Abstract
Increasing studies have demonstrated that microRNAs (miRNAs) are associated with the metastasis of gallbladder carcinoma (GBC). Recently, miR-324-5p has been reported to be a tumour-suppressive miRNA in many types of malignant cancer. However, the biological function and molecular mechanism of miR-324-5p in GBC still remain largely unknown. Here, we found that miR-324-5p expression was notably down-regulated in both GBC tissues and cells compared with that in normal controls. Downregulated miR-324-5p expression was negatively associated with the status of local invasion and lymph node metastasis and predicted a poor prognosis in GBC patients. Further functional assays revealed that restoration of miR-324-5p significantly suppressed GBC cell migration, invasion and epithelial-mesenchymal transition (EMT) in vitro and impeded the metastasis of GBC cells in vivo. Moreover, RNA immunoprecipitation (RIP) and dual-luciferase reporter assay confirmed that the transforming growth factor beta 2 (TGFB2) was a direct target gene of miR-324-5p in GBC cells. Mechanically, small interfering RNA (siRNA)-mediated knockdown of TGFB2 partially phenocopied the inhibitory effects of miR-324-5p overexpression on GBC cell metastatic phenotypes. In summary, our findings demonstrated that miR-324-5p targets TGFB2 expression to inhibit GBC cell metastatic behaviors, and implying miR-324-5p as a potential biomarker for diagnostic and therapeutic strategies in GBC.
Keywords:
Introduction
Gallbladder carcinoma (GBC) is one of the most lethal cancers and the most common bile duct malignancy worldwide [Citation1,Citation2]. The early diagnosis of GBC is very difficult because of the lack of specific clinical symptoms, and a large number of cases with GBC are diagnosed at its advanced stage with serious metastasis [Citation3]. At present, the high rate of tumour recurrence and chemoresistance are the most notable characteristic of GBC and the prognosis of GBC patients with 5-year survival rates of approximately 15% [Citation4,Citation5]. Although diagnostic and surgical treatment methods have been developed over the past decades, however, little success has been achieved in reducing the mortality rates of GBC [Citation6]. Therefore, it is imperative to investigate the molecular and biological mechanisms underlying GBC metastasis, which may help to identify new diagnostic and therapeutic targets to improve prognosis.
MicroRNAs (miRNAs) are class of non-coding RNAs, which are consisted of 18–25 nucleotides and negatively regulate gene expression by binding to the 3′-untranslated region (3′-UTR) of the mRNA of the target gene at the post-transcriptional level [Citation7]. Recently, a number of researches have shown that aberrant expression of miRNAs plays an important role in the regulation of carcinogenesis and metastasis in various types of malignancies [Citation8]. For example, Luo et al. showed that miR-222 promotes colorectal cancer cell migration and invasion by targeting mammalian STE20-like protein kinase 3 [Citation9]. Zhang et al. revealed that miR-216a-5p acts as a tumour suppressor in regulating cell proliferation and metastasis by targeting p21-activated protein kinase 2 gene in breast cancer [Citation10]. Mou et al. showed that miR-345-5p functions as a tumour suppressor in pancreatic cancer by directly targeting C-C motif chemokine ligand 8 [Citation11]. A recent report demonstrated that overexpression of miR-448 inhibits cell proliferation, metastasis and epithelial-mesenchymal transition (EMT) by suppressing insulin receptor substrate 2 expressions in non-small-cell lung cancer [Citation12]. However, the understanding of the roles and mechanisms of miRNAs in the GBC is still in the early stage.
In the present study, the special roles of miR-324-5p in different human cancers have caught our attention. First, decreased miR-324-5p expression had been identified in glioma [Citation13], hepatocellular carcinoma (HCC) [Citation14] and colorectal cancer [Citation15]. These findings have shown that miR-324-5p plays a suppressive role in the progression of human cancers. More importantly, Jiang et al. reported that long non-coding RNA TPT1-AS1 promotes cell growth and metastasis in cervical cancer via acting as a sponge for miR-324-5p [Citation16]. In colorectal cancer, up-regulation of miR-324-5p-mediated tumour cell viability and invasion inhibition was observed [Citation15]. Furthermore, it is well known that EMT is an important process affecting tumour metastasis in human cancers [Citation17,Citation18]. However, the role and mechanism of miR-324-5p in GBC remain unclear. Therefore, we investigated the role of miR-324-5p in GBC, and mainly focussing on cell metastasis and EMT. Here, we first examined the expression profiles of miR-324-5p in GBC tissues and investigated its clinical values. Furthermore, the role of miR-324-5p on GBC cell metastatic behaviours and the underlying molecular mechanism were identified. Our findings suggested that miR-324-5p may serve a new diagnosis and therapeutic target for GBC.
Materials and methods
Cell lines and cultures
Human GBC cell lines (GBC-SD and SGC-996) were bought from the Cell Bank of the Chinese Academy of Science (Shanghai, China) and authenticated though STR typing. GBC cell line NOZ and the non-tumorigenic human intrahepatic biliary epithelial cell line H69 were purchased from the Health Science Research Resources Bank (Osaka, Japan). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM, HyClone, CA, USA) medium supplemented with 10% foetal bovine serum (FBS, HyClone), 100 µg/ml penicillin (Invitrogen, CA, USA) and 100 µg/ml streptomycin. Cells were humidified in an atmosphere with 5% CO2 at 37 °C. The cells in the logarithmic growth period were used for further experiments.
Cell transfection
The miR-324-5p mimics, mimics negative control (mimics NC), transforming growth factor beta 2 (TGFB2) small interfering RNA (si-TGFB2) and control siRNA (si-NC) were synthesized and purified by RiboBio Co., Ltd. (Guangzhou, China). For cell transfection, GBC-SD and SGC-996 cells (2.0 × 106 per well) were seeded and grown overnight in six-well plates. The next day, transfection was performed using Lipofectamine™ 2000 reagent (Life Technologies, CA, USA) following manufacturer’s instructions. The final concentration of miR-324-5p mimics or mimics NC was 100 nM and the si-TGFB2 or si-NC was 50 pmol. The transfected cells were incubated for 6 h in serum-free DMEM, and then normal medium was added and incubated for another 48 h.
Expression of miR-324-5p and TGFB2 was detected before each experiment by quantitative RT-PCR assay or Western blot analysis. Subsequent functional experiments were only performed in case of overexpression rate above 20 times and knockdown rate below 50% compared with control groups.
RNA extraction and quantitative RT-PCR assay
Total RNA was prepared from GBC tissues and cells using a TRIZOL reagent (TaKaRa, Beijing, China). Then, complementary DNA (cDNA) was obtained using a First-Strand cDNA Synthesis kit (TaKaRa) according to the manufacturer’s protocols. Quantitative PCR assay was applied on an ABI 7500 system (Applied Biosystems, CA, USA) using a SYBR Premix Ex TaqTM II kit (TaKaRa). The gene expression levels of miR-324-5p and TGFB2 were normalized to the endogenous reference gene U6 small nuclear RNA (U6) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), respectively. The primers for miR-324-5p and U6 were provided by TaKaRa, and primers for TGFB2 and GAPDH were synthesized and purchased from Sangon Biotech (Shanghai, China). The primer sequences are as follows: TGFB2 forward 5′-TGGTGAAAGCAGAGTTCAG-3′, reverse 5′-CACAACTTTGCTGTCGATGT-3′; and GAPDH forward 5′-GAGTCAACGGATTTGGTCG-3′, reverse: 5′-AATGAAGGGGTCATTGATG-3′. The experiments were performed in triplicate, the relative expression fold changes were calculated by the 2−ΔΔCt method [Citation19].
Specimen collection
Paired GBC tissues and adjacent normal tissues were collected from 35 GBC patients who underwent surgical resection from March 2011 to June 2015 at Tianjin Nankai Hospital (Tianjin, China). After surgical removal, the tissues were immediately frozen using liquid nitrogen and stored at −80 °C. The 35 GBC patients included 13 women and 22 men with an average age of 54.26 years (range from 34–71 years). All patients with GBC did not receive radiotherapy or chemotherapy before surgery. The experiment was approved by Institutional Ethics Committee of Tianjin Nankai Hospital. Signed informed consent was obtained from all participants before the study.
Dual-luciferase reporter assay
TargetScan [Citation20], miRBase [Citation21] and miRanda [Citation22] databases were used to predict the target mRNAs of miR-324-5p. Those consistently identified by the three databases were regarded as potential target mRNAs. According to bioinformatics prediction, the 3′-UTR of wild or mutant TGFB2 was synthesized by RiboBio Co., Ltd. and inserted into a pmirGLO luciferase vector (Promega, WI, USA) for dual-luciferase reporter assay. The GBC-SD and SGC-996 cells were seeded onto 24-well plates (4.0 × 105 per well) and co-transfected with 50 nM miR-324-5p mimics or mimics NC and 200 ng recombinant reporter plasmids by using Lipofectamine™ 2000 reagent (Life Technologies), according to the manufacturer’s protocols. After transfection 24 h, the luciferase activities were detected using a dual-luciferase reporter system (Promega) according to the manufacturer’s instructions in a TD-20/20 luminometer (Turner Designs, Sunnyvale, CA, USA).
RNA immunoprecipitation (RIP) assay
Magna RIP™ RNA-Binding Protein Immunoprecipitation Kit (#17-701; Millipore, CA, USA) was applied to evaluate the interaction between miR-324-5p and TGFB2. In brief, the lysate of SGC-996 cells transfected with miR-324-5p mimics or mimics NC was incubated with protein A/G magnetic beads and anti-Ago2 (argonaute RISC catalytic component 2) (#ab32381; Abcam, Cambridge, MA, USA; 1:1000) antibody, and anti-IgG (#ab133470; Abcam; 1:1000) antibody was used as a negative control. After washing, the RNA in the immunoprecipitated complex was purified using RNase-free DNase I and proteinase K (Life Technologies, CA, USA), followed by the detection of TGFB2 mRNA expression by quantitative RT-PCR assay.
Western blot analysis
Total proteins were extracted from GBC cells using a RIPA buffer (Life Technologies) and quantified using a Modified Bradford reagent (Sangon Biotech). Then, the protein was isolated via 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE, Sangon Biotech) and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore). The membranes were blocked with Tris-buffered saline with Tween-20 (TBST) containing 5% skimmed milk at 37 °C for 1 h. The membranes were then incubated with the corresponding rabbit anti-human TGFB2 (#ab113670; Abcam; 1:500), E-cadherin (#3195; Cell Signaling Technology, Danvers, MA, USA; 1:1000), Vimentin (#5741; Cell Signaling Technology; 1:2000), N-cadherin (#13116; Cell Signaling Technology; 1:1000) and GAPDH (#5174; Cell Signaling Technology; 1:2500) antibodies at 4 °C overnight. On the next day, the membranes were washed with TBST three times and incubated with horseradish peroxidase (HRP) to mark a goat anti-rabbit IgG antibody (Sangon Biotech) for 1 h at 37 °C. The positive bands were visualized using enhanced chemiluminescence (Beyotime Biotechnology, Shanghai, China).
Cell migration and invasion assay
The migration ability of GBC cells was assessed through transwell chambers without matrigel (Corning, NY, USA). For cell invasion assay, before the cells were seeded, the matrigel was mixed with DMEM at a 1:8 ratio and placed on the upper surface of Corning Costar Transwell 24-well plates (Corning). After being washed with PBS, the transfected GBC-SD and SGC-996 cells were detached with trypsin and resuspended in serum-free medium. 200 µl of DMEM without FBS and 2 × 105 cells were added to the upper chamber. 500 µl of DMEM with 20% FBS was added in the lower chamber as a chemoattractant. After incubation for 24 h at 37 °C, the cells remaining in the upper chamber were removed using cotton swabs, while the cells migrating or invade to the lower chambers were fixed with 100% methanol (Sigma-Aldrich, MO, USA) and stained with 0.2% crystal violet (Sigma-Aldrich). Then, an Olympus BX51 microscope (Olympus, Japan) at 200× magnification was used to detect the images of migratory or invasive cells and count their number in five random views.
In vivo metastasis assay
Male BALB/c nude mice (4–6 weeks old) were obtained from Model Animal Research Center of Nanjing University (Nanjing, China) and randomly divided into four groups (10 mice per group). All mice were housed and maintained in laminar airflow cabinets under specific pathogen-free conditions. All procedures were conducted in accordance with the Guidelines for the Care and Use of Laboratory Animals with the approval of the Ethics Committee of the Tianjin Nankai Hospital. To assess the effect of miR-324-5p on metastasis, liver metastasis model was established with intrasplenic injection of 1 × 106 stably transfected SGC-996 cells. Mice were allowed to grow for 6 weeks, and then the mice were sacrificed, and the livers were resected. The micrometastases in the liver were fixed in formalin and sectioned for H&E staining, and a number of liver metastatic foci was counted.
Statistical analysis
Statistical analysis was analysed using the SPSS program (version 17.0; SPSS Inc., Chicago, IL, USA). Each experiment was repeated at least three times. For continuous variables, measurement data were showed as the mean and SD. Student’s t-test or one-way ANOVA followed by post-hoc test were used to analyse the difference among/between sample groups. Clinical pathology parameters were compared using the χ2 test. Survival curves were generated using the Kaplan–Meier method and log-rank tests. The values of p < .05 were considered to be statistically significant.
Results
Reduced expression levels of miR-324-5p in both GBC tissues and cells
To examine whether miR-324-5p was connected with the progression of GBC, we used a quantitative RT-PCR assay to determine the relative expression of miR-324-5p in GBC tissues and adjacent normal tissues. Of the 35 patients, miR-324-5p expression was decreased in 32 GBC tissues (32/35, 91.43%) compared with adjacent normal tissues (). Statistical analysis showed that miR-324-5p was significantly down-regulated in GBC tissues compared with the adjacent normal tissues (, p < .05). Furthermore, we also evaluated the expression of miR-324-5p in various GBC cell lines and the non-tumorigenic human intrahepatic biliary epithelial cell line H69. As shown in , miR-324-5p was markedly decreased in GBC cell lines, such as GBC-SD, SGC-996 and NOZ, compared with H69 cell line (p < .05).
Figure 1. miR-324-5p is downregulated in gallbladder carcinoma (GBC) tissues and cell lines. (A) miR-324-5p expression was validated by quantitative RT-PCR assay in 35 paired GBC tissues and adjacent normal tissues. (B) miR-324-5p was significantly downregulated in GBC tissues compared with the adjacent normal tissues. (C) miR-324-5p was markedly decreased in GBC cell lines, such as GBC-SD, SGC-996 and NOZ, compared with H69 cell line. (D) Decreased miR-324-5p expression was significantly associated with poor overall survival in GBC patients. Data represented the mean and SD of three independent experiments. *p < .05.
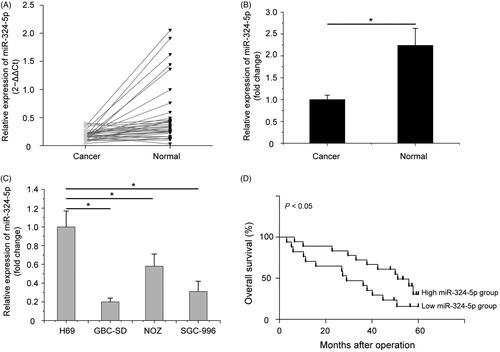
Subsequently, we also analyzed the relationship between miR-324-5p expression and clinical features of patients with GBC in this study. Based on the median value of miR-324-5p expression levels in GBC tissues as a cut-off point, these GBC patients were divided into high miR-324-5p expression group and low miR-324-5p expression group. As shown in , down-regulated miR-324-5p expression was statistically associated with local invasion (p = .001) and lymph node metastasis (p = .042). There was no significant difference in age, gender, tumour size, histological grade, T categories and UICC stages between high and low levels of miR-324-5p expression. In addition, to investigate the value of miR-324-5p expression in the prognosis of GBC patients, Kaplan–Meier survival analysis and log-rank tests were adopted. The results indicated that the downregulation of miR-324-5p predicted a poor prognosis in GBC patients (, p < .05). Altogether, we speculated that reduced expression of miR-324-5p may be involved in the metastasis of GBC.
Table 1. The association of miR-324-5p expression in 35 GBC patients with clinicopathologic characteristics.
Restoration of miR-324-5p suppresses cell migration, invasion and EMT in vitro and impedes the metastasis of GBC cells in vivo
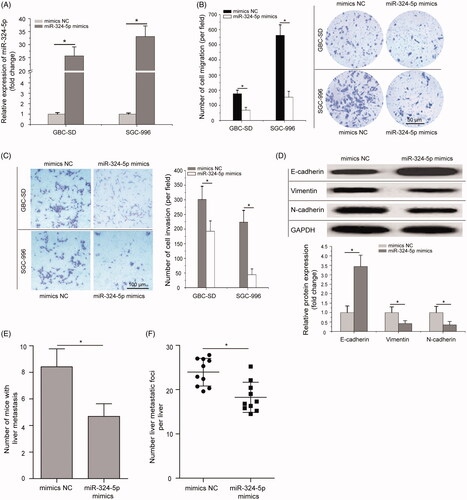
To explore the biological function of miR-324-5p in GBC cell lines, GBC-SD and SGC-996 cells which have the lowest miR-324-5p expression among the three GBC cell lines (GBC-SD, SGC-996 and NOZ), were transfected with the miR-324-5p mimics or mimics NC to induce upregulation of miR-324-5p expression. As shown in , miR-324-5p mimics could efficiently increase the endogenous miR-324-5p expression in GBC-SD and SGC-996 cells compared to the mimics NC (p < .05). The effects of miR-324-5p on cell migration and invasion were analyzed in GBC cells by transwell assays. Compared with the controls, miR-324-5p overexpression significantly inhibited GBC-SD and SGC-996 cells migration (, p < .05). Similarly, miR-324-5p overexpression also dramatically suppressed the invasion of GBC-SD and SGC-996 cells in transwell invasion assay compared to the mimics NC groups (, p < .05). Furthermore, The effect of miR-324-5p on EMT was investigated in SGC-996 cells by Western blot analysis. The results showed that overexpression of miR-324-5p enhanced E-cadherin expression and suppressed the expression of N-cadherin and Vimentin (, p < .05). To investigate the effect of miR-324-5p on GBC metastasis, miR-324-5p stably overexpressed SGC-996 cells were injected through the spleen to establish liver metastasis model in nude mice. Results revealed that ectopic expression of miR-324-5p decreased liver metastatic foci formed by SGC-996 cells (, p < .05). All these data suggested that miR-324-5p could inhibit GBC cell metastatic behaviours.
Figure 2. Overexpression of miR-324-5p inhibits cell migration, invasion and EMT in vitro and impedes the metastasis of GBC cells in vivo. (A) GBC-SD and SGC-996 cells were transfected with miR-324-5p mimics or mimics negative control (mimics NC), and the expression level of miR-324-5p was analyzed by quantitative RT-PCR assay after transfection. (B) Transwell migration assay was performed to compare the cell migration of miR-324-5p overexpression in GBC-SD and SGC-996 cells (200×). (C) Transwell invasion assay demonstrated the suppressive cell invasion ability of miR-324-5p mimics compared to the mimics NC in GBC-SD and SGC-996 cells (200×). (D) The protein levels of E-cadherin, N-cadherin, and Vimentin in SGC-996 cells were determined by Western blot analysis after transfected with miR-324-5p mimics or mimics NC. (E) Number of liver metastasis at the 42nd day after mice following intra-splenic injection of stably transfected with miR-324-5p mimics or mimics NC. (F) H&E staining of the metastatic foci in the liver, and number of metastatic foci per liver was calculated and compared. All experiments were performed three times and data were presented as mean ± SD, *p < .05.
The direct regulation of miR-324-5p on the mRNA 3′-UTR of TGFB2
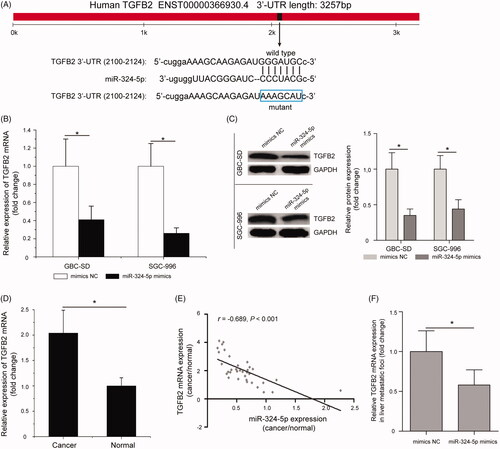
As miRNAs function mainly by inhibiting its target genes, the targets of miR-324-5p in GBC were predicted using TargetScan, miRBase and miRanda databases. Bioinformatics analysis showed that TGFB2 was a potential target of miR-324-5p, complementary sequences existed between miR-324-5p and the mRNA 3′-UTR of TGFB2 (). Among the identified candidates, we selected TGFB2 for subsequent analyses, and previous evidences have demonstrated its involvement in many human malignancies [Citation23]. Quantitative RT-PCR assay and Western blot analysis were applied to examine whether miR-324-5p affects the expression of TGFB2. We found that overexpression of miR-324-5p in GBC-SD and SGC-996 cell lines significantly decreased TGFB2 mRNA and protein expression compared with cells treated with the mimics NC groups (, p < .05).
Figure 3. Restoration of miR-324-5p suppresses the expression of transforming growth factor beta 2 (TGFB2) in GBC cells. (A) Schematic of the mRNA 3′-untranslated region (3′-UTR) of TGFB2 containing the miR-324-5p binding sites. (B) Quantitative RT-PCR assay showed that overexpression of miR-324-5p in GBC cell lines significantly decreased TGFB2 mRNA expression compared with cells treated with the mimics NC groups. (C) The protein levels of TGFB2 in miR-324-5p mimics-transfected GBC-SD and SGC-996 cells were determined by Western blot assay. (D) TGFB2 mRNA was significantly up-regulated in GBC tissues compared with the adjacent normal tissues. (E) The negative correlation between TGFB2 mRNA expression and miR-324-5p levels in GBC patients. (F) Quantitative RT-PCR assay analysis of TGFB2 mRNA expression in liver metastatic foci after injection of miR-324-5p mimics or mimics NC. Data represented the mean and SD of at least three independent experiments, *p < .05.
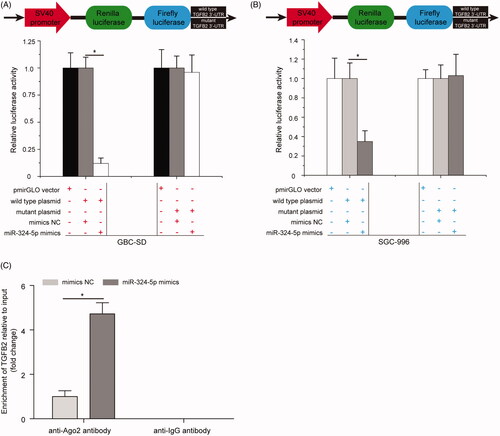
Furthermore, quantitative RT-PCR results showed that TGFB2 mRNA was significantly up-regulated in GBC tissues compared with the adjacent normal tissues (, p < .05). Pearson’s correlation analysis suggested that there was a negative correlation between miR-324-5p expression and TGFB2 mRNA levels in GBC patients (, p < .05). TGFB2 mRNA expression was significantly down-regulated in liver metastatic foci after injection of miR-324-5p mimics compared with the mimics NC group (, p < .05). In addition, dual-luciferase reporter assay was applied to confirm that the direct interaction between miR-324-5p and TGFB2 mRNA 3′-UTR. Interesting, we found that miR-324-5p mimics significantly decreased the luciferase activity of 3′-UTR of wild type TGFB2 reporter plasmid in GBC-SD cells; however, the luciferase activity of the reporter containing 3′-UTR of mutant TGFB2 has no obvious change (, p < .05). Similarly, miR-324-5p mimics were found to inhibit 3′-UTR of wild TGFB2 reporter plasmid luciferase activity in SGC-996 cells, but had no effect on 3′-UTR of mutant TGFB2 reporter plasmid luciferase activity (, p < .05). In addition, the result of RIP assay further indicated that treatment with miR-324-5p mimics and anti-Ago2 antibody enhanced the enrichment of TGFB2 compared to mimics NC group (, p < .05). These data demonstrated that TGFB2 was a direct target of miR-324-5p in GBC.
Figure 4. TGFB2 is a direct target gene of miR-324-5p. (A) miR-324-5p mimics significantly decreased the luciferase activity of 3′-UTR of wild TGFB2 reporter plasmid in GBC-SD cells, while the luciferase activity of the reporter containing 3′-UTR of mutant TGFB2 has no obvious change. (B) Relative luciferase activity in SGC-996 cells co-transfection with 3′-UTR of wild or mutant TGFB2 reporter plasmid and miR-324-5p mimics or mimics NC. (C) RNA immunoprecipitation (RIP) assay analysis of the enrichment of TGFB2 in miR-324-5p mimics or mimics NC-treated SGC-996 cells. Data represented the mean ± SD of three independent experiments, *p < .05.
Knockdown of TGFB2 partially phenocopied the inhibitory effects of miR-324-5p overexpression on GBC cell metastatic behaviours
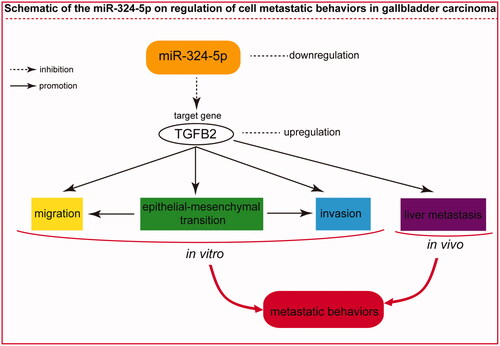
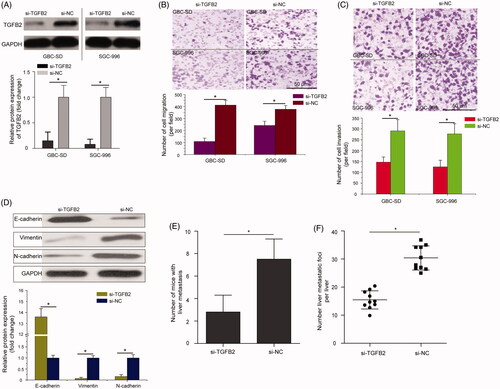
TGFB2 was identified as a direct target of miR-324-5p in GBC, as demonstrated by dual-luciferase reporter assay. Therefore, we investigated whether the knockdown of TGFB2 expression has a similar function in GBC. To examine the effects of TGFB2 on migration and invasion of GBC cells, we decreased TGFB2 expression in GBC-SD and SGC-996 cell lines using a specific TGFB2 small interfering RNA (si-TGFB2) or control siRNA (si-NC). As shown in , TGFB2 protein levels were markedly decreased in the si-TGFB2-transfected cells than that in cells treated with the si-NC (p < .05). Using the transfected cells, transwell migration and invasion assays were performed. The results showed that TGFB2 silencing clearly decreased the cell migration compared with cells transfected with the si-NC (, p < .05). Furthermore, the number of cell invasion in GBC-SD and SGC-996 cells was significantly decreased following transfection with the si-TGFB2 as compared with cells treated with the si-NC (, p < .05). Additionally, si-TGFB2 enhanced the expression of E-cadherin, and inhibited the expression of N-cadherin and Vimentin in SGC-996 cells (, p < .05). In vivo liver metastasis assay revealed that knockdown of TGFB2 decreased liver metastatic foci formed by SGC-996 cells (, p < .05). The schematic of the miR-324-5p on the regulation of cell metastatic behaviours in GBC was thus summarized in . These data strongly indicated that TGFB2 silencing partially phenocopied the inhibitory effects of miR-324-5p overexpression on GBC cell metastatic phenotypes.
Figure 5. Knockdown of TGFB2 partially phenocopied the inhibitory effects of miR-324-5p overexpression on GBC cell metastatic behaviours. (A) Relative protein expression of TGFB2 in TGFB2 small interfering RNA (si-TGFB2) or control siRNA (si-NC) treated GBC-SD and SGC-996 cells was determined by Western blot assay. (B) Transwell migration assay was used to evaluate the cell migration capacity in GBC cells after treatment with si-TGFB2 or si-NC. (C) Transwell invasion assay showed that GBC-SD and SGC-996 cells transfected with the si-TGFB2 had decreased invasive capacities compared to the si-NC groups. (D) si-TGFB2 promoted the expression of E-cadherin and inhibited the expression of N-cadherin and Vimentin in SGC-996 cells. (E) The number of mice with liver metastasis was calculated and compared at the 42nd day following intra-splenic injection. (F) The number of metastatic foci per liver in nude mice was calculated and compared. The mean ± SD of triplicate experiments were plotted, *p < .05.
Discussion
At present, GBC remains malignant tumours with poor prognosis that pose a great threat to people’s life and health worldwide [Citation24]. Finding effective detection and treatment methods are the current focus and difficulty for the prevention of GBC. Our current study demonstrated that the expression of miR-324-5p was significantly downregulated in GBC tissues and cells, and low miR-324-5p expression was negatively associated with local invasion and lymph node metastasis, suggesting that miR-324-5p might associate with the metastasis of GBC. We further demonstrated that overexpression of miR-324-5p inhibited GBC cell migration, invasion and EMT in vitro and impeded the metastasis of GBC cells in vivo. Mechanically, it was confirmed that miR-324-5p directly targeted TGFB2 and suppressed TGFB2 mRNA and protein expression in GBC cell lines. More importantly, knockdown of TGFB2 partially phenocopied the inhibitory effects of miR-324-5p overexpression on GBC cell metastatic phenotypes. Together, our findings suggested that miR-324-5p inhibits GBC cell metastatic behaviours by down-regulation of TGFB2.
Increased study showed that the dysregulation of miR-324-5p was involved in the progression of human malignancies [Citation25]. For example, Xu et al. demonstrated that miR-324-5p can inhibit the proliferation of glioma cells via the targeted regulation of the glioma-associated oncogene 1 [Citation13]. Chen et al. indicated that dysregulation of the miR-324-5p/CUEDC2 axis leads to macrophage dysfunction and is associated with colon cancer [Citation26]. Cao et al. reported that miR-324-5p suppresses HCC cell invasion and might provide new clues to invasive HCC therapy [Citation14]. In addition, miR-324-5p regulates stemness, pathogenesis and sensitivity to bortezomib in multiple myeloma cells by targeting hedgehog signalling. However, the role and mechanism of miR-324-5p in GBC remain unknown [Citation27]. To our knowledge, this is the first paper to evaluate the expression levels of miR-324-5p in GBC patients and cell lines, and our results are in accordance with the previous studies [Citation25]. Statistical analysis in clinical and pathologic characteristics suggested that the low expression level of miR-324-5p was negatively associated with local invasion and lymph node metastasis. Down-regulation of miR-324-5p predicted a poor prognosis in GBC patients. Therefore, we believed that miR-324-5p could be related to GBC metastasis. In the present study, we selected two GBC cell lines (GBC-SD and SGC-996) with relatively lower expression levels of miR-324-5p and restored its expression. The results indicated that overexpression of miR-324-5p inhibited GBC cell migration, invasion and EMT in vitro, and impeded the metastasis of GBC cells in vivo. These results were similar to some studies that reported the inhibitory effect of miR-324-5p on the other types of human cancers, such as glioma [Citation13], HCC [Citation14] and colorectal cancer [Citation15].
Recently, a growing number of reports have suggested that miRNAs can bind to the 3′-UTR of target genes and regulate gene expression at the post-transcriptional level [Citation28]. Lin and his team demonstrated that tetraspanin 8 (TSPAN8) is a functional target of miR-324-5p in gastric cancer, miR-324-5p reduces gastric cancer cell viability and induces apoptosis via downregulating TSPAN8 in SGC-7901 cells [Citation29]. In colorectal cancer, ELAV like RNA binding protein 1 (ELAVL1) is a direct target of miR-324-5p, miR-324-5p plays a suppressive role in colorectal cell viability and invasion through directly targeting ELAVL1 [Citation15]. To understand the mechanism of miR-324-5p actions in GBC, we predicted the potential target genes of miR-324-5p via TargetScan, miRBase and miRanda databases. Among the identified candidates, we selected TGFB2 for subsequent analyses, and previous evidences have demonstrated its involvement in many human malignancies [Citation23]. Through RIP and dual-luciferase reporter assays, we confirmed the direct regulation of miR-324-5p on the mRNA 3′-UTR of TGFB2. As known, TGFB2 is one isoform of TGFB family. Unlike TGFB1 and TGFB3 that can directly bind to TGFB receptor I and TGFB receptor II, TGFB2 shows very low affinity to these receptors, and depends on TGFB receptor III assistance for transduction [Citation30]. Moreover, TGFB2 was found to promote the progression in nasopharyngeal carcinoma [Citation31], glioma [Citation32] and gastric cancer [Citation33]. As a direct target gene, TGFB2 had been reported to be regulated by some miRNAs, including miR-30b [Citation34], miR-141 [Citation35], miR-153 [Citation36] and miR-193a [Citation30]. Here, we found that TGFB2 down-regulation could negatively regulate GBC cell migration, invasion and EMT in vitro and the metastasis of GBC cells in vivo, which was partially phenocopied the inhibitory effects of miR-324-5p overexpression on GBC cells. These data strongly indicated that miR-324-5p directly targets TGFB2 expression to inhibit GBC cell metastatic behaviours.
In summary, our study offered convincing evidence for the first time that the miR-324-5p expression is down-regulated in GBC tissues and cells. More important, we preliminarily demonstrated that decreased miR-324-5p expression plays a suppressive role in regulating GBC cell metastatic behaviours by targeting TGFB2. Along with further research, miR-324-5p may be a diagnostic indicator as well as a therapeutic target for the treatment of GBC.
Author contributions
Ming Chen designed the research. Lei Zhang, Xinrong Zhang, and Dongying Liu performed the experiments. Ming Chen and Xinrong Zhang analyzed the data and wrote the paper.
Disclosure statement
The authors declare no competing financial interests.
References
- Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014;6:99–109.
- Wistuba II, Gazdar AF. Gallbladder cancer: lessons from a rare tumour. Nat Rev Cancer. 2004;4(9):695–706.
- Rakic M, Patrlj L, Kopljar M, et al. Gallbladder cancer. Hepatobiliary Surg Nutr. 2014;3:221–226.
- Goetze TO. Gallbladder carcinoma: prognostic factors and therapeutic options. World J Gastroenterol. 2015;21(43):12211–12217.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30.
- Zhu AX, Hong TS, Hezel AF, et al. Current management of gallbladder carcinoma. Oncologist. 2010;15(2):168–181.
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297.
- Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med. 2014;20(8):460–469.
- Luo F, Zhou J, Wang S, et al. microRNA-222 promotes colorectal cancer cell migration and invasion by targeting MST3. FEBS Open Bio. 2019;9(5):901–913.
- Zhang Y, Lin P, Zou JY, et al. MiR-216a-5p act as a tumor suppressor, regulating the cell proliferation and metastasis by targeting PAK2 in breast cancer. Eur Rev Med Pharmacol Sci. 2019;23(6):2469–2475.
- Mou T, Xie F, Zhong P, et al. MiR-345-5p functions as a tumor suppressor in pancreatic cancer by directly targeting CCL8. Biomed Pharmacother. 2019;111:891–900.
- Gao J, Feng X, Wang F, et al. microRNA-448 inhibits the progression of non-small-cell lung cancer through regulating IRS2. J Cell Biochem. 2019;120(8):13453–13463.
- Xu HS, Zong HL, Shang M, et al. MiR-324-5p inhibits proliferation of glioma by target regulation of GLI1. Eur Rev Med Pharmacol Sci. 2014;18(6):828–832.
- Cao L, Xie B, Yang X, et al. MiR-324-5p suppresses hepatocellular carcinoma cell invasion by counteracting ECM degradation through post-transcriptionally downregulating ETS1 and SP1. PLOS One. 2015;10(7):e0133074.
- Gu C, Zhang M, Sun W, et al. Upregulation of miR-324-5p inhibits proliferation and invasion of colorectal cancer cells by targeting ELAVL1. Oncol Res. 2019;27(5):515–524.
- Jiang H, Huang G, Zhao N, et al. Long non-coding RNA TPT1-AS1 promotes cell growth and metastasis in cervical cancer via acting AS a sponge for miR-324-5p. J Exp Clin Cancer Res. 2018;37(1):169.
- Lu W, Kang Y. Epithelial-mesenchymal plasticity in cancer progression and metastasis. Dev Cell. 2019;49(3):361–374.
- Kang H, Kim H, Lee S, et al. Role of metabolic reprogramming in epithelial(-)mesenchymal transition (EMT). Int J Mol Sci. 2019;20(8): 2042.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods. 2001;25(4):402–408.
- Agarwal V, Bell GW, Nam JW, et al. Predicting effective microRNA target sites in mammalian mRNAs. eLife. 2015;4:e05005.
- Kozomara A, Birgaoanu M, Griffiths-Jones S. miRBase: from microRNA sequences to function. Nucleic Acids Res. 2019;47(D1):D155–D162.
- Enright AJ, John B, Gaul U, et al. MicroRNA targets in Drosophila. Genome Biol. 2003;5(1):R1.
- Zhang C, Zhang X, Xu R, et al. TGF-β2 initiates autophagy via Smad and non-Smad pathway to promote glioma cells’ invasion. J Exp Clin Cancer Res. 2017;36(1):162.
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108.
- Kuo WT, Yu SY, Li SC, et al. MicroRNA-324 in human cancer: miR-324-5p and miR-324-3p have distinct biological functions in human cancer. Anticancer Res. 2016;36(10):5189–5196.
- Chen Y, Wang SX, Mu R, et al. Dysregulation of the miR-324-5p-CUEDC2 axis leads to macrophage dysfunction and is associated with colon cancer. Cell Rep. 2014;7(6):1982–1993.
- Tang B, Xu A, Xu J, et al. MicroRNA-324-5p regulates stemness, pathogenesis and sensitivity to bortezomib in multiple myeloma cells by targeting hedgehog signaling. Int J Cancer. 2018;142(1):109–120.
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233.
- Lin H, Zhou AJ, Zhang JY, et al. MiR-324-5p reduces viability and induces apoptosis in gastric cancer cells through modulating TSPAN8. J Pharm Pharmacol. 2018;70(11):1513–1520.
- Fang C, Dai CY, Mei Z, et al. microRNA-193a stimulates pancreatic cancer cell repopulation and metastasis through modulating TGF-β2/TGF-βRIII signalings. J Exp Clin Cancer Res. 2018;37(1):25.
- Zhao L, Lin L, Pan C, et al. Flotillin-2 promotes nasopharyngeal carcinoma metastasis and is necessary for the epithelial-mesenchymal transition induced by transforming growth factor-β. Oncotarget. 2015;6:9781–9793.
- Frei K, Gramatzki D, Tritschler I, et al. Transforming growth factor-β pathway activity in glioblastoma. Oncotarget. 2015;6(8):5963–5977.
- Ma GF, Miao Q, Zeng XQ, et al. Transforming growth factor-β1 and -β2 in gastric precancer and cancer and roles in tumor-cell interactions with peripheral blood mononuclear cells in vitro. PLoS One. 2013;8(1):e54249.
- Howe GA, Kazda K, Addison CL. MicroRNA-30b controls endothelial cell capillary morphogenesis through regulation of transforming growth factor beta 2. PLOS One. 2017;12(10):e0185619.
- Peng T, Zhang S, Li W, et al. MicroRNA-141 inhibits glioma cells growth and metastasis by targeting TGF-β2. Am J Transl Res. 2016;8(8):3513–3521.
- Guo G, Zhang Y, Hu L, et al. MicroRNA-153 affects nasopharyngeal cancer cell viability by targeting TGF-β2. Oncol Lett. 2019;17(1):646–651.