?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Oxygen tension at 50% haemoglobin saturation (P50), which reflects the degree of peripheral oxygen offloading and tissue oxygenation, plays an important role in the diagnosis and treatment of disease, as well as in transfusion research. Blood gas analysers are commonly used in clinical and obtain P50 values through complex calculations and analysis. Oxygenation-dissociation analysers are specially designed to record the oxygen dissociation curves and obtain P50 values of whole blood, red blood cells (RBCs), and stroma-free haemoglobin. However, whether the two equipment obtain comparable data is still uncertain. Herein, we used both equipment to detect P50 values of blood and stroma-free haemoglobin from human and bovine sources, venous and arterial blood of beagle and rat, and stored rat blood. For human blood, both analysers yielded similar data. P50 of the stroma-free haemoglobin and bovine blood could only be properly detected by oxygenation-dissociation analysers. Blood gas analysers showed different P50 values, while oxygenation-dissociation analysers got similar P50 values for arterial and venous samples. Oxygenation-dissociation analysers distinguished changes in P50 values during RBCs storage. Compared with the blood gas analysers, oxygenation-dissociation analysers had a stronger detection capability in P50 measurement with regard to both sample types and species.
Introduction
Haemoglobin oxygen affinity, which is conveniently characterised in terms of the oxygen tension at 50% saturation (P50), governs O2 binding between pulmonary alveoli or other gas exchange surfaces and the blood, thereby controlling release in peripheral tissues [Citation1]. Understanding how P50 changes will contribute to the study of haemoglobin and haemoglobin-based oxygen carriers (HBOCs), as well as clinical research for drug development and transfusion.
P50 has been widely used in the study of haemoglobin structure and function. The cooperative effect of haemoglobin relies heavily on P50 and the oxygen-dissociation curve (ODC) [Citation2]. In addition, P50 is widely used in the study of abnormal haemoglobin and treated as an important sign of disease. Excessive P50 values, for example of haemoglobin Titusville or Kansas, may worsen hypoxaemia in the setting of acute lung injury [Citation3]. Hereditary erythrocytosis is closely related to high-oxygen-affinity haemoglobin variants, which are associated with left-shifted ODCs (decreased P50) [Citation4]. Thus, P50 is an important indicator and should be brought into clinical diagnosis.
Previous studies of HBOCs refer to different P50 values, such as polymerised bovine haemoglobin (P50 = 54 mmHg) [Citation5], tetrameric cross-linked human haemoglobin (P50 = 33 mmHg) [Citation6], and PEGylated human haemoglobin (P50 = 5 mmHg) [Citation7]. Thus, researchers are exploring the effects of different P50 values on indications and effects of HBOCs. Different products of HBOCs in P50 have different indications, which should be selected in combination with clinical indications [Citation8–11].
The P50 value, which changes significantly with the pH, pO2, and pCO2, plays significant roles in oxygen capture by the human lungs and other pulmonary functions. Haemoglobin possesses higher oxygen affinity (lower P50) to capture more oxygen in alkaline pulmonary alveolar capillary while the haemoglobin’s oxygen affinity might be rapidly reduced (higher P50) in acidic microenvironment such as muscle or brain capillary, which facilitates the oxygen unloading to tissue [Citation12].
Many researchers are looking for substances capable of alleviating or curing disease via regulation of haemoglobin oxygen affinity. GBT1118, a potential drug for the improvement of tissue oxygenation in pulmonary diseases characterised by severe hypoxaemia, works by binding to haemoglobin to produce a concentration-dependent decreased P50 and increased arterial oxygen loading [Citation13–15]. Voxelotor (GBT440) in Phase 3, is an oral drug that modulates Hb affinity for oxygen, thereby inhibiting HbS (Sickle haemoglobin) polymerisation and the resultant sickling of RBCs. Multiple doses of voxelotor in SCD patients from 500 to 1000 mg daily resulted in dose-dependent pharmacodynamic effects, with a dose-dependent increase in Hb-oxygen affinity (decreased P50) [Citation16,Citation17].
Myoinositol trispyrophosphate (ITPP) is a novel investigational drug (approved for Phase I and II clinical use in humans) for tumour treatment; one of its main therapeutic mechanisms is regulating P50. It was found to reverse hypoxia in rodents with pancreatic tumours by increasing P50 [Citation18,Citation19].
P50 is critical to reflect RBCs storage lesion. During blood storage, a series of biochemical and structural changes occur, causing damage [Citation20,Citation21]. In particular, the oxygen-carrying ability of RBCs decreases significantly. Reportedly, P50 values of both whole blood and suspended RBCs decrease significantly during the first 3 weeks of storage [Citation22]. However, the use of rejuvenation methods to increase P50 could improve oxygen release by stored RBCs [Citation23,Citation24].
To date, two types of equipment have been used to obtain P50 values. Blood gas analysers are frequently used in the clinics to detect and export many parameters about blood, including P50 values. However, P50 values exported by blood gas analysers need further clarification. In addition, P50 values can be detected via spectrophotometric methods [Citation25]. The ODC of haemoglobin was recorded using a UV-V spectrophotometer. Deoxygenated haemoglobin was obtained by repeated evacuations and flushing with argon gas (99.99%). A small amount of air was added gradually to deoxygenated haemoglobin before measuring absorption spectra from 450 to 650 nm. The P50 value and Hill’s cooperativity coefficient (n value) were then calculated from ODC and Hill plots, respectively [Citation26]. Oxygenation-dissociation analysers are based on the above principles and specially designed to detect P50 values and parameters reflecting oxygen affinity. This method, however, is not easy to perform; it has only been used in research laboratories, and not been implemented clinically [Citation27].
Whether the P50 value from blood gas analysers and the oxygenation-analysers are comparable, and whether they can be applied in different samples, are still uncertain. Therefore, in this study, we evaluated P50 values of human and bovine blood and haemoglobin solutions, as well as venous blood and arterial blood of beagle and rat, and the stored rat blood. We clarify the accuracy and application range of an oxygenation-dissociation analyser by comparing its results with a blood gas analyser.
Materials and methods
Ethical considerations
All experimental procedures were approved by the Laboratory Animal Centre of the Academy of Military Medical Sciences (Beijing, China). The research protocol adhered to institutional guidelines for the care and use of laboratory animals.
Sample preparation
Blood and haemoglobin of human and bovine
Whole human blood was withdrawn via the median cubital vein of volunteers. A 4-ml sample of blood was placed in a heparin-coated anticoagulation tube (Kangjian Medical Apparatus, Jiangsu, China), while 50 ml of blood was mixed with a solution of citrate phosphate dextrose adenine (CPDA-1; Sigma Aldrich, St Louis, MO, USA). All samples were stored at 4 °C. Bovine whole blood was withdrawn via the carotid vein of cattle (Beijing Created Biotechnology, Beijing, China), mixed with CPDA-1, and stored at 4 °C. Next, human haemoglobin (hHb) and bovine haemoglobin (bHb) were purified from whole blood mixed with CPDA-1 by anion-exchange chromatography, as previously described [Citation28–30]. Haemoglobin solutions were prepared at a concentration of 5 g/dL. After filtration through a 0.22-mm polyether sulphone filter, solutions were stored at −80 °C.
Beagle venous and arterial blood
Healthy male beagles (10–12 kg; Beijing Created Biotechnology, Beijing, China) were anaesthetised with pentobarbital sodium (Beijing Chemical Agent, Beijing, China) to establish an arteriovenous access in the right lower extremity. Next, 1 ml of arterial blood and 1 ml of venous blood were withdrawn and immediately used for experiments.
Fresh and stored rat blood
Healthy male Wistar rats (220–260 g; Vital River, Beijing, China) with ad libitum access to food and water were anaesthetised by intraperitoneal injection of 50 mg/kg sodium pentobarbital sodium, placed in the supine position on a warming pad (TMS-202, Softron Biotechnology, Beijing, China), with temperature maintained at 37 ± 0.1 °C. The carotid artery, right femoral artery, and right femoral vein were cannulated.
Heparin (400 U/kg; Chinese Medicine Group Chemical Agent, Beijing, China) was administered via the carotid artery to inhibit coagulation. Blood was withdrawn individually from the femoral artery and femoral vein.
Blood collection and storage were performed as previously described by Wang et al. [Citation31]. Briefly, blood was withdrawn via the carotid artery and mixed with CPDA-1 to yield a final concentration of 14% CPDA-1. Blood was leukoreduced by passage through a leukoreduction filter (BengBu Medical College, BengBu, China), centrifuged for 15 min at 400 × g to remove the supernatant, and stored at 4 °C.
Equipment
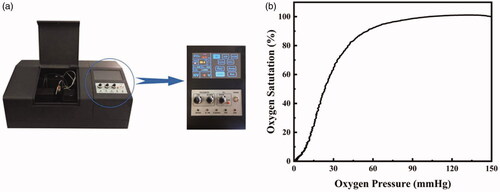
Samples were analysed using a blood gas analyser (ABL90 FLEX, Radiometer, Brønshøj, Denmark) [Citation32,Citation33] and an oxygenation-dissociation analyser (BLOODOX-2018 Analyser, Softron Biotechnology, Beijing, China) as shown in . BLOODOX-Solution buffer includes 130 mM NaCl (Chinese Medicine Group Chemical Agent, Beijing, China), 30 mM TES (Sigma Aldrich, St Louis, MO, USA) and 5 mM KCl (Chinese Medicine Group Chemical Agent, Beijing, China), at pH 7.4 ± 0.01.
P50 measurement
Blood gas analyser
Samples were sealed in a 1 ml syringe and analysed according to the standard instrument operation. The blood gas analyser calculated the P50 value primarily according to the following formula (1). However, there are special cases in the series of calculations. If one or more default values have been used in the calculation, the result may deviate significantly from the true value. The deviation on “estimated” oxygen status parameters then, might become particularly significant if default values are used instead of measured blood oximetry data. In some cases, however, the default value is not accepted as the input for the calculation. This is because the actual values of the missing parameter may deviate significantly from the default value, thus making the estimation clinically inappropriate.
(1)
(1)
Table
Bloodox-2018 analyser
Three milligrams of haemoglobin or an equal volume of RBCs were used to calculate haemoglobin content according to formula (2) after dilution in 4 ml of BLOODOX-Solution buffer mixed with 20 μL bovine serum albumin (A7034, Sigma Aldrich, St Louis, MO, USA), and 20 μL of anti-foaming agent (Sigma Aldrich, St Louis, MO, USA). The sample-buffer was drawn into a cuvette, equilibrated and brought to 37 °C, and oxygenated to 100% with air at the same time. After adjustment of the pO2 value, the sample was deoxygenated with nitrogen. A Clark oxygen electrode was used to detect changes of oxygen tension during the deoxygenation process on the x-axis of an x-y recorder, while the deoxyhemoglobin fraction was simultaneously monitored by dual-wavelength spectrophotometry at 560 and 570 nm, and displayed on the y-axis. Finally, the ODC was automatically recorded on graph paper, as shown in . The P50 value was extrapolated on the x-axis as the point at which O2 saturation is 50%.
(2)
(2)
Statistical analysis
Data are expressed as mean ± standard deviation (SD). Statistical analysis was performed using SAS 9.2 (IBM, Armonk, NY, USA). All data are consistent with a normal distribution. Differences between groups were analysed using a t-test or paired t-test. Changes were considered statistically significant if p < .05.
Results
P50 value detection of human and bovine blood and haemoglobin
As shown in , the average P50 value of human heparin-anticoagulated venous blood detected by the blood-gas analyser was 25.42 ± 0.81 mmHg, while the oxygenation-dissociation analyser yielded an average value of 23.83 ± 2.96 mmHg. There was no statistical difference between these values (paired t-test, p > .05). As shown in , the average P50 value of bovine venous blood detected by the blood gas analyser was 43.92 ± 2.72 mmHg, while the oxygenation-dissociation analyser yielded a value of 35.30 ± 0.48 mmHg, which was statistically significant (p < .05).
Figure 2. P50 value detection of human (a) and bovine (b) blood by blood gas analyser and oxygenation-dissociation analyser. P50 value detection of human (c) and bovine (d) blood and purified haemoglobin solution by the oxygenation-dissociation analyser. P50 value detection of blood (e) and purified haemoglobin (f) of human and bovine.
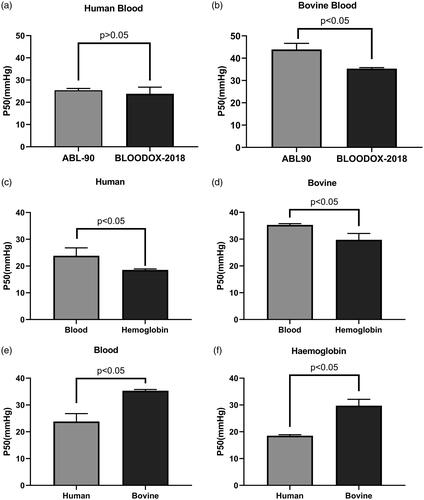
After purification, the P50 of hHb solutions was 18.55 ± 0.39 mmHg and could only be evaluated by the oxygenation-dissociation analyser, as shown in . Moreover, there was a statistical difference between the human blood and hHb (p < .05). Upon testing with the blood gas analyser, the P50 value could not be calculated. The same result occurred for bHb solutions, as shown in . After purification, the average P50 of bHb solutions was 29.75 ± 2.37 mmHg and could only be evaluated by the oxygenation-dissociation analyser. As shown in , P50 values of both human blood and haemoglobin were lower than that of bovine, and there was a statistical difference between them.
P50 value detection of arterial and venous whole blood from beagle and rat
As shown in , P50 values of beagle arterial and venous blood as detected by the blood-gas analyser were 28.21 ± 1.32 mmHg and 37.60 ± 4.80 mmHg, respectively. There was a statistical difference between these values (p < .05). However, there was no statistical difference (p > .05) between P50 values detected by the oxygenation-dissociation analyser, 46.47 ± 1.20 mmHg and 44.61 ± 0.36 mmHg, respectively.
Figure 3. P50 value detection of arterial (A) and venous (V) blood from beagle (a), P50 value detection arterial (A) and venous (V) blood from rat (b) and stored rat RBCs (c). The ODC of stored rat RBCs (d).
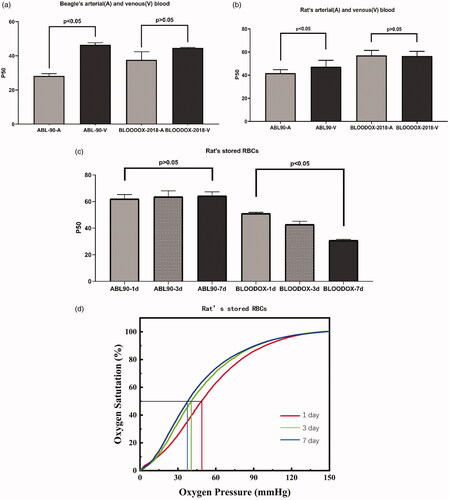
As shown in , P50 values of rat arterial and venous blood as detected by the blood gas analyser were 41.72 ± 2.99 mmHg and 47.17 ± 5.68 mmHg, respectively. There was a statistical difference between these values (p < .05). However, the P50 values of rat arterial and venous blood detected by the oxygenation-dissociation analyser were 57.09 ± 4.25 mmHg and 56.48 ± 4.13 mmHg, respectively (p > .05).
P50 value detection of stored rat RBCs
As shown in , P50 values of stored rat RBCs, as detected by the blood gas analyser at 1, 3, and 7 days, were 62.13 ± 3.07 mmHg, 63.72 ± 4.35 mmHg, and 64.34 ± 2.94 mmHg, respectively(p > .05). Values detected by the oxygenation-dissociation analyser were 51.16 ± 0.71 mmHg, 42.92 ± 2.29 mmHg, and 30.99 ± 0.60 mmHg, respectively (p < .05). Obviously, values detected by the blood-gas analyser did not change, while those detected by the oxygenation-dissociation analyser showed a rapid and significant downward trend.
Discussion
Both the blood-gas analyser and oxygenation-dissociation analyser could detect P50 values. However, the results showed a difference, likely because the testing principles of the two equipment are different. P50 values determined by the blood-gas analyser are based on the reference P50 value of humans under standard conditions. This reference value is 3.578 kPa (26.8 mmHg) and the standard condition is defined as temperature = 37 °C, pH 7.4, partial pressure of carbon dioxide (pCO2) = 5.33KPa, fraction of carboxyhaemoglobin (FCOHb) = 0, fraction of methaemoglobin (FMetHb) = 0, fraction of foetal haemoglobin (FHbF) = 0, and concentration of diphosphoglycerate (cDPG) = 5 mmol/L. The actual P50 value is calculated according to the values of parameters such as pCO2, the partial pressure of oxygen (pO2), saturation of oxygen (sO2), FCOHb, FMetHb, and FHbF. If the blood gas analyser fails to gain some of the required parameters, the P50 value cannot be accurately calculated.
Determination of P50 values by the oxygenation-dissociation analyser is based on dual-wavelength spectrophotometry to measure the sO2 of Hb, and a Clark electrode to measure pO2 in the process of oxygenation/deoxygenation. Measurements were carried out at 37 °C and pH 7.4. P50 values are calculated using the ODC. Therefore, the oxygenation-dissociation analyser is capable to test whole blood, RBCs, haemoglobin solutions, and HBOCs. The present results provide some important details regarding these methods.
The oxygenation-dissociation analyser could detect P50 values of blood from human and other species
The P50 value of human blood reported by the oxygenation-dissociation analyser was similar to that of the blood-gas analyser and close to the reference value [Citation34]. This provides sufficient evidence that the oxygenation-dissociation analyser can accurately detect P50 values. Furthermore, the P50 value of bovine whole blood exported by the oxygenation-dissociation analyser was close to the reference value [Citation35]. However, P50 values from the blood gas analyser deviated from the reference value significantly. This likely occurred because the blood gas analyser is prepared to detect natural whole blood of human, and the characteristics of bovine blood are different from that of human blood. For example, bHb shares 85% similarity with hHb according to sequence alignment [Citation36]. Moreover, the P50 of bHb is not primarily regulated by 2,3-DPG, but is instead highly sensitive to chloride ions (Cl−) [Citation37]. These unique characteristics lead them to possess different oxygen affinities with reference P50 values of 26.60 mmHg and 30 mmHg for human and bovine RBCs, respectively [Citation38–40]. Therefore, the P50 value measured by blood gas analyser is accurate for human blood, but inaccurate for blood from other species if their blood shows different characteristics from human blood. In contrast, the oxygenation-dissociation analyser was capable of better detecting the P50 values of other animals, if their haemoglobin has similar visible light spectrum as human.
The oxygenation-dissociation analyser could obtain P50 value of stroma free haemoglobin
The P50 of purified stroma-free haemoglobin could only be accurately evaluated by the oxygenation-dissociation analyser. During testing with the blood-gas analyser, many parameters including pCO2, which is the main parameter used to calculate P50 value, were different from the reference value of the equipment significantly. Thus, the blood gas analyser failed to accurately detect the P50 value of stroma-free haemoglobin. Notably, P50 values of stroma-free haemoglobin detected by the oxygenation-dissociation analyser were lower than that of corresponding whole blood because of a lack of appropriate regulation by 2,3-DPG [Citation41].
The oxygenation-dissociation analyser could detect P50 values independent of the different blood gases between arterial and venous blood
Arterial and venous blood results from beagles and rats showed statistically significant differences in blood gas analyser detection. Indeed, pH, pO2, and pCO2 are significantly different between arterial and venous blood. The P50 value is affected by protons and CO2 (which enhance O2 release in metabolising tissues via the Bohr effect), the red cell organic phosphate 2,3-DPG, and Cl− [Citation36]. According to the principles of the blood gas analyser, differences in pH, pCO2, and other possible parameters produce different P50 values between arterial and venous blood. Therefore, detection with the blood gas analyser could indicate transient P50 values under some certain states, as this temporary P50 value changes reversibly with circulation, it cannot be regarded as characteristic of haemoglobin. The P50 values from arterial and venous get similar data by the oxygenation-dissociation analyser. And it obtains the P50 values by simulating the oxygenation/deoxygenation process of gas exchange in physiological conditions, thus reflecting the characteristic of haemoglobin.
Experimental procedures were found to affect P50 measurements, including anaesthesia, anticoagulation, experimental season, sample preparation, etc. [Citation42,Citation43]. In the present study, we did not use anticoagulant components for beagle RBCs, which may result in higher values compared with previous literature [Citation44].
Changes of P50 values during RBCs storage were distinguished by the oxygenation-dissociation analyser
Changes in oxygen affinity of RBCs with storage time have been verified in many studies in the literature [Citation45,Citation46]. In this study, the oxygenation-dissociation analyser can distinguish changes of P50 values during RBCs storage, while the blood gas analyser failed to reflect the changes.
Firstly, the blood gas analyser is only recommended for the detection of fresh heparin-anticoagulated samples. Concentrations of pO2, pCO2, sO2, and 2,3-DPG in RBCs suspension changed gradually during storage because of storage lesion. Furthermore, CPDA-1, which contains citric acid, sodium citrate, glucose, sodium dihydrogen phosphate, and adenine, accelerate changes of pH and pCO2, which may disturb P50 measurement by blood gas analyser.
During the measurement of stored RBCs, some parameters, such as FCOHb and FMetHb, could not be detected by the blood gas analyser. According to the principles of blood gas analysis, default values (e.g. for FCOHb = 0.4% and FMetHb = 0.4%) are used to calculate the P50 value when such parameters could not be obtained. Thus, the blood gas analyser reports a P50 value based on these default values, which do not reflect the changed oxygen affinity during storage.
Conclusion
First, both the equipment can detect the P50 values of human blood. The oxygenation-dissociation analyser can detect P50 value from different species with similar visible light spectrum as human blood. Second, the oxygen-dissociation analyser could obtain P50 values of stroma free haemoglobin. Third, it could detect P50 values as characteristics of haemoglobin independent of differences in blood gas between arterial and venous blood. Finally, it can reflect changing P50 value during RBCs storage. Compared with blood gas analyser, the oxygenation-dissociation analyser had stronger detection capability in P50 measurement with regard to both sample type and species.
Limitation
The P50 value may be affected by a variety of factors, such as anticoagulants, anaesthetics, experimental season, ambient temperature, age and status of the animal, etc. Further research is needed in the future.
Disclosure statement
The authors declare no competing financial interest.
Additional information
Funding
References
- Mairbaurl H, Weber RE. Oxygen transport by hemoglobin. Compr Physiol. 2012;2(2):1463–1489.
- Weiss JN. The Hill equation revisited: uses and misuses. FASEB J. 1997;11(11):835–841.
- Mangin O. High oxygen affinity hemoglobins. Rev Med Interne. 2017;38(2):106–112.
- Oliveira JL, Coon LM, Frederick LA, et al. Genotype-phenotype correlation of hereditary erythrocytosis mutations, a single center experience. Am J Hematol. 2018;93(8):1029–1041.
- Wang Y, Wang L, Yu W, et al. A PEGylated bovine hemoglobin as a potent hemoglobin-based oxygen carrier. Biotechnol Prog. 2017;33(1):252–260.
- Cole R, Vandegriff K, Szeri A, et al. Targeted O2 delivery by blood substitutes: in vitro arteriolar simulations of first- and second-generation products. Microvasc Res. 2008;76(3):169–179.
- Winslow RM. Targeted O2 delivery by low-p50 hemoglobin: a new basis for hemoglobin-based oxygen carriers. Artif Cells Blood Substit Immobil Biotechnol. 2005;33(1):1–12.
- Hare GMT, Harrington A, Liu E, et al. Effect of oxygen affinity and molecular weight of HBOCs on cerebral oxygenation and blood pressure in rats. Can J Anesth/J Can Anesth. 2006;53(10):1030–1038.
- Song BK, Nugent WH, Moon-Massat PF, et al. Effects of a hemoglobin-based oxygen carrier (HBOC-201) and derivatives with altered oxygen affinity and viscosity on systemic and microcirculatory variables in a top-load rat model. Microvasc Res. 2014;95:124–130.
- Kawaguchi AT, Okamoto Y, Kise Y, et al. Effects of liposome-encapsulated hemoglobin on gastric wound healing in the rat. Artif Organs. 2014;38(8):641–649.
- Winslow RM. MP4, a new nonvasoactive polyethylene glycol-hemoglobin conjugate. Artif Organs. 2004;28(9):800–806.
- Kang M-Y, Hua-Huy T, Günther S, et al. Individual modeling of oxygen capture by the human lungs. Respir Physiol Neurobiol. 2019;270:103256.
- Geng X, Dufu K, Hutchaleelaha A, et al. Increased hemoglobin-oxygen affinity ameliorates bleomycin-induced hypoxemia and pulmonary fibrosis. Physiol Rep. 2016;4(17):e12965.
- Dufu K, Yalcin O, Ao-Ieong ESY, et al. GBT1118, a potent allosteric modifier of hemoglobin O2 affinity, increases tolerance to severe hypoxia in mice. Am J Physiol Heart Circ Physiol. 2017;313(2):H381–H391.
- Putz ND, Shaver CM, Dufu K, et al. GBT1118, a compound that increases the oxygen affinity of hemoglobin, improves survival in murine hypoxic acute lung injury. J Appl Physiol. 2018;124(4):899–905.
- Vichinsky E, Hoppe CC, Ataga KI, et al. A phase 3 randomized trial of voxelotor in sickle cell disease. N Engl J Med. 2019;381(6):509–519.
- Howard J, Hemmaway CJ, Telfer P, et al. A phase 1/2 ascending dose study and open-label extension study of voxelotor in patients with sickle cell disease. Blood. 2019;133(17):1865–1875.
- Kieda C, El Hafny-Rahbi B, Collet G, et al. Stable tumor vessel normalization with pO2 increase and endothelial PTEN activation by inositol trispyrophosphate brings novel tumor treatment. J Mol Med. 2013;91(7):883–899.
- Limani P, Linecker M, Schneider MA, et al. The allosteric hemoglobin effector ITPP inhibits metastatic colon cancer in mice. Ann Surg. 2017;266(5):746–753.
- Adams F, Bellairs G, Bird AR, et al. Biochemical storage lesions occurring in nonirradiated and irradiated red blood cells: a brief review. Biomed Res Int. 2015;2015:968302.
- Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB: red blood cell transfusion thresholds and storage. JAMA. 2016;316(19):2025–2035.
- Li Y, Xiong Y, Wang R, et al. Blood banking-induced alteration of red blood cell oxygen release ability. Blood Transfus. 2016;14(2):238–244.
- Srinivasan AJ, Morkane C, Martin DS, et al. Should modulation of p50 be a therapeutic target in the critically ill? Expert Rev Hematol. 2017;10(5):449–458.
- Srinivasan AJ, Kausch K, Inglut C, et al. Estimation of achievable oxygen consumption following transfusion with rejuvenated red blood cells. Semin Thorac Cardiovasc Surg. 2018;30(2):134–141.
- Asakura T, Kawai Y, Yoneyama Y, et al. Use of sodium borohydride in determination of oxygen dissociation curves of hemoglobin. Anal Biochem. 1964;7:393–400.
- Hill A. The possible effects of the aggregation of the molecules of haemoglobin on its dissociation curves. J Physiol (Lond). 1910;40:4–7.
- Jandaruang J, Siritapetawee J, Songsiriritthigul C, et al. Purification, characterization, and crystallization of Crocodylus siamensis hemoglobin. Protein J. 2014;33(4):377–385.
- Manjula BN, Acharya SA. Purification and molecular analysis of hemoglobin by high-performance liquid chromatography. Methods Mol Med. 2003;82:31–47.
- Wang Q, Zhang R, You G, et al. Influence of polydopamine-mediated surface modification on oxygen-release capacity of haemoglobin-based oxygen carriers. Artif Cells Nanomed Biotechnol. 2018;46(Sup2):484–492.
- Weber RE, Fago A, Campbell KL. Enthalpic partitioning of the reduced temperature sensitivity of O2 binding in bovine hemoglobin. Comp Biochem Physiol A Mol Integr Physiol. 2014;176:20–25.
- Wang Y, You G, Chen P, et al. The mechanical properties of stored red blood cells measured by a convenient microfluidic approach combining with mathematic model. Biomicrofluidics. 2016;10(2):024104.
- Zanardo V, Mari G, de Luca F, et al. Lactate in cord blood and its relation to fetal gluconeogenesis in at term deliveries. Early Hum Dev. 2015;91(3):165–168.
- Dukić L, Milevoj Kopčinović L, Dorotić A, et al. Blood gas testing and related measurements: national recommendations on behalf of the Croatian Society of Medical Biochemistry and Laboratory Medicine. Biochem Med. 2016;26(3):318–336.
- Siggaard-Andersen O, Siggaard-Andersen M. The oxygen status algorithm: a computer program for calculating and displaying pH and blood gas data. Scand J Clin Lab Invest Suppl. 1990;203:29–45.
- Mahoney JJ, Wong RJ, Van Kessel AL. Reduced bovine hemoglobin solution evaluated for use as a blood gas quality-control material. Clin Chem. 1993;39(5):874–879.
- Perutz MF, Fermi G, Poyart C, et al. A novel allosteric mechanism in haemoglobin. Structure of bovine deoxyhaemoglobin, absence of specific chloride-binding sites and origin of the chloride-linked Bohr effect in bovine and human haemoglobin. J Mol Biol. 1993;233(3):536–545.
- Fronticelli C, Sato T, Orth C, et al. Bovine hemoglobin as a potential source of hemoglobin-based oxygen carriers: crosslinking with bis(2,3-dibromosalycyl)fumarate. Biochim Biophys Acta. 1986;874(1):76–81.
- Kawaguchi AT, Salybekov AA, Yamano M, et al. PEGylated carboxyhemoglobin bovine (SANGUINATE) ameliorates myocardial infarction in a rat model. Artif Organs. 2018;42(12):1174–1184.
- Wang Q, Sun L, Ji S, et al. Reversible protection of Cys-93(β) by PEG alters the structural and functional properties of the PEGylated hemoglobin . Biochim Biophys Acta. 2014;1844(7):1201–1207.
- Webster KD, Dahhan D, Otto AM, et al. “Inside-Out” PEGylation of bovine β-cross-linked hemoglobin. Artif Organs. 2017;41(4):351–358.
- Roamcharern N, Payoungkiattikun W, Anwised P, et al. Physicochemical properties and oxygen affinity of glutaraldehyde polymerized crocodile hemoglobin: the new alternative hemoglobin source for hemoglobin-based oxygen carriers. Artif Cells Nanomed Biotechnol. 2019;47(1):852–861.
- Guarnone R, Centenara E, Barosi G. Performance characteristics of Hemox-Analyzer for assessment of the hemoglobin dissociation curve. Haematologica. 1995;80(5):426–430.
- Hess JR. Measures of stored red blood cell quality. Vox Sang. 2014;107(1):1–9.
- Cambier C, Wierinckx M, Clerbaux T, et al. Haemoglobin oxygen affinity and regulating factors of the blood oxygen transport in canine and feline blood. Res Vet Sci. 2004;77(1):83–88.
- Li Q, Ma H, Zhang Y, et al. Study on oxidation stability and oxygen affinity of hemoglobin during storage. Artif Organs. 2018;42(12):1185–1195.
- Garcia-Roa M, Del C V M, Bobes A M, et al. Red blood cell storage time and transfusion: current practice, concerns and future perspectives. Blood Transfus. 2017;15(3):222–231.