation, biodegradability, and biocompatibility [Citation13–16]. The organogels based on these gelators are always thermal-reversible and stable for months [Citation17,Citation18]. Such properties make it possible to prepare various functional gel systems. The injectable in situ-forming implant is easy to administrate and can prolong the retention time of drugs, reduce the undesired side effect, and improve patient compliance [Citation19–21]. Up to now, there has much successful application of organogels in the in situ forming system [Citation16,Citation22,Citation23]. To make the organogel injectable at room temperature, a small amount of biocompatible amphiphilic solvent is added into the preparation before administration, such as ethanol, N-methyl pyrrolidone (NMP), and dimethyl sulfoxide (DMSO), which could also disrupt the interactions between gelator molecules, thus this kind of solvent was also named antigelation solvent. Upon subcutaneous injection, it can diffuse to surrounding tissues rapidly, and the depot containing active compounds would form in situ. Then, the drug release from this depot through drug diffusion and material degradation.
However, the addition of the antigelation solvent bring some problems in the meanwhile, such as the initial burst of drugs and unclear degradation mechanism of the organogels. As the diffusion rate and dosage volume affect the diffusion time of the antigelation solvent, the uncontrollable drug release before the drug depot formed severely restricted its further application. For the most drugs with a narrow therapeutic window, the burst release can lead to a different degree of toxicity and side effects. The solution to these problems focussed on two aspects. One is shrinking the gelation time after administration, such as increasing the gelator concentration, modifying gelator structure or adding crosslinker [Citation24–26]. The other is preloaded the drug into some vesicles such as microemulsions, nanoparticles or microspheres [Citation27,Citation28]. Then these drug vesicles were loaded into gels to achieve the sustained release aim and avoid the burst release. While this form of preparation tremendously increases the difficulty in research and preparation development, especially in the large-scale manufacture process. Because the preparation process is complex and drug-specific, it is necessary to design appropriate new preparations for specific drugs. Thus it is more feasible to explore the properties of each component in the gel system in analyzing the burst release.
In our previous work, a series of amino acid based gelators were synthesized to explore the role of the gelator structure on functional properties, and found that the in situ organogel-forming implant has excellent biodegradability and biocompatibility, which indicates great potential for safe in situ forming drug delivery [Citation29–32]. However, it is still questionable how the burst release could be modified through the formulation composition. Such an understanding would provide a better design of systems in which molecular modification would control the gel assembles and ultimately execute specific materials function. To address these issues, four new gelators based on l-alanine acid, l-glutamic acid and l-serine acid were designed and synthesized. Their gelation behaviour in the injectable oils was characterized. To obtain a suitable in situ depot, the thermal behaviours, rheological properties, and in vitro drug release of organogels were investigated. Finally, the release behaviour of risperidone (RIS) chosen as a lipophilic model drug was explored in vivo in the perspective of their potential application in drug delivery.
Materials and methods
Materials
Eicosanoic acid were obtained from Aladdin Industrial Corporation (China). Dimethyl l-glutamate hydrochloride, l-alanine methyl ester hydrochloride, methyl-dl-serine hydrochloride, l-alanine ethyl ester hydrochloride, 1-(3-dimethylamino propyl)-3-ethylcarbodiimide HCl (EDCI) and 1-hydroxybenzotriazole (HOBt) were obtained from GL Biochem Ltd. (Shanghai, China). 1-Methyl-2-pyrrolidinone was from Sigma-Aldrich LLC. Injectable soybean oil (LCT) was obtained from Zhonghang Tieling Pharmaceuticals Co., Ltd., Tieling, China. All other materials were available commercially.
Synthesis of amino acid based gelators
The four amino-based gelator: N-icosanoyl-l-alanine methyl ester (C20-Ala-Me) N-icosanoyl-l-glutamate dimethyl ester (C20-Glu-Me), N-icosanoyl-l-serine methyl ester (C20-Ser-Me) and N-arachidonoyl-l-alanine ethyl ester (C20-Ala-Et) were synthesized by the corresponding amino acids with eicosanoic acid. All functionalized amino-based gelators were synthesized using the method we reported before [Citation29]. The full synthetic route and characterization are described in the supporting information.
Preparation of organogel
The organogel was prepared by dissolving the amount of gelator in 1 ml LCT at 80 °C. The solution was cooled to the room temperature and inversed vertically to see whether the organogels formed. For example, the concentration of 8% blank organogel was prepared by dissolving 80 mg gelator 1 ml LCT at 80 °C. The concentration of 5% and 10% blank organogel were prepared by the same method. In this paper, risperidone (RIS) was used as the model drug, due to its hydrophobic character and long term administration. To load a drug into a gel, a certain amount of drug was first dissolved in the oily solution. RIS is an atypical antipsychotic, which need long-term administration. To reduce frequency of administration combat patient discomfort, the sustained release property of organogels shows advantages in its application. As the RIS was oil-soluble, the drug can completely loaded into the preparation under prescription dosage. Then the organogels were prepared as described above. Some anti-gelation agent was added into the system to maintain a sol state before administration. The prepared samples were stored at 4 °C.
Gelation study
As the solubility of gelator increased with temperature, the 3 D-network formed with the insolubility in solvents at room temperature leading to the formation of organogels. Upon a certain concentration, the gelators can gelate the oil phase. This concentration is the critical gelation concentration (CGC), which is a fundamental parameter that can reflect the gelator property. The CGC was determined using a dilution method by measuring the minimum amount of gelator required for the formation of a stable gel at 25 °C. 50 mg gelator and 0.5 ml solvent were placed in a glass tube heating to 80 °C for a solution. And then they were maintained under the temperature of 25 °C for 15 min. The glass vial was put upside down to identify the organogels were formed or not. If not, a small amount of gelator was added to the system, and the heating/cooling process was repeated until the gel state appeared. All determinations were conducted in triplicate.
Thermal stability of gels
The gel-to-sol transition temperatures (Tgs) is an important parameter in determining the gel function, which should higher than the body temperature to maintain the gel state after administration. The dropping-ball method was applied in to obtain this parameter. In practice, the organogels were prepared in the tube. A steel ball with a diameter of 2 mm was put on the surface of the organogel gently with the temperature slowly increased by 2 °C/min from 25 °C. The temperature when the ball began dropping was recorded as Tgs of the gel system.
The thermal properties of RIS, blank organogel, and the RIS-organogels were measured by differential scanning calorimeter (DSC1, METTLER-TOLEDO Co., Switzerland) to gain the heating and cooling curves.
Rheological measurements
The rheological property of organogel was carried out on an AR2000 rheometer (TA, Co Ltd.) with parallel-plate geometry (diameter was 20 mm). The gap between the plates was fixed at 1000 μm and the frequency sweep was performed over a range of 0.1–10 Hz with a particular stain (0.5%) at 37 ± 1 °C.
Scanning electron microscopy (SEM)
The morphology of the organogel was obtained using an S-4800 field emission scanning electron microscope (SEM, Hitachi, Japan) operated at 5 kV. In this part, the self-assembled morphology of gelators was investigated under xerogels state. The xerogels were obtained by preparing organogels in toluene at a concentration of 8 w/v% and dried in a vacuum for 24 h. Then, the xerogels were placed on a carbon tape and coated with a thin layer of gold.
In vitro release evaluation
The drug-loaded organogels were prepared by risperidone, amnio acid derivative gelators and injectable soybean oil, which were allowed to evaluate the in vitro release property [Citation33,Citation34]. After the addition of NMP into organogel (10:90, w/w), the system turned to be a sol state and easily administrated through the syringe. It was then transferred into the dialysis bags (8000–14,000 Da), which was then placed in the bottles with 100 ml of phosphate buffer (104 mM NaH2PO4, 36 mM NaOH, 0.5 w/v% SDS, 0.1%w/v NaN3, pH 7.4). The temperature was maintained at 37 °C in a water bath with an oscillator (100 rpm). An oily solution consisting of the drug without gelators were also prepared. At predetermined time intervals, 3 ml of releasing solution was withdrawn for analysis and replaced by fresh PBS. Release kinetics were measured under sink conditions. Each formulation was prepared in triplicate. The samples of Risperidone were analyzed by a Shimadzu UV-1750 spectrophotometer at a maximum absorbance of 279 nm. The risperidone calibration curve was used, as given in EquationEquation (1)(1)
(1) :
(1)
(1)
where y is the absorbance at 279 nm and x is the concentration of risperidone (µg/ml) and the r2 value was 0.9999.
Optimation of risperidone organogel
A response surface methodology (RSM) was used to obtain the optimal formulation of RIS organogels. Three experimental factors: amounts of gelator (X1), NMP (X2) and RIS (X3) in three-level were applied to evaluate the influence on drug release. Box–Behnken design (BBD) [Citation35,Citation36] was used to optimise the formulation of organogel loaded RIS. To investigate the degree of burst release and sustained release time of organogels, the cumulative release amounts of RIS in 24 h (R24h) and 14 days (R14d) were chosen as the response values, respectively. The response and formation variables of all model formulations were analysed by Design-Expert software (Design Expert® v 8.0.1, Stat-Ease, USA). Details of studied levels and the response variables are shown in .
Table 1. Structure information of gelators and the corresponding property in organogels.
In vivo release of risperidone organogels
In vivo drug release performance was carried out in male Sprague–Dawley rats weighted 200–250 g obtained from Liaoning Changsheng Biotech Corporation (PR China). Animal experiments were approved by the Animal Ethics Committee of Shenyang Pharmaceutical University. The rats were randomly divided into two groups (five rats per group). For group A, 0.07 ml optimal organogel preparations (about two weeks’ dosage) were subcutaneously injected in the dorsal area using a 25-gauge syringe. Group B was subcutaneously injected 0.1 ml oil solutions of RIS (2.6 mg/ml, two days’ dosage). For the pharmacokinetic study, 0.5 ml blood samples were obtained from the retro-orbital plexus of each rat in group A at the interval of 0.5, 1, 2, 6, 12, 24 h and 34,678 days. While for group B, the blood samples were obtained at an interval of 0.083, 0.25, 0.5, 1, 2, 6, 12, 24 h and two days. The plasma was separated by centrifugation (containing disodium EDTA, 5000 rpm × 10 min) and stored at −20 °C for further analysis. In this method, 200 μl plasma mixed with 20 μl berberine, as an internal standard, were added to the plastic centrifugal tube. After 750 μl of ethyl acetate added to the tube, this mixture was vortexed for 3 min and centrifuged at 13,000 rpm for 10 min. The supernatant was transferred to a test tube and evaporated to the dryness under gentle steam of nitrogen. The residue was reconstituted with 200 μL of the mobile phase and centrifuged at 13,000 rpm for 10 min. An aliquot of the 20 μL was then withdrawn for the assay.
Drug concentration was monitored by the HPLC, equipped with a Hitachi 10-AT pump connected with a UV detector set as 280 nm. The analytical column of Thermo ODS-2 Hypersil (250 mm × 4.6 mm, 5 μm, USA) was applied with the mobile phase consisted of acetonitrile–water–ammonium acetate (60:40:0.1, v/v/v) at a flow rate of 1 ml/min. The area under each peak was calculated. The concentration of RIS was calculated through the internal standard.
Results and discussion
Gelator structure and organogel property
Gelator is the inevitable composition of organogels. The gel properties significantly depend on the structure of the organogelators. In this paper, we synthesized a series of amino acid-based gelator, which was based on an amide bond to link the alkyl chain and the amino acid esters. Their molecular structure information and critical gelation concentration (CGC) in injectable soybean oil (LCT) are listed in . The R1 represents various amino acid residues. R2 group is the amino acid esters in methyl or ethyl. These group mainly defined the number of H-bonding, Van der Waals force or other weak inter-chain interactions that stabilized the gel network. According to our former research [Citation32], Van der Waals force is produced by the different length (carbon atom: 12–20) of the alkyl chain. The longer alkyl chain, the stronger the hydrophobic force formed between the gelators. Therefore, the alkyl chain length of the gelators were all fixed in 20 carbon atoms. The values of c log P among the gelators differ little, which means the effect of hydrophobicity on the gelation ability seems similar to the four gelators.
According to the CGC results, the number of H-bonding determines gelation ability. Among them, C20-Ser-Me shows a CGC value of 0.7%, displaying the best gelation ability among the gelators. This is determined by the number of potential H-bonding sites, as the C20-Ser-Me possesses three sites of H-bonding higher than others. According to the results of C20-Ala-Me and C20-Ala-Et, the effect of the R2 group on gelator ability was depicted. The ethyl group showed a lowered gelation ability, compared with the methyl group. The gelation ability of C20-Ala-Et decreased dramatically, which possibly due to the obstruction of molecular packing. This phenomenon was also observed in the R1 site. The steric bulk of the R1 residues were calculated, and represented as Vm in cm3/mol, and were as follows: Glu (90.53) > Ser (37.21) > Ala (28.64) presented in . C20-Glu-Me shows the highest value of Vm due to its large R1 structure. The CGC value of C20-Glu-Me shows an extraordinarily high value compared with others. As the methyl group in C20-Ala-Me and the hydroxymethyl group in C20-Ser-Me provided smaller steric exclusion during molecular packing, they showed a better gelation ability.
Meanwhile, the thermal stability of gels is listed in . The Tgs of organogels at the concentration of 8% in LCT were compared by different gelators. Different from the CGC results, the Tgs does not show a similar law in the relationship of gelator structure. As the thermal stability is influenced by gelator-gelator interactions and gelator-solvent interactions, the gelator structure is not the only aspect that affects the property of organogels. The solvent’s property should be considered in this process.
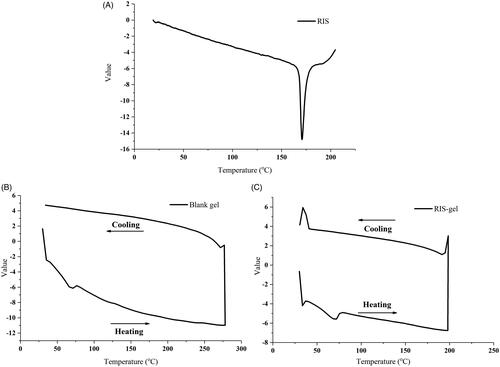
shows the DSC curve of RIS, which has an obvious and sharp endothermic peak at 170.8 °C. This is coincident with its melting point. However, after loaded into the organogels, the melting point disappeared (. The thermograms of RIS-gel does not show any endothermic peak at 170.8 °C, demonstrated that in organogels the RIS presented as a non-crystal state.
Rheology
Rheology can directly show the physical and structural stability of organogels. In this part, we prepared a series of organogels to investigate the relationship of gelator structure, drug and rheological performance. and Citation3 show the rheology of various blank organogels in LCT at gelator concentration of 10%. For all organogels tested, the storage modulus (G′) was higher than the loss modulus (G″) over frequency ranges at 0.5% strain (lower than the yield strain). The loss tangent (tan δ = G″/G′) indicated the organogel strength.
Figure 2. Variation of storage modulus (G′) and loss modulus (G″) of organogel loaded with 0, 5, 10 and 15 mg RIS in C20-Glu-Me organogels. (■ and □ corresponds to the G′ and G″ in 15 mg; ● and ○ corresponds to the G′ and G″ in 10 mg; ▲ and △ corresponds to the G′ and G″ in 5 mg; ◆ and ◇ corresponds to the G′ and G″ in blank organogel).
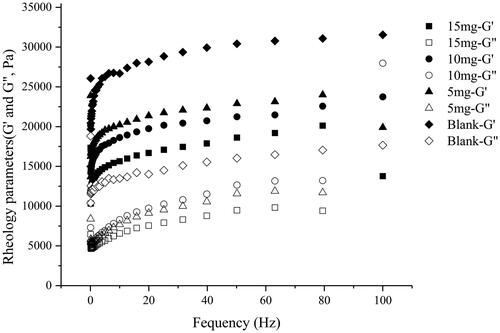
is the rheology profile of C20-Glu-Me organogels loaded with different amount of risperidone. As shown in the profile, G′ was stably higher than the G″ over frequency ranges at 0.5% strain (lower than the yield strain), indicating that the organogels performed a quality of the semi-solid property. The rheology parameters of drug-loaded C20-Glu-Me gels are listed in . Compared with the blank organogels, the parameters such as G′, G″ and η* of drug-loaded organogels show much lower value. According to the formerly reports, the strength of the organogels is mainly affected by the 3 D-network made up by gelator–gelator interaction. The stronger the hydrophobic forces or the more H-bonding formed, the more stable the intermolecular interactions, leading to the 3 D-network more compact, thus forming the gels with strong strength. The gels in this part were prepared by C20-Glu-Me. The formation of the gel network derived from the hydrophobic force of the alkyl chain and the H-bonding site in amide bond. When the drug was loaded in the gel, the H-bonding between gelators could be affected by the O and N atoms in risperidone molecules which were more prone to be the H-bonding accepter. This declined the number of H-bonding formed between gelator molecules, resulting in a sparse network. Thus the gel strength tends to be weak, and the increase of the drug loading aggravate it. Although the G′ and G″ decreased, gels can still be in a strong gel strength to remain in the gel state at 37 °C. This indicates that the gel system can form a stable drug delivery system in vivo.
Table 2. Rhelogy parameters of C20-Glu-Me blank oragnogel and drug-loaded organogel at 37 °C.
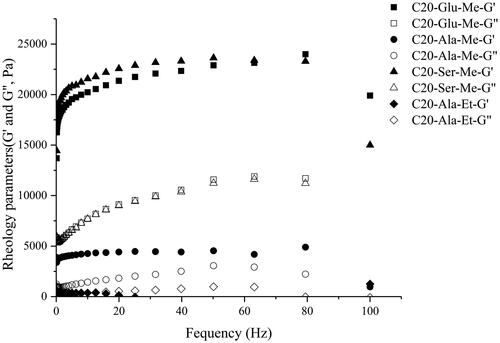
Then, to investigate the influence of gelator structure, we fixed the loading risperidone in 10 mg. shows the gelator structures and the corresponding G′ and G″. According to the rheology profile, C20-Ser-Me shows the strongest gel strength due to the H-bonding interaction upon the hydroxyl group and the better molecular packing. Moreover, compared with C20-Ala-Me, the strength of gels prepared by C20-Ala-Et decreased dramatically to 500 Pa, which is not suitable to be applied in the drug carrier as it could retain its formulation. As the R2 varied in methyl and ethyl, consistent with the gelation ability, the gel strength was also caused by the steric repulsion.
Figure 3. Variation of storage modulus (G′) and loss modulus (G″) of organogel loaded 10 mg RIS prepared by different gelators. (■ and □ corresponds to the G′ and G″ in C20-Glu-Me; ● and ○ corresponds to the G′ and G″ in C20-Ala-Me; ▲ and △ corresponds to the G′ and G″ in C20-Ser-Me; ◆ and ◇ corresponds to the G′ and G″ in C20-Ala-Et).
The morphology of organogels
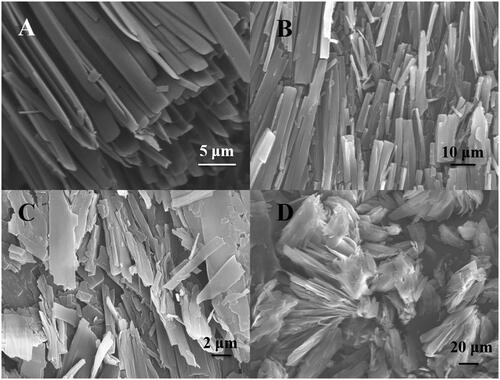
To investigate the influence of gelator structure on its self-assembled morphology, the xerogels of C20-Glu-Me, C20-Ser-Me, C20-Ala-Me, and C20-Ala-Et were prepared. presents SEM images of the four gelators. The importance of the gelator structure on the assembly pattern of the xerogels is evident. The morphology of C20-Ser-Me and C20-Glu-Me showed a similar assemble pattern. The gelators were fibrous parallel assembled. It was found that the self-assembly patterns were similar with the same amino acid residues. In C20-Ala-Me and C20-Ala-Et, they both showed a lamellar structures, disorderly assembled. Meanwhile, in a same amino acid residues, C20-Ala-Et presents a larger cluster compared with C20-Ala-Me. This is probably related with the gelation process. The gel samples were gelated under room temperature, which is a gelator-related process. Consistent with the gelation ability results, C20-Ala-Me has better gelation ability. Thus, it gelate faster, leaving little time for nucleation and fibre branching, which obtained a shorter fibre length.
In vitro drug release evaluation
As a drug carrier, the drug release behaviour is an important aspect. In vitro, drug release behaviour can reflect its sustained-release property, which could be affected by various issues such as the gelator structure, gelator concentration, anti-gelating agent, and drug amount. To obtain the optimum organogel formulation, we chose the Box-Behnken tests by the Design-Expert software to fit the regression model.
Gelator type
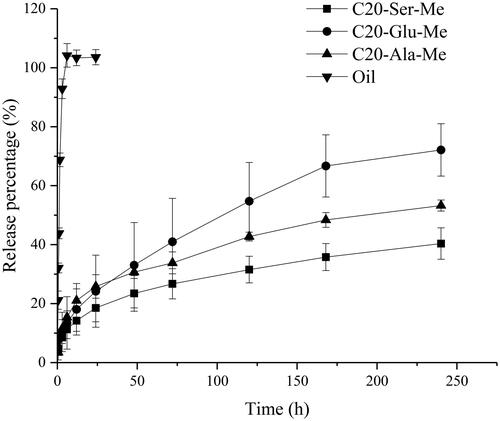
shows the in vitro release profiles of RIS organogels prepared by different gelators. The oil solution of RIS shows a quick release, reaching 100% in 12 h. Compared with the oil solution, the organogel preparations all performed a sustained release tendency, which can extend the release time to over 20 days. Meanwhile, the performance of organogels depends on gelator structure. It was observed that the release rate followed the order C20-Ser-Me < C20-Ala-Me < C20-Glu-Me, consistent with the above CGC and rheology results. As the drug release is a complex process of gel erosion and drug diffusion. Risperidone is a typically lipophilic drug. It can diffuse directly into the release medium from oil phase in the initial period of administration. This lead to the fast release of drug in the early period, which is inevitable and can be detected in the three types of organogels. Gel erosion is a process related to gelator type and solvent property [Citation29,Citation30], this process is the common method to manipulate the release rate of the drug carrier. As the gelator structure directly related to the drug release process, the release properties of organogels could be adjusted by the varying the gelator structure, providing a theoretical basis for controllable drug delivery implants.
Gelator concentration
Gelator concentration is another factor that affects drug release. According to the results of the gelator type, we prepared different concentration of C20-Ser-Me organogels to investigate the influence of gelator concentration on drug release. is the release profile of RIS organogels prepared by C20-Ser-Me at different concentrations.
Figure 6. The release profile of drug from RIS organogels prepared by C20-Ser-Me at different concentrations.
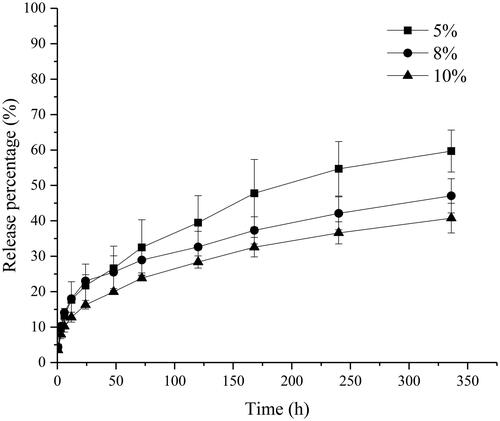
With the increase of the gelator concentration, the release rate of the RIS decrease. In higher concentration, the gelator number increased in per unit volume, resulting in a dense and compact 3 D-network. Thus the drug will encounter greater resistance during the release process. Furthermore, the burst release among different concentrations shows concentration relativity. With the gelator concentration increased, the burst release weakened, which could be related to the gelation time. Combining the above results of gelation time, gelation time shows obvious concentration dependence. This means that the burst release appeared higher in low concentration.
Amount of anti-gelating agent
The drug delivery system in this paper was prepared by the oil, gelator, and drug. To complete the administration process successfully, a kind of anti-gelation agent need be added to the system, such as ethanol, DMSO or NMP, which were some hydrophilic solvent that can interrupt the formation of a 3 D network of gelators. As the gelator can easily gelate the oil phase into a semisolid state in room temperature for thermogel, this phenomenon hindered the administration process. To obtain a liquid system before administration, the amount of anti-gelating agent were added. After administration, the anti-gelation solvent can quickly diffuse into the surrounding tissues leaving the remaining form organogels. While the addition of anti-gelating agent leads to another problem in the application. It extends the gelation time after administration, resulting in the burst release of the drug. It can interrupt the formation of the 3 D-network before administration, while diffuse instantly into the surrounding tissues after administration. This process is directly related to the burst release of injectable organogels.
In this study, we chose NMP as the anti-gelation agent, which could make the gel in a sol state under room temperature. shows the release profile of RIS prepared by C20-Ser-Me organogels with different amount of NMP.
Figure 7. The release profile of drug from RIS organogels prepared by C20-Ser-Me with different amount of NMP.
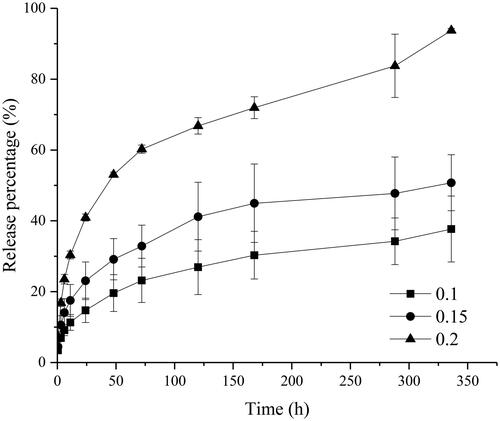
As shown in , with the amount of NMP increase, the burst release aggravated. When the amount of the NMP was 20% (v/v), the burst release of risperidone reached 40% after 24 h, apparently higher than the formulation of 15% (23%) and 10% (14%). As the anti-gelation agent, NMP controls the gelation by interrupting the formation of H-bonds among gelators. The more it been added, the greater impact it acted on the gelator. Thus it takes a long time to diffuse into surroundings, extending the gelation time. In this period, many drugs will escape to the surroundings. As for the in vivo implants, the burst release is inevitable and vital to their quality. For some drug, the burst release can quickly achieve the therapeutic concentration through the burst release. While for the most drugs with a narrow therapeutic window, the burst release can lead to a different degree of toxicity and side effects. On the whole, in the designation of preparations, the amount of NMP should be considered comprehensively.
The amount of drug loading
The amount of drug loading in organogels is another factor that could affect the rate of the drug release. presents the release profile of drug from organogels prepared by C20-Ser-Me with different amount of RIS.
Figure 8. The release profile of drug from organogels prepared by C20-Ser-Me with different amount of RIS.
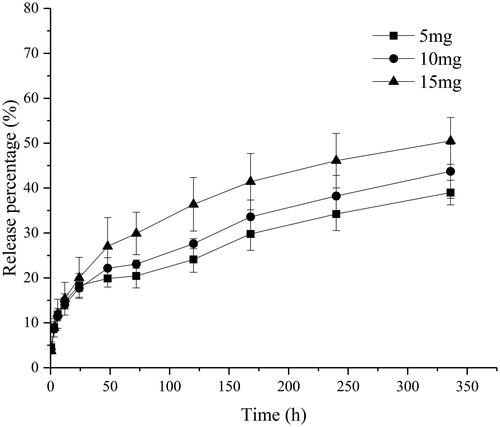
As shown in , the release behaviour could be affected by the amount of drug loading. As the amount of the NMP is identical, the burst release appears similar among the three formulations. Along with the drug release, the preparation of 15 mg RIS shows a faster release rate, which cumulative release of risperidone reached 50.5% after 336 h, larger than the other two dosage formulations. As for the dosage of 5 mg and 10 mg, the release profile presented a similar tendency, indicating that the organogel shows a similar sustained-release ability in a certain range of the drug loading amount.
Optimization
To formulate the optimal organogel prescription, design expert 6.0.4 software was applied based on response surface methodology (RSM) to screen a formulation with low burst release and long release time. CCD Design involves three variables: amounts of gelator (X1), NMP (X2) and drug RIS (X3). Their levels and ranges were listed in . To optimize the drug release, cumulative release of RIS in 24 h (Y24h) and 15 days (Y14d) were chosen as the response values.
Table 3. Independent variables and their levels in codes and physical form.
shows the design layout of the corresponding runs. According to the data processed by the BBD, two mathematical models were obtained. EquationEquations (2)(2)
(2) and Equation(3)
(3)
(3) were the mathematical model in 24 h (R24h) and 14 days (R14d) of the RIS release from organogels. According to EquationEquation (2)
(2)
(2) , the drug release in 24 h follows a linear model. The coefficient of NMP values in the model performs a highly positive on the response, which means a greater influence on the release results at 24 h. While for 14 days, it tends to be a quadratic model suggested by the software. After deleting some coefficient with poor fitness, we obtained the modified model. According to EquationEquation (3)
(3)
(3) , the drug release in 14 days depends on the NMP value and gelator concentration mostly.
Table 4. The response values for the different levels of experimental design.
From EquationEquations (2)(2)
(2) and Equation(3)
(3)
(3) , the optimum conditions to produce lower drug release organogels were listed in . The optimisation criteria have two options: minimum R24h and R14d values for the lower burst release and longer drug release time. According to the above results, the optimum formula contained gelator (100 mg), NMP (0.1 ml) and RIS (14.32 mg).
(2)
(2)
(3)
(3)
X1, X2, X3-different amounts of C20-Ser-Me, NMP and RIS.R24h and R14d represent the drug release percent at 24 h and 14 d.
In vivo pharmacokinetic study
To investigate the in vivo performance of organogels in this study, the organogels prepared by the optimal formulation and the oily drug solution were subcutaneously injected in rats. shows the in vivo plasma concentration-time curves of RIS oily solution and corresponding organogel formulation. lists the pharmacokinetic parameters obtained from plasma concentration-time curves. As shown in , in vivo release of the two preparations, have different performance. The plasma concentration of RIS in oily solution reached 0 in 12 h indicating the complete release from oil. Meanwhile, the organogel formulation could maintain a sustained release in 7 days. After injection, both preparations have a significant increase in drug concentration in plasma as the RIS is a BCS class II, low solubility and high permeability drug. Compared to its oil solution, the organogel shows a significant (p<.05) prolongation of the release time, which Cmax of 3.628 μg/mL at Tmax of 0.313 h vs. 6.961 μg/mL at 0.5 h, respectively. As the drug loading of the organogel is four-fold than oil solution, the Cmax of organogel formulation exceeded only by about two-fold than that of the oily solution. The AUC(0–t) values of RIS organogel formulation exceeded by about 29-fold than that of the oily solution and the MRT prolonged from 1.48 to 58.4 h.
Figure 9. In vivo plasma concentration–time curves of drug oily solution (■) and organogel formulation (●).
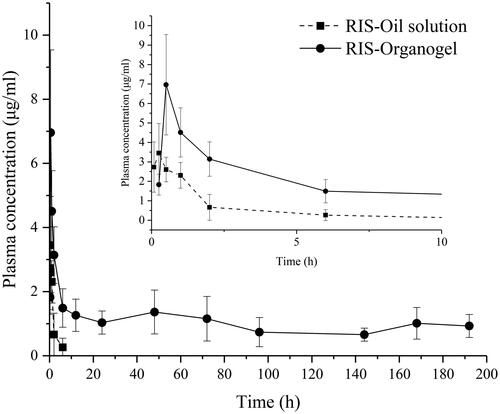
Table 5. Pharmacokinetic parameters after subcutaneous administration of RIS oil solution and organogel.
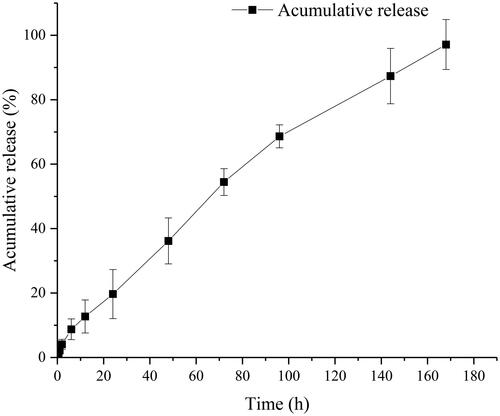
As the subcutaneous preparation can avoid the first-pass effect, the bioavailability can be regarded as 100%. The in vivo cumulative release-time curve was shown in . The release curve fitted Riger–Peppas model very well (r = 0.9919). The diffusion index was 0.7736, indicating that the release mechanism was determined by both drug diffusion and frame erosion. A content of burst release can be seen in , as the organogel formation depends on the process of NMP diffusion, which could lead to the release of oil-carrying with the drug.
Conclusion
In this study, based on the amino acid derives gelators, their gelation, gel strength, in vitro and in vivo drug release properties were investigated. The gel properties significantly depend on the chemical structure of the organogelators. Understanding the role of gelator structure and solvent parameter helps in elucidating how the organogel property was affected, such as gelation ability, gel strength, and especially the drug release. The drug-loaded properties of the organogel have been utilised for preparing a formulation with a model lipophilic risperidone, to finding out their influences on the properties of the organogels and the release mechanism. The results indicated that the drugs had the deep influence to the properties of organogels, such as the thermal stability decreased with a lower transition temperature and the mechanism of organgoel was also weakened as the values of G′ and G″ was much small. The factors which may affect release profiles were investigated, such as gelator structure, concentration, the amount of drug, and gelation inhibitor. Based on the single factor investigation and CCD experiment, the optimal formulation was investigated. According to the optimal formulation, the RIS-loaded organogel was prepared, and then studied the in vitro and in vivo behaviour and explored the nature of the sustained-release drug delivery and release principle of organogel. The in vitro results showed that RIS-loaded organogel prolonged the period of drug release obviously, which could provide a sustained release up to 20 days. Comparing with the drug oil solution, the blood drug concentration was prolonged from 1 day to 7 days. In addition, the release profile of organogel formulation was fitted to the Ritger–Peppas release model and showed that the release behaviour was a combined action of drug diffusion and erosion. It can be concluded that the in situ organogel based on amino acid derivatives was biocompatible and biodegradable. The relationship between gel properties, gelator structures, and release behaviours of organogels was investigated, which provided a scientific basis for the design and further optimisation of the organogel preparation.
Supplementary_File.docx
Download MS Word (36.6 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Wang C, Li Z, Wang X, et al. Gelation mechanism and microstructure of organogels formed with L-Valine dihydrazide derivatives. Colloid Surf A. 2011;384(1–3):490–495.
- Su MM, Yang HK, Ren LJ, et al. Solvent-mediated gel formation, hierarchical structures, and rheological properties of organogels. Soft Matter. 2015;11(4):741–748.
- Martin-Illana A, Cazorla-Luna R, Notario-Perez F, et al. Freeze-dried bioadhesive vaginal bigels for controlled release of Tenofovir. Eur J Pharm Sci. 2019;127:38–51.
- Singh VK, Pramanik K, Ray SS, et al. Development and characterization of sorbitan monostearate and sesame oil-based organogels for topical delivery of antimicrobials. AAPS PharmSciTech. 2015;16:293–305.
- Behera B, Sagiri SS, Pal K, et al. Modulating the physical properties of sunflower oil and sorbitan monopalmitate‐based organogels. J Appl Polym Sci. 2013;127(6):4910–4917.
- Sravan B, Kamalakar K, Karuna MSL, et al. Studies on organogelation of self assembling bis urea type low molecular weight molecules. J Sol-Gel Sci Technol. 2014;71(2):372–379.
- Cao X, Zhao X, Gao A, et al. Organogel formation based on bis-urea derivative. Supramol Chem. 2014;26(10–12):804–808.
- Wang D, Zhao J, Liu X, et al. Parenteral thermo-sensitive organogel for schizophrenia therapy, in vitro and in vivo evaluation. Eur J Pharm Sci. 2014;60:40–48.
- Sagiri S, Behera B, Rafanan R, et al. Organogels as matrices for controlled drug delivery: a review on the current state. Soft Mater. 2014;12(1):47–72.
- Jiao T, Wang Y, Zhang Q, et al. Regulation of substituent groups on morphologies and self-assembly of organogels based on some azobenzene imide derivatives. Nanoscale Res Lett. 2013;8(1):1–8.
- Pal A, Dey J. Gelation of organic solvents by N-(n-tetradecylcarbamoyl)-l-amino acids. Supramol Chem. 2015;27(1–2):127–135.
- Vigato AA, Querobino SM, de Faria NC, et al. Synthesis and characterization of nanostructured lipid-poloxamer organogels for enhanced skin local anesthesia. Eur J Pharm Sci. 2019;128:270–278.
- Wang K, Jia Q, Han F, et al. Self-assembled L-alanine derivative organogel as in situ drug delivery implant: characterization, biodegradability, and biocompatibility. Drug Dev Ind Pharm. 2010;36(12):1511–1521.
- Samai S, Dey J, Biradha K. Amino acid based low-molecular-weight tris (bis-amido) organogelators. Soft Matter. 2011;7(5):2121–2126.
- Bastiat G, Plourde F, Motulsky A, et al. Tyrosine-based rivastigmine-loaded organogels in the treatment of Alzheimer's disease. Biomaterials. 2010;31(23):6031–6038.
- Vintiloiu A, Lafleur M, Bastiat G, et al. In situ-forming oleogel implant for rivastigmine delivery. Pharm Res. 2008;25(4):845–852.
- Dou QQ, Liow SS, Ye E, et al. Biodegradable thermogelling polymers: working towards clinical applications. Adv Healthcare Mater. 2014;3(7):977–988.
- Jangdey MS, Kaur CD, Saraf S. Efficacy of Concanavalin-A conjugated nanotransfersomal gel of apigenin for enhanced targeted delivery of UV induced skin malignant melanoma. Artif Cells Nanomed Biotechnol. 2019;47(1):904–916.
- Hatefi A, Amsden B. Biodegradable injectable in situ forming drug delivery systems. J Control Release. 2002;80(1-3):9–28.
- Zhang T, Luo J, Peng Q, et al. Injectable and biodegradable phospholipid-based phase separation gel for sustained delivery of insulin. Colloids Surf B Biointerfaces. 2019;176:194–201.
- Chaulagain B, Jain A, Tiwari A, et al. Passive delivery of protein drugs through transdermal route. Artif Cells Nanomed Biotechnol. 2018;46(sup1):472–487.
- Yang Y, Xu L, Gao Y, et al. Improved initial burst of estradiol organogel as long-term in situ drug delivery implant: formulation, in vitro and in vivo characterization. Drug Dev Ind Pharm. 2012;38(5):550–556.
- Ohsedo Y, Taniguchi M, Oono M, et al. Creation of thixotropic multicomponent alkylamide organogels containing non-volatile oil as potential drug release host materials. RSC Adv. 2014;4(67):35484–35488.
- Bae KH, Lee F, Xu K, et al. Microstructured dextran hydrogels for burst-free sustained release of PEGylated protein drugs. Biomaterials. 2015;63:146–157.
- Thedrattanawong C, Manaspon C, Nasongkla N. Controlling the burst release of doxorubicin from polymeric depots via adjusting hydrophobic/hydrophilic properties. J Drug Deliv Sci Tec. 2018;46:446–451.
- Geng Z, Luo X, Zhang Z, et al. Study of an injectable in situ forming gel for sustained-release of Ivermectin in vitro and in vivo. Int J Biol Macromol. 2016;85:271–276.
- Din FU, Kim DW, Choi JY, et al. Irinotecan-loaded double-reversible thermogel with improved antitumor efficacy without initial burst effect and toxicity for intramuscular administration. Acta Biomater. 2017;54:239–248.
- Li Y, Angelova A, Liu J, et al. In situ phase transition of microemulsions for parenteral injection yielding lyotropic liquid crystalline carriers of the antitumor drug bufalin. Colloids Surf B Biointerfaces. 2019;173:217–225.
- Hu B, Wang W, Wang Y, et al. Degradation of glutamate-based organogels for biodegradable implants: in vitro study and in vivo observation. Mater Sci Eng C Mater Biol Appl. 2018;82:80–90.
- Li Z, Cao J, Hu B, et al. Studies on the in vitro and in vivo degradation behavior of amino acid derivative-based organogels. Drug Dev Ind Pharm. 2016;42(11):1732–1741.
- Li Z, Cao J, Li H, et al. Self-assembled drug delivery system based on low-molecular-weight bis-amide organogelator: synthesis, properties and in vivo evaluation. Drug Deliv. 2016;23(8):3168–3178.
- Hu B, Sun W, Li H, et al. Systematic modifications of amino acid-based organogelators for the investigation of structure-property correlations in drug delivery system. Int J Pharm. 2018;547(1–2):637–647.
- Qiao Z, Yuan Z, Zhang W, et al. Preparation, in vitro release and antibacterial activity evaluation of rifampicin and moxifloxacin-loaded poly(D,L-lactide-co-glycolide) microspheres. Artif Cells Nanomed Biotechnol. 2019;47(1):790–798.
- USP. Semisolid drug products–performance tests. USP 37/NF 32. Rockville, USA: The United States Pharmacopeial Convention; 2014. p. 1273–1284.
- Fachel FNS, Medeiros-Neves B, Dal Pra M, et al. Box-Behnken design optimization of mucoadhesive chitosan-coated nanoemulsions for rosmarinic acid nasal delivery-in vitro studies. Carbohydr Polym. 2018;199:572–582.
- Sabbagh F, Muhamad II, Nazari Z, et al. From formulation of acrylamide-based hydrogels to their optimization for drug release using response surface methodology. Mater Sci Eng C Mater Biol Appl. 2018;92:20–25.