Abstract
Neoadjuvant chemoradiotherapy has been established as the standard treatment for patients with locally advanced rectal cancer. However, the role of radiotherapy (RT) has not been fully confirmed in advanced colon cancer (LACC). We postulated that patients with pathological T4N2 locally advanced colon cancer would benefit more from RT. 6715 pT4N2M0 colon cancer patients were included in the Surveillance, Epidemiology, and End Results (SEER) database. The primary endpoints were 5-year overall survival (OS) and cancer-specific survival (CSS). Propensity score matching (PSM) with Kaplan–Meier and Cox proportional hazards’ models was performed to estimate prognosis. Before PSM, patients underwent RT had better OS and CSS as compared to patients did not receive RT (OS: 40.1% vs 27.6%, p < .001; CSS: 49.6% vs 41.1%, p = .002). After PSM, 239 matched pairs were formed for further analysis. RT group also presented significantly improved prognosis (OS: 40.1% vs 25.7%, p = .008; CSS: 49.6% vs 38.2%, p = .042). Multivariable Cox regression analysis showed that RT was a protective factor [OS:Hazard ratio (HR) =0.677, 95% Confidence interval (CI): 0.532–0.862, p = .002; CSS: HR = 0.708, 95% CI: 0.533–0.941, p = .018]. For pT4N2M0 colon cancer patients, the addition of RT seems to confer survival benefit as compared to patients who did not receive RT.
Background
The incidence of colon cancer in the world was estimated to be 1,100,000, with approximately 550,000 deaths in 2018 [Citation1]. In the United States, the estimated incidence and mortality of colon cancer were both in third place in 2019 [Citation2]. American Joint Committee on Cancer (AJCC) divided colon cancer into T1–4 stage according to the tumour infiltration of the intestines. Locally advanced colon cancer (T3, T4 or lymph node positive) occupied 10–20% of colon cancer patients [Citation3]. Comparing to patients with T1 or T2 stage, the 5-year survival rate of LACC patients was more dismal (89.9% vs 71.3%) [Citation4]. Therefore, more attention should be paid on the early diagnosis and therapeutic regimes of LACC patients.
Nowadays, multiple therapeutic strategies (operation, chemotherapy and radiotherapy (RT)) have been adopted for the treatment of locally advanced rectal cancer (LARC), in which RT was demonstrated superior local control for LARC [Citation5–6]. The addition of chemotherapy to adjuvant RT could improve 3-year of local control and pathologic complete response (pCR) rates compared with RT alone [Citation7]. Neoadjuvant chemoradiotherapy followed by total mesorectal excision (TME) and adjuvant chemotherapy has been recommended by National Comprehensive Cancer Network (NCCN) guidelines as a standard treatment for LARC. For the treatment of LACC, the role of adjuvant RT and neoadjuvant chemoradiotherapy have only been evaluated in a few studies [Citation8–13].
Because of small sample sizes, selection bias and lack of comparison group, no consistent conclusion was drawn from these studies. As expected, the latest edition of the NCCN guideline for colon cancer omit any recommendation or discussion of RT as neoadjuvant or adjuvant treatment for resectable colon cancer. The panel just discussed neoadjuvant RT with concurrent fluoropyrimidine-based chemotherapy for unresectable nonmetastatic colon cancer to help convert to resectability. However, it has not been established if adjuvant RT is useful in improving the prognosis of LACC?
In 2004, the intergroup-0130 planned to recruit 700 LACC patients who received complete resection to evaluate the value of postoperative chemoradiotherapy in the treatment of colon patients. Finally, 222 patients were enrolled. The results showed that similar OS, disease-free survival (DFS), local control between chemoradiotherapy and chemotherapy alone, while high toxicity was observed in the chemoradiotherapy group. However, because of the high number of ineligible patients and poor accrual (222 vs 700), the results must be interpreted with caution [Citation11]. Recently, a retrospective study included 21,789 pathological T4 (pT4) colon cancer patients from the Surveillance, Epidemiology, and End Results (SEER) database, all patients underwent surgery and 1001 patients received adjuvant RT. The results indicated that pT4 colon cancer patients who received adjuvant RT after surgery had a decreased risk of death from colon cancer and showed a significant CSS benefit as compared to those who did not receive adjuvant RT. However, the survival benefit is limited and the relative decreased risk of death from cancer was only 11.51% [Citation4]. It is necessary to identify subgroup who benefit more adjuvant RT and those who does not benefit from adjuvant RT from pT4 colon patients. Based on the former studies, we wonder that if the patients with a more advanced stage can benefit more from adjuvant RT, therefore we hypothesised that LACC patients with severe local lesions with a high extent of lymph node involvement could benefit more from adjuvant RT. Then we performed the analysis of survival benefit of adjuvant RT in pT4N2M0 colon cancer patients from the SEER database.
Materials and methods
Patients selection
Patients with pT4N2M0 colon cancer were identified in the SEER database (2004–2015) which was established by the National Cancer Institute. The inclusion criteria were listed as follows: (a) the age of patients was older than 18 and colon was the first primary site; (b) patients were diagnosed from 2004 to 2015; (c) the site code represented “colon (018)”; (d) surgery was performed in primary site; (e) patients with pathological stage T4N2 without distant metastases (M0) according to AJCC 7th edition; (f) information about OS, CSS and survival months were available.
The following variables were gathered: age at diagnosis, gender, marital status, race, serum CEA level, histologic type, differentiation status, tumour size, CSS and survival months. Because it is hard to distinguish unknown chemotherapy from no chemotherapy and the regime of chemotherapy is unavailable, information about chemotherapy was not included in our final analyses.
Ethics approval
This study was conducted on the basis of SEER database, which is publicly accessible. We have got permit for the research purpose before study. No personal identifying information was used. The informed consent was not required for our study any more.
Statistical analyses
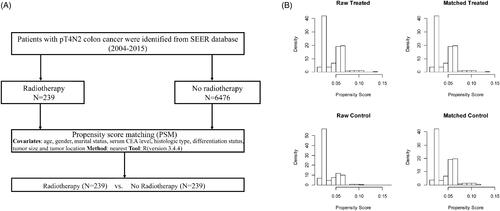
To lessen the selection bias in a retrospective study, propensity score matching at a 1:1 ratio was performed between the two groups to adjust for age, gender, marital status, year of diagnosis, tumour location, serum CEA level, histologic type, differentiation status and tumour size. The PSM was performed by “Match It” R package and the “nearest neighbor matching” method (ratio = 1:1). The details of PSM process are presented in . We calculated the frequency and percentage of categorical variables and adopted a chi-square test to compare between groups. Kaplan–Meier survival curves were assessed by log-rank test. OS and CSS were two end points of the study. Univariate analysis was performed on all variables in the study. We estimated the prognostic effect of covariates using OS and CSS multivariate Cox regression models. Two-tailed p < .05 was considered statistically significant. SPSS 22.0 and GraphPad Prism 7.0 were used to analyse data.
Figure 1. (A) Flow diagram of the PSM process. (B) Distribution of propensity score before and after propensity score analysis. Left upper and lower panel show the distribution of the propensity score for patients with receiving radiotherapy or without receiving radiotherapy before the matching procedure. Right upper and lower panel demonstrate the distribution of the propensity score after full propensity score matching.
Results
Patients characteristics
A total of 6715 patients with pT4N2M0 colon cancer were included. The details of patients’ characteristics are shown in the . In the cohort, most patients were older than age 60 years (4658, 69.3%), female (3460, 51.5%), married (3365, 50.1%) and diagnosed colon cancer between 2010-2015 (3694, 55.0%). Most of the primary tumours arose in the right-sided colon (4450, 66.2%) with tumour size less than 6 cm (3555, 53.0%). The histologic type of tumours was mainly adenocarcinoma (4778, 71.5%). Poor and undifferentiated differentiation pathological grade (3392, 51.0%) was examined in most of the patients.
Table 1. Clinical characteristics of pathological T4N2M0 colon cancer patients with or without radiotherapy.
In terms of treatment, there were 239 (3.5%) patients received RT after surgery. The data seemingly indicated patients less than age 60 years, diagnosed between 2004 and 2009, with left-sided colon cancer and whose tumour size larger than 6 cm were more likely to receive RT. We performed PSM at a 1:1 ratio between patients received RT or no-RT and no patients’ characteristics differed significantly between two groups except the year of diagnosis. All of the above were shown in .
Survival analysis
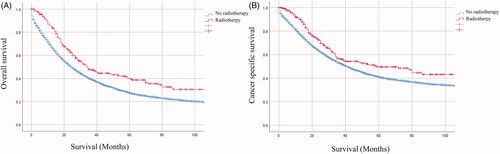
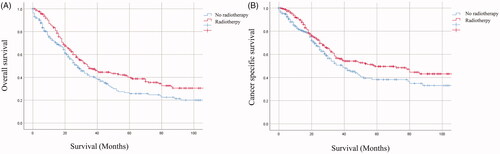
The Kaplan–Meier survival curves of the entire cohort and matched populations according to the administration of RT are shown in and , respectively. Before PSM, patients who received RT had significantly better survival compared with those received no-RT (OS: 25 months vs 34 months, p < .001: CSS: 41 months vs 59 months, p = .002, ). After PSM, patients who received RT also still had significantly better survival compared with those received no-RT (OS: 28 months vs 34 months, p = .008;CSS: 39 months vs 59 months, p = .042, ).
Prognostic factors
After PSM, the results of univariate and multivariate analyses for OS and CSS in the entire cohort were shown in the and , respectively. In the multivariate analyses, age > 60 years (HR = 2.021, 95% CI: 1.571–2.599, p < .001), signet ring cell carcinoma (HR = 1.802, 95% CI: 1.013–3.206, p = .045), and poor and undifferentiated (HR = 1.305, 95% CI: 1.047–1.741, p = .021) were associated with inferior OS. Married status (HR = 0.727, 95% CI: 0.562–0.941, p = .015), left-sided colon cancer (HR = 0.754, 95% CI: 0.584–0.975, p = .031), and RT (HR = 0.677, 95% CI: 0.532–0.862, p = .002) were independent protective factors. The multivariate analysis for CSS showed that patients with age >60 years (HR = 1.539, 95%CI: 1.147–2.065, p = .004), and signet ring cell carcinoma (HR = 2.179, 95% CI: 1.108–4.287, p = .024) had poor prognosis. Patients who received RT (HR = 0.708, 95% CI: 0.533–0.941, p = .018) had significantly better prognosis than those who did not receive RT.
Table 2 Univariate and multivariate analysis for overall survival (OS).
Table 3. Univariate and multivariate analysis for cancer-specific survival (CSS).
Discussion
There were about 32.9% of T4 colon cancer patients who received multi visceral R0 resection, resulting in survival benefit in United States [Citation14]. However, high 5-year locoregional tumour recurrence rate (6.5%) and distant metastases (24.2%) after R0 resection were still observed in these patients [Citation15]. To improve the situation, adjuvant RT had been introduced to the treatment of LACC. As the radiation therapy planning and delivery techniques evolved, adjuvant RT has been extensively used in both pre- and postoperative settings.
Recently, a cohort analysis included 131 T4 colon cancer patients and 23 received neoadjuvant RT before complete resection. The results showed a downstaging in terms of T-stage in the neoadjuvant RT group. Comparing with the non-neoadjuvant RT group, the neoadjuvant RT group had an improved R0 resection rate and decreased local recurrence [Citation10]. Hawkins et al. performed a retrospective study with the National Cancer Database. Finally, 15,207 T4 colon cancer patients were included and 195 received neoadjuvant RT. The results showed the neoadjuvant RT group had superior R0 resection rates and increased 5-year overall survival rates [Citation16]. Furthermore, Cukier et al. found neoadjuvant chemoradiotherapy could lead to high R0 resection rates and excellent local control [Citation12]. All these studies showed T4 colon cancer patients benefitted from preoperative RT, especially for R0 resection.
Postoperative RT usually performed on locoregional sites or abdomen. They had similar efficacy, but the toxicity of abdominal RT was more remarkable [Citation17]. Studies focussed on the role of postoperative RT in T4 colon cancer patients were limited and the results were controversial as described before [Citation4,Citation11]. Since the NCCN guideline does not recommend neoadjuvant or adjuvant RT as a treatment for resectable colon cancer, it is unreasonable to adopt RT to all pT4 patients. To identify the subgroup which benefit more from radiotherapy and avoid excessive medical treatment for patients with a limited survival benefit, we hypothesised that LACC patients with severe local lesions and with a high extent of lymph node involvement could benefit more from postoperative adjuvant RT.
Evidence indicated that lymph node metastasis in colorectal cancer patients is a major predictor of oncologic outcome [Citation18,Citation19]. Comparing to node-negative T4 lesions patients who received adjuvant chemotherapy alone, the 3-year DFS rates of node-positive T4 lesions patients were much lower (39.3% vs 100%) [Citation20]. Ludmir et al. also found T4 colon cancer patients with node-positive would have poor locoregional control and DFS rates if received with adjuvant chemotherapy alone [Citation8]. All these studies indicated that T4 colon cancer patients with node-positive who received adjuvant chemotherapy alone were insufficient. In the present study, we performed an analysis based on lymph node status and found adjuvant RT could be a protective factor for pT4N2M0 colon cancer patients. Patients who received adjuvant RT would have better rates of OS and CSS than those who did not receive adjuvant RT. After PSM, the RT group also presented significantly improved prognosis as compared to the no-RT group.
Recently, Mclaughlin et al. performed a retrospective study that included a high proportion of node-positive T4 colon cancer patients (13,342, 65%). The result of the study showed postoperative adjuvant RT can only decrease the relative risk of death from colon cancer by 11.51% at 5 years. It is unreasonable to routinely adopt adjuvant RT for all T4 non-rectal colon cancer [Citation4]. Comparing to this research, we included 6715 pT4N2M0 colon cancer patients and found adjuvant RT can decrease the risk of death from colon cancer by near 30%, which was much higher than 11.51% of T4 colon cancer. Furthermore, our results were more reliable because we lessen the selection bias by PSM.
However, there were some inevitable limitations in our study. Firstly, the information of chemotherapy was not included in our study because we could not distinguish unknown chemotherapy to no chemotherapy and the absence of the regime of chemotherapy. Secondly, after PSM, the difference still existed in terms of the year of diagnosis, which might result from alterations of AJCC editions and the treatment concept of colon cancer. All of these limitations may lead to selection bias.
In conclusion, our study showed that pT4N2M0 colon cancer patients who received adjuvant RT could have significantly better OS and CSS. It can reduce the risk of death from colon cancer by near 30%. Adjuvant RT should be actively considered for pT4N2M0 colon cancer patients, while RT should be recommended for pT4 colon cancer with caution.
Author contributions
The study was designed by CJF and WW. All authors except FGS were involved in collection of study data. HY and GKX were involved in data analysis and interpretation. GX developed the manuscript. All authors reviewed and revised drafts of the manuscript, and approved the final version for submission.
Acknowledgements
We acknowledged the efforts of the Surveillance, Epidemiology, and End Results (SEER) Programme tumour registries in the creation of SEER database.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets are available in the SEER repository and can be obtained from https://seer.cancer.gov.
References
- Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–1953.
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34.
- Rosander E, Nordenvall C, Sjovall A, et al. Management and outcome after multivisceral resections in patients with locally advanced primary colon cancer. Dis Colon Rectum. 2018;61(4):454–460.
- McLaughlin C, Kim NK, Bandyopadhyay D, et al. Adjuvant radiation therapy for T4 non-rectal colon adenocarcinoma provides a cause-specific survival advantage: a SEER database analysis. Radiother Oncol. 2019;133:50–53.
- Smith CA, Kachnic LA. Evolving treatment paradigm in the treatment of locally advanced rectal cancer. J Natl Compr Canc Netw. 2018;16(7):909–915.
- Peeters KC, Marijnen CA, Nagtegaal ID, et al. The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg. 2007;246(5):693–701.
- Bonnetain F, Bosset JF, Gerard JP, et al. What is the clinical benefit of preoperative chemoradiotherapy with 5FU/leucovorin for T3-4 rectal cancer in a pooled analysis of EORTC 22921 and FFCD 9203 trials: surrogacy in question?. Eur J Cancer. 2012;48(12):1781–1790.
- Ludmir EB, Arya R, Wu Y, et al. Role of adjuvant radiotherapy in locally advanced colonic carcinoma in the modern chemotherapy era. Ann Surg Oncol. 2016;23(3):856–862.
- Willett CG, Fung CY, Kaufman DS, et al. Postoperative radiation therapy for high-risk colon carcinoma. J Clin Oncol. 1993;11(6):1112–1117.
- Krishnamurty DM, Hawkins AT, Wells KO, et al. Neoadjuvant radiation therapy in locally advanced colon cancer: a cohort analysis. J Gastrointest Surg. 2018;22(5):906–912.
- Martenson JJ, Willett CG, Sargent DJ, et al. Phase III study of adjuvant chemotherapy and radiation therapy compared with chemotherapy alone in the surgical adjuvant treatment of colon cancer: results of intergroup protocol 0130. J Clin Oncol. 2004;22(16):3277–3283.
- Cukier M, Smith AJ, Milot L, et al. Neoadjuvant chemoradiotherapy and multivisceral resection for primary locally advanced adherent colon cancer: a single institution experience. Eur J Surg Oncol. 2012;38(8):677–682.
- Huang CM, Huang MY, Ma CJ, Yeh Y, et al. Neoadjuvant FOLFOX chemotherapy combined with radiotherapy followed by radical resection in patients with locally advanced colon cancer. Radiat Oncol. 2017;12(1):48.
- Govindarajan A, Coburn NG, Kiss A, et al. Population-based assessment of the surgical management of locally advanced colorectal cancer. J Natl Cancer Inst. 2006;98(20):1474–1481.
- Croner RS, Merkel S, Papadopoulos T, et al. Multivisceral resection for colon carcinoma. Dis Colon Rectum. 2009;52(8):1381–1386.
- Hawkins AT, Ford MM, Geiger TM, et al. Neoadjuvant radiation for clinical T4 colon cancer: a potential improvement to overall survival. Surgery. 2019;165(2):469–475.
- Mendenhall WM, Amos EH, Rout WR, et al. Adjuvant postoperative radiotherapy for colon carcinoma. Cancer. 2004;101(6):1338–1344.
- Chang GJ, Rodriguez-Bigas MA, Skibber JM, et al. Lymph node evaluation and survival after curative resection of colon cancer: systematic review. J Natl Cancer Inst. 2007;99(6):433–441.
- Ceelen W, Van Nieuwenhove Y, Pattyn P. Prognostic value of the lymph node ratio in stage iii colorectal cancer: a systematic review. Ann Surg Oncol. 2010;17(11):2847–2855.
- Taieb J, Tabernero J, Mini E, et al. Oxaliplatin, fluorouracil, and leucovorin with or without cetuximab in patients with resected stage III colon cancer (PETACC-8): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(8):862–873.