 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Medical support for traumatic haemorrhage is lacking for far-forward combat units. VIR-HBOC (haemoglobin-based oxygen carrier) is a novel biological therapeutic under development as a field-stable resuscitation fluid. HBOCs have a long history of complications, chief among them is vasoconstrictive hypertension, which must be resolved before efficacy testing. As such, VIR-HBOC was compared against Lactated Ringers (LRS; vehicle) and a cross-linked haemoglobin (ααHb; a known vasoactive HBOC) in a rat topload model. Twenty-three male, Sprague Dawley rats were randomly assigned to receive a 10% infusion (estimated total blood volume) of one test article while normotensive and under anaesthesia. Cardiovascular, blood chemistry and oximetry, microvascular arteriolar diameters, and interstitial tissue oxygenation parameters were measured. Circulatory half-life was calculated by plasma total haemoglobin. Treatment with ααHb caused immediate increases in mean arterial pressure compared to LRS and VIR-HBOC groups, and corresponding arteriolar vasoconstriction (p < .05), which did not occur for LRS or VIR-HBOC. Circulatory half-lives for VIR-HBOC and ααHb were calculated as 340 and 157 min, respectively. This first report of VIR-HBOC showed no evidence of a hypertensive or vasoactive effect. It was well-tolerated over the eight-hour time course of this topload model, which warrants further investigation in studies of haemorrhagic shock.
Introduction
There is a dire, unmet need for transfusion alternatives to whole blood. Haemoglobin-based oxygen carriers (HBOCs), commonly manufactured from purified human or bovine haemoglobin (Hb), are designed to relieve hypoxia in biological systems. They are positioned to meet needs in the following areas: organ preservation, transfusion when blood is not an option (e.g. cases of religious exemption, autologous hemolysis), and pre-hospital resuscitation, particularly in rural (civilian) communities and far-forward (military) settings with tight logistical constraints.
HBOCs can have several distinct advantages over stored blood, including universal usage, longer shelf-life, and reduced cold-chain support [Citation1–3]. However, regulatory concerns have prevented earlier generations from approval in the United States. For example, one meta-analysis of sixteen unrelated clinical trials of five different products suggested that HBOC infusions in physiologically compromised human patients increased mortality and incidence of myocardial infarction as compared to controls [Citation4]. While the meta-analysis was widely contested [Citation5], a hypertensive effect of early-generation HBOCs was well-documented [Citation6]. The result is an automatic burden to evaluate all novel purified Hb products for vasoactive and hypertensive side-effects.
Early HBOCs contained exclusive or significant amounts of single, dissociable Hb tetramers, which readily extravasated from the vascular lumen. Newer generations utilize additional processing (e.g. Hb hyperpolymerization or PEGylation) to minimize free αβ Hb dimers or α2β2 Hb tetramers and create larger macromolecules, which increased circulatory half-life. In addition to better efficacy, larger molecules avoid the extravasation that produces the observed vasoactive effect of HBOCs by subendothelial scavenging of the endothelial-derived relaxing factor, nitric oxide (NO).
Endothelial NO is an endogenous vasodilator constitutively produced by the vascular endothelium and, when sequestered by acellular Hb, leads to arteriolar constriction [Citation7–10] of the smooth endothelial muscle surrounding arterioles [Citation11–13]. Research has shown that minimising the ratio of Hb αβ dimers or α2β2 tetramers [Citation8,Citation14] and increasing the molecular size of the HBOC [Citation9,Citation13,Citation15,Citation16] may decrease the rate of extravasation and attenuate vasoconstriction.
VIR-HBOC (VirTech Bio, Natick, MA) is a novel product under development that uses hyperpolymerized human Hb to achieve an average molecular weight of 1.6 MDa. Other novel properties include above-average Hb concentration (11 g/dL), low solution colloid osmotic pressure (12 mmHg), and high viscosity (12 cPs).
VIR-HBOC has already shown promise in ex vivo organ perfusion studies [Citation17,Citation18] and is further explored here in its first in vivo pre-clinical study. Aims were to determine any vasoconstrictive and hypertensive effects and circulatory half-life in a well-established topload (TL) model that has been employed to evaluate second- and third-generation HBOCs and perfluorocarbon emulsions for over a decade [Citation19–22]. The rat model has been useful because it supports comprehensive physiological and microcirculatory instrumentation for simultaneous monitoring of systemic and localized responses to treatment. The TL approach is more appropriate than hemodilution or haemorrhagic shock (efficacy) models due to the potentially confounding impact of trauma on determining whether VIR-HBOC is vasoactive and hypertensive.
For the present study, we hypothesized that the novel chemical and solution characteristics of VIR-HBOC would result in reduced arteriolar constriction and pressor response when compared with a cross-linked haemoglobin (ααHb), which is known to be vasoactive [Citation23]. Based on previous experience with various HBOC TL studies, we did not anticipate enhanced interstitial fluid oxygenation (PISFO2) because of the small infusion volume. However, any observed decrease in PISFO2 would be expected if prolonged occurrences of vasoconstriction and hypertension were to result in partial ischaemia.
Methods and materials
Animals
The following protocol and experimental procedures were at Song Biotechnologies, LLC, Baltimore, MD. SoBran Biosciences, Inc IACUC approved the studies (Fairfax, VA; Protocol # SON-001-2015), which are consistent with the National Institutes of Health guidelines for the humane treatment of laboratory animals, as well as the American Physiological Society’s Guiding Principles in the Care and Use of Animals. SoBran Biosciences operated the animal care vivarium and administered the IACUC. Subjects were twenty-three male Sprague Dawley rats (300−350 g; Charles River Laboratories, Inc., Wilmington, MA) housed in pairs and maintained on a 12/12 light/dark cycle with free access to rat chow and water.
Surgical preparation
Animals were induced to anaesthesia with 1–5% isoflurane in air for initial pre-operative preparation and cannulations. Anaesthetic, alfaxalone acetate (Alfaxan, Schering-Plough Animal Health, Welwyn Garden City, UK), was infused continuously intravascularly at 0.1 mg/kg/min through a femoral vein cannula. Alfaxan is ideal for physiological studies focussed on cardiopulmonary and vascular function since it is minimally depressive per an appropriate anaesthetic depth—heel but not toe pinch response. The continuous rate of infusion allows for better control and consistency compared to bolus infusions. It has been used by our group in multiple topload and long term rat studies [Citation19,Citation20,Citation24–27] and recently in an unpublished swine model of haemorrhagic shock. A femoral artery cannula, which was not used for infusion and kept patent with heparinized phosphate-buffered saline (20 IU heparin/mL), was connected to a pressure transducer for monitoring of systemic circulatory variables with a multichannel physiological monitoring system (BIOPAC MP-150, BIOPAC Systems, Goleta, CA). The jugular vein was cannulated for TL fluid infusions. A tracheal tube (PE-240) was inserted to maintain airway patency; animals continued to inspire room air and were not artificially ventilated. After experimentation, animals were euthanized with a dose of Euthasol (150 mg/kg, pentobarbital component, intravenously; Delmarva, Midlothian, Virginia).
Spinotrapezius preparation
The rat spinotrapezius muscle was exteriorized as described by Grey [Citation28] with some modification to accommodate measurements of microvascular activity and PISFO2 on a thermostable animal platform adapted for intravital and phosphorescence quenching microscopy as described here [Citation27,Citation29] and in the supplemental section of Nugent et al. [Citation24]. Animals and exteriorized spinotrapezius muscle—isolated from atmospheric contamination by a transparent barrier film—were maintained at 37 °C during experimentation, which was monitored by a rectal probe (BIOPAC; Part # SS7L, BIOPAC Systems, Goleta, CA).
Microcirculatory parameters
Observation and measurement of the exteriorized spinotrapezius preparation were carried out with an intravital microscope (Axioimager2m, Carl Zeiss, Jena, Germany) configured for trans-illumination through a 20X/0.8 objective (Plan-APOCHROMATE, Zeiss) and custom modified for phosphorescence quenching microscopy (PQM). Trans-illumination was used to select measurement sites, establish appropriate focal planes, and verify flow conditions. Measurements of arteriolar diameter in the spinotrapezius muscle were made using a 10X/0.25 objective (N-Achroplan, Zeiss). The objective was focussed in the diametral plane and the image displayed on an ultra-high-definition screen (XBR43X830C, Sony, Tokyo, Japan). Images of the microvasculature were captured in real-time by a colour CMOS camera (Axiocam 305 colour, Zeiss, Germany) and stored digitally. Internal (luminal) vessel diameters for first-order arterioles (>80 μm) were measured by converting from pixels to µm using a calibration based on pixelization of a standard microscope micrometer (Stage Micrometer, Zeiss). Arterioles were used to assess vasoactivity.
Phosphorescence quenching microscopy
Phosphorescence quenching for the detection of oxygen (O2) in biological systems was initially described by Wilson et al. [Citation30,Citation31]. Adaptation of this technology for microscopic measurements of the PISFO2 followed our previous reports [Citation29,Citation32–34]. Calibration of our PQM equipment and O2 probe was performed as described by Golub and Pittman [Citation35]. Each measurement consisted of ten curves over ten seconds at a 1 Hz collection rate. Three interstitial sites were monitored per experiment.
Topload solutions
LRS (Hospira Inc., Lake Forest, IL) is a sterile, balanced electrolyte solution infusate used in hospitals to treat dehydration, act as a vehicle for drug delivery, or for temporary relief of hypotension. It is hypo-oncotic to blood and contains no specific O2 carrier. In this study, it served as a negative vehicle control to account for the effects of volume alone.
VIR-HBOC (VirTech Bio, Inc., Natick, MA) is a hyperpolymerized cell-free human Hb therapeutic developed for the treatment of blood loss in veterinary and human indications when blood is not an option. The Hb content with a p50 of 36 mm Hg is designed to facilitate O2 delivery, while blood volume is maintained through its colloidal properties. The average polymer molecular weight is 1.6 MDa at a concentration of ∼11 g/dL in a preparation of LRS modified with the addition of N-acetylcysteine as an antioxidant during storage. As a result, it has a low solution colloid osmotic pressure (12 mmHg) and high viscosity (12 cPs) to maintain blood volume without promoting fluid shifts or altering blood-flow shear effects.
ααHb is a first-generation tetrameric αα-crosslinked human Hb formulated at 8 g/dL with a p50 typically reported to be 26 mmHg, low viscosity (1.5 cPs) and high colloid osmotic pressure (42 mmHg). It is a known, vasoactive HBOC and served in this study as a positive control.
Experimental protocol
Following surgical preparation, anaesthetized animals were randomly assigned to receive a controlled, single-bolus infusion (10% of estimated total blood volume [TBV]) of either VIR-HBOC (N = 7), Lactated Ringer’s solution (LRS; volume control and vehicle; N = 7) or alpha-alpha cross-linked HBOC (ααHb; a highly-characterized first-generation HBOC; N = 9). Infusion occurred through the jugular vein at a rate of 0.25 ml/kg/min via a syringe pump (Genie TouchTM, Kent Scientific, Torrington, CT). Total infusion volume was 10% of the estimated TBV based on the following equation:
as reported here [Citation36]. After infusion, animals were observed for eight hours, challenged with the potent vasoconstrictor phenylephrine (PE; 1 mg/kg; West-Ward, Eatontown, NJ), and euthanized. Inclusion into the study required animals to tolerate surgical preparation and proceed through TL infusion without evidence of adverse events. The exclusion criteria were animal demise before or during TL infusion, evidence of adverse events associated with surgery or infusion, lack of response to PE challenge, or discrepancies in test articles. Evidence of external factors (e.g. human procedural error during measurements) during the observation phase was an additional exclusion factor.
Baseline (BL) measurements of mean arterial pressure (MAP), pulse pressure (PP), heart rate (HR), and temperature (BIOPAC), arteriolar diameter, and PISFO2 (microscopy) were recorded once the animal had stabilized from surgery (15−30 min). Next, 200 µL of whole blood was collected through the femoral artery cannula. 65 µL was used for blood gas/chemistry analysis via ABL90 Flex (Radiometer; Copenhagen, Denmark). The remaining volume was centrifuged and 65 µL of plasma drawn off for analysis via the ABL90 Flex.
Measurements through the BIOPAC data acquisition equipment were recorded continuously. Imaging of arteriolar diameters, measurement of PISFO2, and blood sampling were conducted at discrete time points throughout each experiment according to the following schedule: BL, Post-Infusion (PIn) where n is time from the end of infusion in minutes. Measurements occurred at 0, 60, 120, 180, 240, and 480 min PI. Continuous data were plotted to align with those discrete time points.
Statistics
Data are expressed as mean ± standard error of the mean (SEM). One-way ANOVA (Prism 6, GraphPad Software) was used to detect changes between groups for single measurements (e.g. weight, contractile response to PE). In cases where a significant difference was detected, an appropriate multiple comparison test (Tukey’s HSD) was conducted. Comparisons between groups were conducted using a Two-way ANOVA Mixed Effects Analysis (due to a missing data point at PI480) where appropriate and further analysed by Tukey’s HSD multiple comparison test or Dunnet’s test in cases for comparisons to BL or the VIR-HBOC group.
Data availability
Data are presented in and in mean ± standard error form. Please contact [email protected] for additional granularity.
Table 1. Systemic variables.
Table 2. Arterial blood chemistry (Whole Blood).
Results
Topload
Average animal weights were 323 ± 9.6, 325 ± 7.4, and 325 ± 8.3 g for LRS, VIR-HBOC, and ααHb, respectively, and were not significantly different. All animals tolerated TL infusion well and were free of adverse events. One animal in the ααHb group died at 422 min post-infusion, which was not determined to be associated with procedural error.
Mean arterial pressure
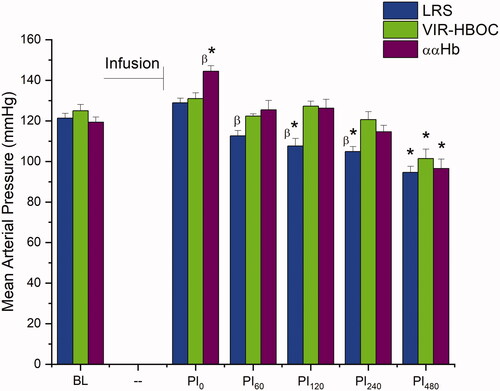
BL MAPs showed no statistical difference between the three treatment groups, 121 ± 2 for LRS, 125 ± 3 for VIR-HBOC, and 119 ± 3 mmHg for ααHb (). Following TL infusion, ααHb MAP showed a significant elevation to 144 ± 3 mmHg versus BL (p < .001) and VIR-HBOC (p < .01), which was not observed in the other two groups. By 60 min post-infusion (PI60), MAP for ααHb returned to BL (125 ± 5 mmHg) and remained unchanged for LRS and VIR-HBOC. PI120 showed hypotension in the LRS group with a MAP of 108 ± 4 mmHg compared to BL (p < .01) and VIR-HBOC (p < .05), while VIR-HBOC and ααHb groups remained at BL levels. LRS remained depressed for the remaining observation period. At PI480, all groups were lower than BL (p < .05).
Figure 1. Mean Arterial Pressure. Blood pressure was assessed continuously and plotted at hourly time points to align with discrete microcirculatory measurements. LRS-treated animals showed hypotension after 2 h, whereas VIR-HBOC kept MAP stable until 8 h. ααHb produced an immediate hypertensive response, which disappeared after 1 h. BL: Baseline; PI: Post-Infusion; Pin: n in minutes. *Indicates p < .05 compared to BL. βIndicates p < .05 compared to VIR-HBOC.
Arteriolar diameters
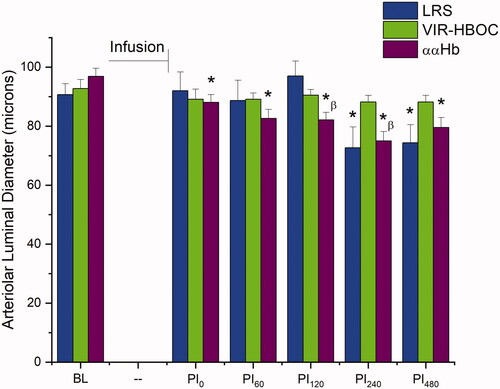
First-order arterioles, containing luminal diameters of 120 > 80 µm, were not different at BL for LRS, VIR-HBOC, and ααHb groups 92 ± 4, 93 ± 3, and 97 ± 3 µm, respectively (). ααHb treated animals showed significant vasoconstriction (compared to BL) following infusion (p < .001) and remained so until the end of the observation period (p < .0001), with the smallest diameter recorded at PI240 (75 ± 3 µm), a 23% decrease from BL. LRS animals began to show constriction at PI240, which endured until the end. In contrast, VIR-HBOC showed no significant changes to vessel diameter following infusion or during the 8-h observation period.
Figure 2. Arteriolar Luminal Diameter. Arteriolar luminal diameters within the spinotrapezius microcirculation. 10% TL infusion VIR-HBOC did not produce significant vasoactive effects. LRS became constricted four hours into the observation period. ααHb -treated animals, however, showed an early vasoconstrictive response to infusion that persisted until the end. BL: Baseline; PI: Post-Infusion; Pin: n in minutes. *Indicates p < .05 vs BL. βIndicates p < .05 compared to VIR-HBOC.
Peripheral tissue oxygenation (PISFO2)
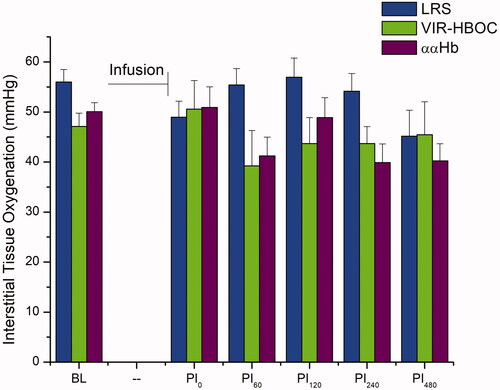
BL values for PISFO2 were as follows: LRS: 56 ± 2.5, VIR-HBOC: 41 ± 2.6, and ααHb: 50 ± 1.8 mmHg, and were not different (). TL infusion did not significantly change PISFO2 in any treatment group during the subsequent eight-hour observation phase, nor were differences detected between groups. A negative trend for LRS (p = .143) and ααHb (p = .203) compared to BL was visually evident towards the end of observation, whereas VIR-HBOC (p = .9996) remained steady.
Figure 3. Peripheral Interstitial Oxygenation. Interstitial oxygenation of spinotrapezius muscle. PISFO2 was not significantly affected by the TL protocol across or between groups. However, ααHb-treated animals appeared to trend downward after PI120 but remained well within the range of normoxia. BL: Baseline; PI: Post-Infusion; Pin: n in minutes.
Systolic, diastolic, and pulse pressures, heart rate, calculated cardiac output, and respiration
Systolic and diastolic blood pressures (SBP and DBP) were not different between groups at BL and generally followed the MAP profile. Some differences in intergroup comparisons are noted in .
Pulse pressures at BL were 43 ± 2.0, 49 ± 4.0, and 41 ± 1.0 mmHg for LRS, VIR-HBOC, and ααHb groups, respectively (). No significant changes were noted for LRS or VIR-HBOC treated animals, whereas, in contrast, ααHb showed an elevated pulse pressure at PI0 (60 ± 2.0 mmHg; p < .0001) that returned to BL at subsequent time points.
BL heart rates were 446 ± 10, 454 ± 6, and 449 ± 8 BPM for LRS, VIR-HBOC, and ααHb groups, respectively. No significant changes compared to BL were noted for LRS or ααHb groups. VIR-HBOC produced bradycardia at PI0 and PI60 (419 ± 8 and 407 ± 8 BPM; p < .001), which subsequently returned to BL.
Spontaneous respiration rates at BL were 116 ± 7, 117 ± 4, and 114 ± 6 per minute for LRS, VIR-HBOC, and ααHb groups, respectively. LRS and ααHb did not show differences during the observation phase compared to their BLs, but VIR-HBOC-treated animals showed an elevation in the rate at PI480 (p < .01; ). Intergroup comparison revealed no differences at any time point.
Blood chemistry and oximetry
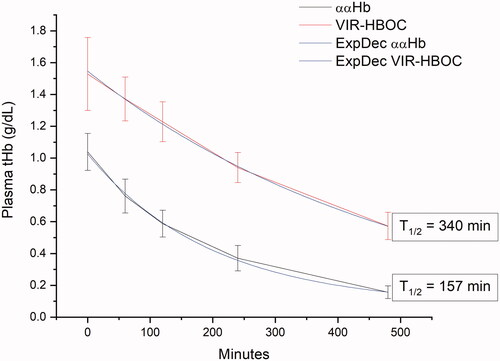
Complete results are listed in . Total Hb (tHb) at BL was equivalent across groups at 15.3 ± 0.6, 15.7 ± 0.2, and 15.8 ± 0.3 g/dL for LRS, VIR-HBOC, and, ααHb respectively. Whole blood tHb for ααHb was lower than VIR-HBOC after infusion. Plasma tHb rose differentially from non-detectable to 1.5 ± 0.09 and 1.0 ± 0.04 g/dL for VIR-HBOC and ααHb HBOCs, respectively (p < .01). As expected, tHb remained non-detectable in the plasma fraction of LRS-treated animals throughout the experiment. By PI480, LRS tHb in whole blood tHb for LRS was significantly lower than VIR-HBOC (p < .05). In the plasma fraction, tHb decreased with an exponential decay rate yielding half-life values of 340 and 157 min for VIR-HBOC and ααHb groups, respectively ().
Figure 4. Circulatory Half-life in Plasma. Total Haemoglobin (tHb; g/dL) was measured post-infusion from the plasma fraction of blood samples during the eight-hour observation period. LRS is not shown, but consistently reported non-detectable levels of Hb. Raw data was fit to a mono-exponential decay function and half-life calculated based on the fitting. Different formula concentrations of Hb in ααHb (8 g/dL) and VIR-HBOC (11 g/dL) explain why post-infusion tHb values were different.
For both VIR-HBOC and ααHb HBOCs, oxidized (Fe3+) Hb (%methaemoglobin, metHb), levels rose following infusion and remained elevated until the end of experimentation (p < .05). By PI120, levels for VIR-HBOC were higher than ααHb and LRS (p < .0001) and persisted (). HbO2 saturation (SO2) values dropped after infusion for VIR-HBOC and ααHb and then returned to BL for VIR-HBOC by PI60 and ααHb by PI120. In ααHb treated animals only, intravascular O2 tension (pO2) decreased after infusion and remained significantly lower than VIR-HBOC until PI120 (p < .01). Lactate rose above BL for all groups at PI120 (p < .05), while glucose remained at BL levels for LRS but decreased from BL after infusion for ααHb and VIR-HBOC. Glucose returned to BL by PI120 for VIR-HBOC but remained low for ααHb.
PE challenge
New microvascular sites were selected for the final PE challenge to exclude possible photo-interference from measurements during the observation phase. Before PE infusion, luminal diameters were 91 ± 5, 80 ± 5, and 87 ± 4 µm for LRS, VIR-HBOC, and ααHb groups, respectively, that were not significantly different from each other. Intravascular injection of PE caused rapid vasoconstriction to 40 ± 8, 28 ± 6, and 38 ± 4 µm of the respective groups (p < .0001 for all). Change in diameter was not different between groups. PE infusion induced hypertension as assessed by MAP. Starting pressures (LRS, 98 ± 6; VIR-HBOC, 102 ± 4; ααHb, 97 ± 5 mmHg) increased significantly within 30 s of infusion (LRS, 156 ± 5; VIR-HBOC, 162 ± 3; ααHb, 155 ± 5 mmHg). The hypertensive response from PE was not different between the groups in all aspects.
Discussion
In this first in vivo pre-clinical assessment of the novel VIR-HBOC, we report no adverse effects of a TL bolus in rats. Given the history of HBOC vasoactivity, primary interests were changes to blood pressure and arteriolar diameter. Infusion of ααHb, a first-generation HBOC, produced hypertension, consistent with previous reports [Citation30–32,Citation37], which corresponded to vasoconstriction in the microvasculature. VIR-HBOC, however, showed no evidence of localized vasoconstriction nor hypertension beyond the volume artefact seen with a 10% bolus infusion.
The single-bolus TL protocol afforded the study a robust physiological and microcirculatory instrumentation package to measure reactions and outcomes. Comparator groups, ααHb (positive control for vasoactivity) and LRS (vehicle), defined the model sensitivity to the vasoactive and hypertensive effects of HBOC infusions and volume artefacts of vehicle fluids. The protracted (eight hours) post-infusion observation phase allowed for the development of delayed reactions (e.g. damage to the vascular endothelium or myocardial effects) to infusion, along with establishment of circulatory half-life in rats.
Investigation of the microcirculation involved exteriorisation of the rat spinotrapezius—a true postural muscle—as performed in previous studies of vasoactivity [Citation33,Citation34], tissue oxygenation [Citation27,Citation35], and HBOC topload [Citation19–21]. This procedure, although minimally impactful to the microcirculation [Citation38], was more invasive than, for example, non-surgical visualisation of the corneal microcirculation, which is also amenable to fibre-optic phosphorescence quenching [Citation39]. However, exteriorisation allowed for homogenous loading of the phosphorescent probe and isolation from deeper oxygen sources. Additionally, transillumination provides superior optical clarity for both intravital microscopy due to optical clarity, which is important for detecting small changes in arteriolar diameter and targeting of specific points along the oxygen gradient, respectively.
One of the leading contraindications for HBOCs is pre-clinical hypertension, and systemic vasoconstriction [Citation40,Citation41], which have been observed in both humans and animals [Citation6,Citation19,Citation20]. VIR-HBOC produced neither hypertension nor evidence of vasoconstriction. In comparison, the infusion of ααHb was rapidly hypertensive and associated with developing vasoconstriction at PI0 that persisted throughout the experimental time course (). Vascular constriction with LRS at later time points is speculated to be the cumulative result of blood sampling (∼300 µL per sample) and the fact that crystalloids extravasate faster than protein solutions (HBOCs), which is supported by a corresponding decrease in tHb at PI480.
Mechanisms of HBOC-induced vasoconstriction are still incompletely described but may involve the disruption of tonic vasodilatory tone that is known to develop soon after infusion [Citation42]. Nitric oxide (NO) scavenging is the leading culprit since Hb’s affinity for NO is considerably higher than O2. Nevertheless, while the kinetics of NO binding is rapid (essentially diffusion-limited for this discussion), the localisation of cell-free Hb can vary. Hb is most effective in disrupting NO signalling when it extravasates into the region between the vascular endothelium and the sheath of vascular smooth muscle that determines contractile tone [Citation11]. The rate of protein extravasation is relatively slow and would explain why vasoconstriction develops over tens of minutes instead of seconds as it would with a rapid-diffusing pharmaceutical vasoconstrictor.
The observed hypertensive response to ααHb infusion was transient. MAP returned to BL by PI60, but the microvascular arterioles remained vasoconstricted. One possibility is the tendency of HBOCs to induce bradycardia [Citation37]. Trends were noted in this study for VIR-HBOC (significantly so at PI60) and ααHb, but there did not appear to be an association with MAP. A more likely explanation involves larger arterial compensation to the increase in total peripheral resistance throughout the vasculature. The diastolic pressure for ααHb was elevated at PI0 and returned to BL by PI60.
Later time points showed hypotension beginning with LRS at PI120 and all groups at PI480, which is supported by corresponding arterial vasoconstriction but not changes in PP or HR. This suggests a cumulative effect of blood sampling, fluid loss over 8 h (replacement fluids were not provided), and some degree of polytrauma from numerous surgical procedures that triggered and ultimately overwhelmed hypotensive compensatory mechanisms. LRS, which did not contain water-retaining protein, rapidly extravasated to the tissues and poorly compensated for the progressive fluid loss. Expectedly, groups treated with protein-containing colloids (i.e. HBOCs) were more resistant to hypotension, due to better intravascular fluid retention.
A critical element of biologic therapeutic efficacy is circulatory half-life. VIR-HBOC’s manufacturer predicted a half-life of four to six hours in rats for their product based on experience with other similar products, and testing fell within that range. ααHb’s measured half-life of 157 min was consistent with previous measurements of ααHb in rats [Citation43]. Since LRS served as a volume control and contained no Hb, the half-life was not measured in this study. However, LRS is known to be oedematous with a relatively short circulatory half-life in humans (20−40 min [Citation44]) as it is a non-colloidal solution, and this is consistent with the early hypotension and vasoconstrictive compensation seen in the LRS-treated animals.
It is of interest that PISFO2 of the spinotrapezius muscle was not different between or within groups. The TL model provided an uncomplicated view of healthy rodent physiology reacting to foreign biologics with intact compensatory responses. Under these conditions, the O2 transport chain operates with a large reserve to prevent hypoxia and, thus, O2 delivery to tissues is at functional excess to meet sudden changes in metabolic demand [Citation27]. So, it is unsurprising that changes in PISFO2 levels were not detected for any groups despite changes to blood flow evidenced by vasoconstriction in the ααHb group. This predicts that reserve O2 delivery capacity was not exceeded for any of the groups. If the tested HBOCs or LRS solutions were significantly disruptive to the vasculature, the developed pathology would have manifested in part as decreases in PISFO2. This underscores the need for higher severity models to assess HBOCs in their intended roles. Since O2 delivery and plasma expansion are critical features of HBOC products, an O2 and volume deficiency model (e.g. haemolytic anaemia, exchange transfusion, or haemorrhagic shock) is useful in efficacy studies of HBOC O2 delivery.
Summary
A novel HBOC O2 therapeutic, VIR-HBOC, was tested for the first time in vivo. Infused as a 10% TL bolus in rats, it was compared to volume-matched infusions of LRS and first-generation ααHb HBOC. The rat model was sensitive to the known hypertensive and vasoconstrictive effects of ααHb. VIR-HBOC did not induce vasoconstriction or hypertension, and there was no evidence of toxic effects in systemic, microvascular, oximetry, or blood chemistry measurements. VIR-HBOC was well-tolerated and is indicated for future pre-clinical safety and efficacy studies investigating its capacity to function as a red blood cell replacement solution.
Limitations
This pre-clinical study of acute product top-loading was performed in surgically-prepared, anaesthetized rats under aseptic conditions. Instrumentation, including exteriorisation of the spinotrapezius muscle, was not supportive of a survival study, which limited resolution on chronic complications. Robust data collection focussed on the proximal effects of product infusion in an animal that was not recovered from surgery. The HBOC product was laboratory grade and not the cGMP material used for pre-investigational new drug safety studies. Longer-term, GLP-compliant toxicology, and safety studies are still needed to characterize any chronic effects of treatment. Although the study did directly examine peripheral PISFO2, lactate, and other clinical blood chemistry parameters, critical organ function was mostly assumed, and sub-clinical damage may not have been evident. Future safety and toxicology studies using cGMP products will better evaluate detrimental tissue and organ effects through histopathology and biosampling. Also, depletion of platelet inhibiting factors was not measured.
Acknowledgments
The authors are grateful to the United States Special Operations Command and the U.S. Department of Defense for study funding and conceptual assistance.
Disclosure statement
Authors Richard Light, Kim Vandegriff and Joe Tucker are employees of VirTech Bio, Inc., which produces VIR-HBOC.
Additional information
Funding
References
- Moore EE. Blood substitutes: the future is now. J Am Coll Surg. 2003;196(1):1–17.
- Dube GP, Vranckx P, Greenburg AG. HBOC-201: the multi-purpose oxygen therapeutic. EuroIntervention: journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the. EuroIntervention. 2008;4(1):161–165.
- Riess JG. Oxygen carriers (“blood substitutes”)-raison d’etre, chemistry, and some physiology. Chem Rev. 2001;101(9):2797–2920.
- Natanson C, Kern SJ, Lurie P, et al. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis. JAMA: J Am Med Assoc. 2008;299(19):2304–2312.
- Golub RM. Hemoglobin-based blood substitutes and risk of myocardial infarction and death. letters section. JAMA: J Am Med Assoc. 2008;300(11):5.
- Chen JY, Scerbo M, Kramer G. A review of blood substitutes: examining the history, clinical trial results, and ethics of hemoglobin-based oxygen carriers. Clinics. 2009;64(8):803–813.
- Schultz SC, Grady B, Cole F, et al. A role for endothelin and nitric oxide in the pressor response to diaspirin cross-linked hemoglobin. J Lab Clin Med. 1993;122(3):301–308.
- Thompson A, McGarry AE, Valeri CR, et al. Stroma-free hemoglobin increases blood pressure and GFR in the hypotensive rat: role of nitric oxide. J Appl Physiol. 1994;77(5):2348–2354.
- Gould SA, Moss GS. Clinical development of human polymerized hemoglobin as a blood substitute. World J Surg. 1996;20(9):1200–1207.
- Doherty DH, Doyle MP, Curry SR, et al. Rate of reaction with nitric oxide determines the hypertensive effect of cell-free hemoglobin. Nat Biotechnol. 1998;16(7):672–676.
- Kavdia M, Tsoukias NM, Popel AS. Model of nitric oxide diffusion in an arteriole: impact of hemoglobin-based blood substitutes. Am J Physiol Heart Circ Physiol. 2002;282(6):H2245–H2253.
- Matheson B, Kwansa HE, Bucci E, et al. Vascular response to infusions of a nonextravasating hemoglobin polymer. J Appl Physiol. 2002;93(4):1479–1486.
- Olson JS, Foley EW, Rogge C, et al. No scavenging and the hypertensive effect of hemoglobin-based blood substitutes. Free Radic Biol Med. 2004;36(6):685–697.
- Rice J, Philbin N, Light R, et al. The effects of decreasing low-molecular weight hemoglobin components of hemoglobin-based oxygen carriers in swine with hemorrhagic shock. J Trauma. 2008;64(5):1240–1257.
- Hare GM, Harrington A, Liu E, et al. Effect of oxygen affinity and molecular weight of HBOCs on cerebral oxygenation and blood pressure in rats. Can J Anesth/J Can Anesth. 2006;53(10):1030–1038.
- Yu B, Liu Z, Chang TM. Polyhemoglobin with different percentage of tetrameric hemoglobin and effects on vasoactivity and electrocardiogram. Artif Cells Blood Substit Immobil Biotechnol. 2006;34(2):159–173.
- Light W, Malavalli A, Vandegriff K, et al. Liver preservation with machine perfusion and a new human-derived hemoglobin-based oxygen carrier solution (HDHBOC). Philidelphia (PA): American Transplant Congress; 2018.
- Light W, Malavalli A, Vandegriff K, et al. Development of a new hemoglobin-based oxygen carrier solution (VIR-XV1) for liver allograft preservation in combination with machine perfusion Int Symposium Blood Substitutes & Oxygen Therapeutics Meeting; Montreal, Canada2017.
- Song BK, Nugent WH, Moon-Massat PF, et al. Effects of top-loading a zero-link bovine hemoglobin, OxyVita, on systemic and microcirculatory variables. Mil Med. 2013;178(5):570–577.
- Song BK, Nugent WH, Moon-Massat PF, et al. Effects of a hemoglobin-based oxygen carrier (HBOC-201) and derivatives with altered oxygen affinity and viscosity on systemic and microcirculatory variables in a top-load rat model. Microvasc Res. 2014;95:124–130.
- Sheppard FR, Macko AR, Song BK, RA. Evaluation of third generation Hemoglobin-Based Oxygen Carriers (HBOCs) on systemic and microcirculatory vasoactivity in a top-load rat model. Namru-SA Dtic. 2013:33.
- Macko AR, Sheppard FR, Song BK, RA. Evaluation of novel perfluorocarbon-based oxygen carriers (PFCs) on systemic and microcirculatory vasoactivity in a top-load rat model. Namru-SA Dtic. 2013:15.
- Caron A, Malfatti E, Aguejouf O, et al. Vasoconstrictive response of rat mesenteric arterioles following infusion of cross-linked, polymerized, and conjugated hemoglobin solutions. Artif Cells Blood Substit Immobil Biotechnol. 2001;29(1):19–30.
- Nugent WH, Cestero RF, Ward K, et al. Effects of sanguinate(R) on systemic and microcirculatory variables in a model of prolonged hemorrhagic shock. Shock. 2017;52(1S Suppl 1):108–115.
- Nugent WH, Jubin R, Buontempo PJ, et al. Microvascular and systemic responses to novel PEGylated carboxyhaemoglobin-based oxygen carrier in a rat model of vaso-occlusive crisis. Artif Cells Nanomed Biotechnol. 2019;47(1):95–103.
- Nugent WH, Sheppard FR, Dubick MA, et al. Microvascular and systemic impact of resuscitation with pegylated carboxyhemoglobin-based oxygen carrier or hetastarch in a rat model of transient hemorrhagic shock. Shock. 2019;52(1s suppl 1):108–115.
- Nugent WH, Song BK, Pittman RN, et al. Simultaneous sampling of tissue oxygenation and oxygen consumption in skeletal muscle. Microvasc Res. 2016;105:15–22.
- Gray SD. Rat spinotrapezius muscle preparation for microscopic observation of the terminal vascular bed. Microvasc Res. 1973;5(3):395–400.
- Golub AS, Pittman RN. Thermostatic animal platform for intravital microscopy of thin tissues. Microvasc Res. 2003;66(3):213–217.
- Baylis C. Acute and long-term effects of modified hemoglobin (HBOC-201) in a rat model of hypertension and chronic kidney disease. Transfusion. 2006;46(7):1104–1111.
- Gotshall RW, Hamilton KL, Foreman B, et al. Glutaraldehyde-polymerized bovine hemoglobin and phosphodiesterase-5 inhibition. Critical Care Medicine. 2009;37(6):1988–1993.
- Buehler PW, Mehendale S, Wang H, et al. Resuscitative effects of polynitroxylated alphaalpha-cross-linked hemoglobin following severe hemorrhage in the rat. Free Radic Biol Med. 2000;29(8):764–774.
- Xiang L, Naik JS, Hester RL. Functional vasodilation in the rat spinotrapezius muscle: role of nitric oxide, prostanoids and epoxyeicosatrienoic acids. Clin Exp Pharmacol Physiol. 2008;35(5–6):617–624.
- Craig JC, Colburn TD, Hirai DM, et al. Sexual dimorphism in the control of skeletal muscle interstitial Po2 of heart failure rats: effects of dietary nitrate supplementation. J Appl Physiol. 2019;126(5):1184–1192.
- Golub AS, Tevald MA, Pittman RN. Phosphorescence quenching microrespirometry of skeletal muscle in situ. Am J Physiol Heart Circ Physiol. 2011;300(1):H135–43.
- Lee HB, Blaufox MD. Blood volume in the rat. J Nucl Med. 1985;26(1):72–76.
- Ning J, Wong LT, Christoff B, et al. Haemodynamic response following a 10% topload infusion of HemolinkTM in conscious, anaesthetized and treated spontaneously hypertensive rats. Transfus Med. 2000;10(1):13–22.
- Bailey JK, Kindig CA, Behnke BJ, et al. Spinotrapezius muscle microcirculatory function: effects of surgical exteriorization. Am J Physiol Heart Circ Physiol. 2000;279(6):H3131–7.
- Guerci P, Ergin B, Kandil A, et al. Resuscitation with PEGylated carboxyhemoglobin preserves renal cortical oxygenation and improves skeletal muscle microcirculatory flow during endotoxemia. Am J Physiol Renal Physiol. 2020;318(5):F1271–F1283.
- Irwin DC, Foreman B, Morris K, et al. Polymerized bovine hemoglobin decreases oxygen delivery during normoxia and acute hypoxia in the rat. Am J Physiol Heart Circ Physiol. 2008;295(3):H1090–H1099.
- Sakai H, Hara H, Yuasa M, et al. Molecular dimensions of Hb-based O2 carriers determine constriction of resistance arteries and hypertension. Am J Physiol Heart Circ Physiol. 2000;279(3):H908–15.
- Alayash AI. Oxygen therapeutics: can we tame haemoglobin? Nat Rev Drug Discov. 2004;3(2):152–159.
- Manning LR, Morgan S, Beavis RC, et al. Preparation, properties, and plasma retention of human hemoglobin derivatives: comparison of uncrosslinked carboxymethylated hemoglobin with crosslinked tetrameric hemoglobin. Proc Natl Acad Sci USA. 1991;88(8):3329–3333.
- Hahn RG, Lyons G. The half-life of infusion fluids: an educational review. Eur J Anaesth. 2016;33(7):475–482.
