?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The green approachable of metal nanoparticles is treated to be an eco-friendly path and cost-effectiveness. In this present study, nano copper was synthesized profitably by Cissus vitiginea. The synthesized nano copper was used to evaluate the antioxidant and antibacterial activity against urinary tract infections pathogens. The resulting constructed nanoparticles were characterized by using ultraviolet spectroscopy absorbance around 370 nm. Scanning electron microscopy results showed the distribution of nanoparticles and particles sizes are found to be in the range of 5–20 nm. X-ray diffraction spectrum characteristic diffraction peaks for copper nanoparticles were observed at 2θ ranges 35.5 and 43.2° correspond to lattice planes (1 1 1) and (2 0 2), respectively. X-ray photoelectron spectroscopy shows that two distinct peaks at binding energy resulted that the chemical states of copper. The results serve the evidence that the green mediated nano copper might indeed be the potential source to treat urinary tract infections caused by E. coli, Enterococcus sp., Proteus sp. and Klebsiella sp. This fact-finding conclusion is that C. vitiginea leaf extract based green synthesis nano copper particles proved to effectively kill it or significantly inhibit activity contra to urinary tract infection pathogens and exhibit excellent antioxidant activity.
Introduction
Nanotechnology and nanoscience is a multidisciplinary field to exploit the wide spectrum of the emerging area from fundamental sciences such as physics, chemistry electronics, material science with novel techniques and produces unusual and unique properties of a nanomaterial at nano range [Citation1,Citation2]. These studies provide support for the utilizing of copper nanoparticles as a unique class of biocidal agents because of the profitable copper as -a great antibacterial agent known for a long time [Citation3]. In the ancient period were used copper compounds in different forms [Citation4]. This noble metal copper nanoparticle was manipulated by common methods such as metal vapours synthesis [Citation4], exploding wire method [Citation5], vacuum vapour deposition [Citation6], laser irradiation [Citation7] and microemulsion [Citation8]. These techniques need high cost, pressure, radiation and toxic chemicals. But the usages of green or eco-friendly materials like the plant, enzymes, microbes, etc. not only ignore the hazards of noxious materials but also much restraint, simple and low cost [Citation9]. This greener method generating metal nanoparticles for being easily available, environmentally benign and main reason for less tedious purification steps [Citation10–13]. Several studies reported that bioactive of green mediated copper nanomaterials are providing higher environmental mobility and allowing interact closely and penetrate inside with bacterial membranes also. Copper nanoparticles were synthesized using various plant extracts including tea leaf [Citation14], Murraya Koenigii leaves [Citation15], Eclipta prostrata leaves [Citation16], tomato [Citation17], Punica granatum peel extract [Citation18] and Aegle marmelos leaf [Citation19], etc.
Cissus vitiginea belongs to the family Vitaceae consists of about 14 genera and 900 species primarily distributed in tropical regions [Citation20]. It is a common medicinal value perennial climber and is distributed throughout India, particularly in tropical regions. It has a rich number of antioxidants [Citation21]. Antioxidants may be for great benefit in improving the quality of life by preventing or postponing the onset of degenerative diseases [Citation22]. This plant was used in Ayurveda as an alternative, dyspeptic, digestive, tonic analgesic in the eye and ear diseases and the treatment of irregular menstruation and asthma [Citation23]. This present study was attempted to find out the antibacterial and antioxidant activity of green synthesized nano copper by using C. vitiginea leaf extract as a reducing agent against the urinary tract pathogens and 2,2-diphenylpicrylhydrazyl (DPPH) free radical.
Materials and methods
Plant collection
The traditional and medicinally important C. vitiginea plant was collected from Tamil Nadu and its plant authentication number is PAR/2016/3578.
Preparation of plant extract
Fresh leaves of C. vitiginea was weighed (25 g) and washed with double distilled water. Washed leaves were mixed with 100 ml of distilled water and boiled at 60 °C for 10 min. The boiled leaf sample was cooled and filtered to obtain an aqueous extract of leaves. Thus, the prepared extract was stored at 4 °C for one week and further used for synthesis of copper nanoparticles.
Phytochemical analysis of plant extract
Phytochemicals such as carbohydrates, proteins, flavonoids, saponins, alkaloids, polyphenol, anthraquinone, steroids, terpenoids and tannins of aqueous extract of C. vitiginea were performed by following the method is adopted from Harborne [Citation24].
Preparation of plant-mediated copper nanoparticles
In the typical synthesis of copper nanoparticles, 10 ml of C. vitiginea plant leaf extract was added into 90 ml of 10 mM of copper sulphate solution and kept in stirring for constant mixing under room temperature. A colour change of the solution was noted by visual examination, this confirmed the synthesis of copper nanoparticles.
Characterization techniques of synthesized nanoparticles
The characterization of synthesized green mediated nanoparticles was analyzed by different techniques such as, the surface plasmon resonance (SPR) band for nano copper was identified by ultraviolet- visible (UV-Vis) spectroscopic analysis. Shape and size were analyzed using scanning electron microscope (SEM) and the crystalline structure of the synthesized nano copper analyzed using X-ray diffraction method (XRD). Elemental composition and chemical states of synthesized copper nanoparticles were characterized by X-ray photoelectron spectroscopy (XPS). Transmission electron microscopy (TEM) techniques utilized for the visualization of structural characteristics of green nano copper nanoparticles and topographical structure of the synthesized nano copper analyzed by using atomic force microscopy (AFM). The Fourier transform infrared spectroscopy (FT-IR) used to analyze phytochemicals responsible for nanoparticle synthesis.
Antibacterial activity of synthesized nano copper
The antibacterial activity of synthesized nano copper was performed by the agar disc diffusion method against urinary tract infection pathogens like E. coli, Enterococcus sp., Proteus sp., and Klebsiella sp. Fresh overnight culture of each strain was swabbed uniformly onto the individual plates containing sterilized and cooled Luria Bertani Agar. Then, 6 mm diameter sterile discs were impregnated into the copper nanoparticles solution at different concentrations (25, 50 and 75 µl, respectively). The impregnated discs were placed onto the plates and incubated for 24 h at 37 °C. Commercial antibiotic discs (ampicillin) were placed as control. After incubation, different levels of zonation formed around the discs were measured.
Antioxidant activity of synthesized nano copper
The antioxidant activity of green synthesized copper nanoparticles was examined by DPPH free radical scavenging assay. This assay was performed according to the method described by Liyana-Pathiranan and Shahidi [Citation25]. Briefly, the sample i.e. green synthesized copper nanoparticles were dissolved in methanol at different concentrations (100–500 mg). About 0.5 ml of dissolved plant-mediated nano copper particles were added to 3 ml of 0.5 mM DPPH in methanol solution and this reaction mixture was incubated for 30 min in the dark conditions at room temperature. The color change of the reaction mixture was noted from violet to yellow indicates that copper nanoparticles reduced the DPPH by donating a hydrogen atom. The absorbance of the reaction mixture was subsequently measured at 517 nm using a UV- Vis spectrophotometer. The percentage of DPPH inhibition was calculated by using the following formula
Result and discussion
Visual identification
Nanosized materials are having a great interest due to their unique optical properties. Nanoparticles were exhibit different array of colours while the synthesis process. Plant extract contains several phytochemicals that react with copper sulphate and converts into copper nanoparticles was primarily identified by the change of colour from blue to brown in the reaction mixture observed within 1 h. The phytochemicals present in the plant extract shown in . After 24 h reaction, the colour change was stopped and precipitation was observed indicates that the nanoparticles synthesis process was completed. Ashtaputrey et al. [Citation15] were similarly observed the colour change during the synthesis of copper nanoparticles at different incubation times.
Table 1. Phytochemical analysis of C. vitiginea.
UV-Vis spectroscopic analysis of copper nanoparticles
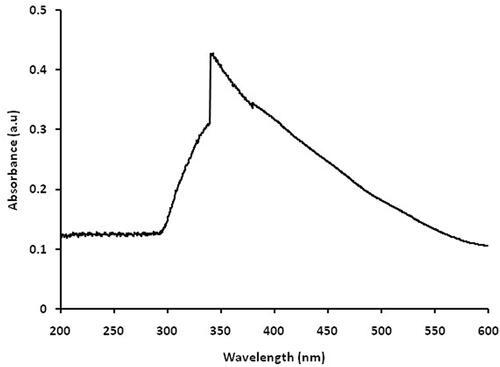
UV-Vis absorption spectra of the green synthesized copper nanoparticles were recorded at a different wavelength from 200–600 nm shown in . The copper nanoparticles are synthesized using copper sulphate and C. vitiginea leaf extract as a reducing agent displays an absorption peak at 340 nm. This peak can be assigned to be synthesized copper nanoparticles using plant extract [Citation15]. Broadened SPR peak was observed in this UV-Vis spectrum confirmed that polydispersed nanosized particles are synthesized [Citation26].
SEM and EDX of copper nanoparticles synthesized using C. vitiginea
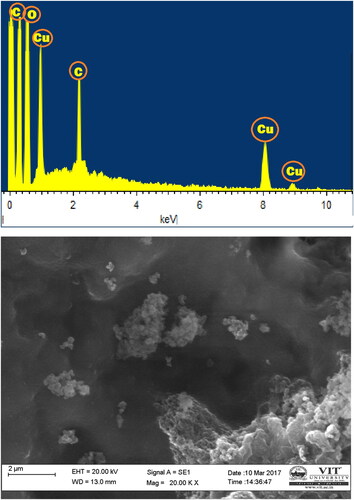
SEM and EDX images of nano copper particles synthesized using C. vitiginea leaf extract shown in . The surface morphology and size of the nano-copper by this eco-friendly method showed the nearly monodispersed distribution of particles sizes. The average particle size of the nano copper was observed around 20 nm. It shows mostly spherical nano copper, as well as the number of aggregates, synthesized nanoparticles and some of them, show the undefined shape nanoparticles. The EDX spectrum confirms the copper elements with carbon and oxygen. This aggregation may be due to the presence of secondary metabolites in the leaf extract of C. vitiginea.
XRD spectrum of green mediated copper nanoparticles
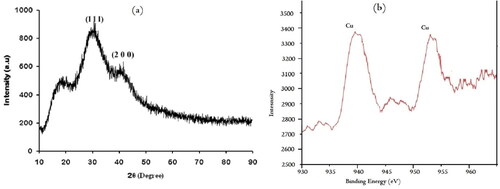
XRD spectrum provides an insight into the crystallinity of the nanoparticle represent. The nano copper synthesized using leaf extract of C. vitiginea was confirmed by X-ray diffraction analysis and the oxidation state of the nanoparticles was confirmed using XPS (). There are two lattice planes are (111) and (200) was observed for copper nanoparticles at the De Bragg’s reflection angles of 35.5 and 43.2°, respectively (JCPDS File No. 89-7102). These characteristic peaks represented that synthesized copper nanoparticles are crystalline. Here some unassigned peaks were present, it may fewer bio compounds. Similarly, Nasrollahzadeh et al. [Citation27] reported that crystalline copper nanoparticles synthesized by leaf extract (aqueous extract) of Euphorbia esula. L. Thus, XRD used to newly synthesized product is purely nano copper with high crystalline.
XPS analysis
XPS analysis of copper nanoparticles as shown in . It shows two peaks at the binding energy of 939.9 and 951.6 eV corresponding to Cu2p3/2 and Cu2p1/2, respectively, assigned for Cu(0) nanoparticles. These peaks were confirmed that the presence of copper. Similarly, Song et al. [Citation28] reported that the XPS spectrum of copper nanoparticles obtained two distinct peaks which are characterized for Cu(0).
AFM analysis of copper nanoparticles synthesized using C. vitiginea
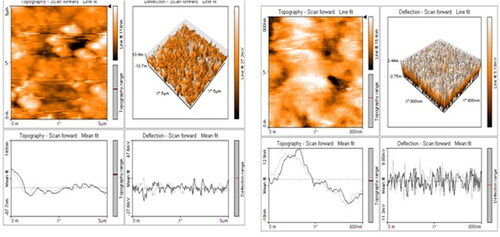
AFM characterization stated about morphological structure of green synthesized copper nanoparticles in three dimesional forms. This technique was used to analyse the surface structure and topography roughness of the synthesized copper nanoparticles. shows three-dimensional images in the particles were approximately spherical with aggregations was observed in different magnification ranges of 5–25 µm. Highly dispersed nanoparticles have an average size range of 10–50 nm ().
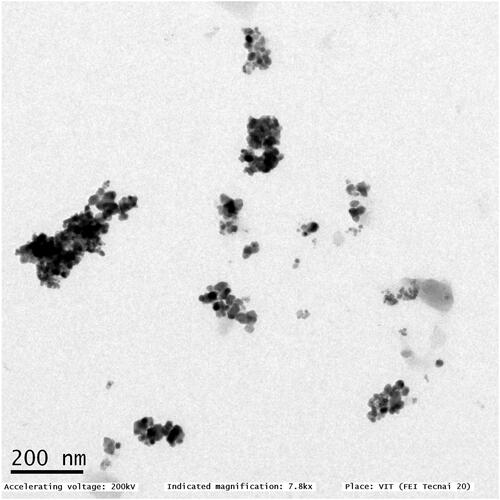
TEM and FT-IR analysis of copper nanoparticles synthesized using C. vitiginea
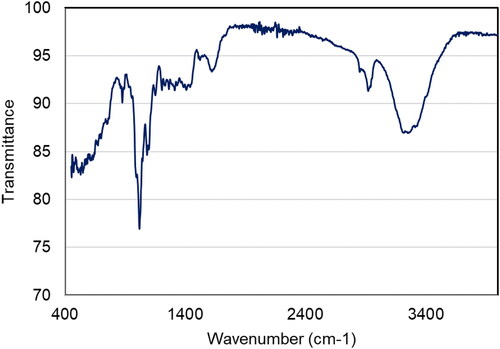
TEM is the most common tool in the conviction of structure, size, morphology, dispersion, and orientation of biological and physical samples. shows the typical TEM images of nano copper; this appearance is spherical and had broad size distribution [Citation29]. With a size range between 10–20 nm, these are medium-sized and widely dispersed. As Figure 5 illustrates the strong aggregation observed may be enhanced electrostatic attraction between particles. The size distribution of nano copper tends to broad while meaning an increase in the role of the C. vitiginea phytochemicals. Finally, we found the capping agents to control the diameter and particle size of nano copper. shows the FT-IR spectrum of copper nanoparticles synthesized using C. vitiginea. The peak at 1018, 1096, 1640, 2923 and 3294 cm−1 corresponds to the C–O stretch ethers or esters, N–H bends primary amines, H–C–H asymmetric and symmetric stretch alkanes and hydrogen bonded O–H stretch alcohols and phenols respectively. The FT-IR results indicate the phytochemicals of plant extract responsible for nanoparticle synthesis.
Antibacterial activity of copper nanoparticles against urinary tract infection (UTI) pathogens
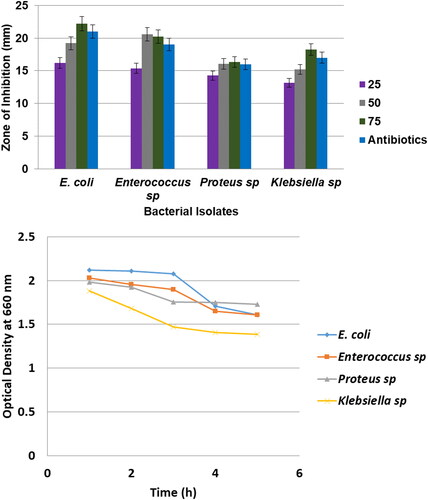
Now a day non-conventional antioxidant and antibacterial agents are becoming key in the pharmaceutical research field. The results of antibacterial action of spherical shaped nano copper treated against UTI pathogens, namely E.coli, Enterococcus sp., Proteus sp. and Klebsiella sp. and the zone of inhibition around the copper nanparticles loaded disc was noted and measured (). From our results, we observed that copper nanoparticles highly active against E. coli and Enterococcus sp. it shows the high zone of inhibition around 22.2 and 20.3 mm in diameter, respectively. Klebsiella sp. exhibit moderately inhibition (18.5 mm) whereas Proteus sp. exhibit less sensitivity (16.33 mm) to copper nanoparticles. Likewise, Ruparehia et al. [Citation30] reported that E. coli showed higher sensitivity to nano copper. It was the strains of E. coli and Enterococcus sp. was the more resistant microorganisms being higher than 80 and 70%, respectively. This nano copper appears to push its killing effect by introducing reactive hydroxyl radicals that can cause irrespirable damage such as the oxidation of proteins, separation of RNA and DNA molecules and cell membrane mutilation due to lipid peroxidation. Similarly, Sivaraj et al. [Citation31] proved that Tabernaemontana divaricate leaves mediated synthesized copper oxide nanoparticles were very effective nanomaterials against UTI pathogen especially E. coli. The nano copper proved that forcible inhibits the growth of UTI pathogens namely E. coli, Enterococcus sp., Proteus sp. and Klebsiella sp.
Antioxidant activity of copper nanoparticles
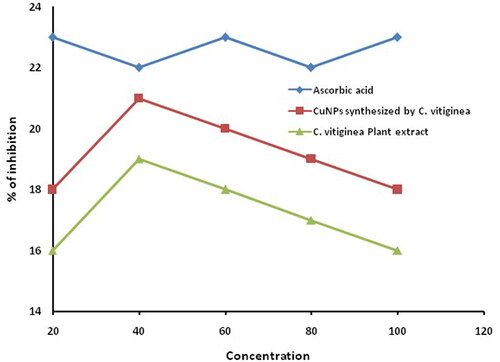
Naturally, antioxidants are product humans against many degenerative diseases and disorders. Among the various by nature products, phenolic compounds are general antioxidants that have the character of quenching oxygen-derived free radicals by donating hydrogen atom or an electron to the free radicals [Citation21]. Antioxidant activity of green synthesized copper nanoparticles against DPPH radical was assessed and compared with standard ascorbic acid. The percentage of inhibition of green synthesized copper nanoparticles was found at different concentrations. The inhibition was visually identified colour change from violet to yellow indicates that DPPH was reduced by donating hydrogen atom exhibit high scavenging activity. Maximum inhibition was found to be 21 and 19% for copper nanoparticles and plant extract, respectively (). C. vitiginea leaves extract which encompasses rich polyphenols, flavonoids and they are associated with nanoparticles. These phytochemicals may be enhancing the antioxidant activity of copper nanoparticles, which may discover a new way to ripening traditional medicine and treat many incurable diseases.
Conclusion
This investigation concluded that C. vitiginea leaf extract-based green synthesized copper nanoparticles have strong antioxidant and antibacterial activity against DPPH free radical and urinary tract infection pathogens. Green synthesis of copper nanoparticles was initially confirmed by the position of SPR band at 340 nm in UV-Vis spectra. The particle size of about 20 nm with a spherical shape. XRD spectrum shows crystalline nanostructured copper particles and AFM techniques illustrate the spherical shape of nanoparticles with aggregation. TEM image shows poly dispersed nanoparticles with a size of around 10−20 nm. The copper nanoparticles exhibited stronger antibacterial and antioxidant activity against UTI pathogens and DPPH free radical, respectively.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Koch CC. Nanostructured materials, processing, properties and applications. Norwich, New York: Noyes Publications; 2002.
- Gardea-Torresdey JL, Parsons JG, Gomez E, et al. Formation and growth of Au nano particles inside live alfalfa plants. Nano Lett. 2002;2(4):397–401.
- Panigrahi S, Kundu S, Ghosh SK, Nath S, et al. General method of synthesis for metal Nanoparticle. J Nanopart Res. 2004;6(4):411–414.
- Mari G, Bajpai SK, Chand N. Copper alginate- cotton cellulose (CACC) fibers with excellent antibacterials properties. J Eng Fiber Faber. 2009;4:24–35.
- Li M, Xiang K, Luo G, et al. Preparation of monodispersed copper nanoparticles by an environmentally friendly chemical reduction. Chin J Chem. 2013;31(10):1285–1289.
- Liu Z, Bando Y. A novel method for preparing copper nanorods and nanowires. Adv Mater. 2003;15(4):303–305.
- Yeh MS, Yang YS, Lee YP, et al. Formation and characteristics of Cu colloids from CuO powder by laser irradiation in 2-propanol. J Phys Chem B. 1999;103(33):6851–6857.
- Yallappa S, Manjanna J, Sindhe MA, et al. Microwave assisted rapid synthesis and biological evaluation of stable copper nanoparticles using T. arjuna bark extract. Spectrochim Acta A Mol Biomol Spectrosc. 2013;110:108–111.
- Rajeshkumar S. Anticancer activity of eco-friendly gold nanoparticles against lung and liver cancer cells. J Genet Eng Biotechnol. 2016;14(1):195–202.
- Ponnanikajamideen M, Rajeshkumar S, Vanaja M, et al. In-vivo anti-diabetic and wound healing effect of antioxidant gold nanoparticles synthesized using insulin plant (Chamaecostus cuspidatus). Can J Diabetes. 2019;43(2):82–89.e6.
- Rajeshkumar S, Rinitha G. Nanostructural characterization of antimicrobial and antioxidant copper nanoparticles synthesized using novel Persea americana seeds. Open Nano. 2018;3:18–27.
- Rajeshkumar S, Malarkodi C, Paulkumar K, et al. Algae mediated green fabrication of silver nanoparticles and examination of its antifungal activity against clinical pathogens. Int J Metals. 2014;2014:1–8.
- Sidjui LS, Ponnanikajamideen M, Malini M, et al. Lovoa trichilioides root back mediated green synthesis of silver nanoparticles and rating of its antioxidant and antibacterial activity against clinical pathogens. J Nanosci Nanotechnol. 2016;2(1):32–36.
- Mohindru JJ, Garg UK. Green synthesis of copper nanoparticles using tea leaf extract. IJESRT. 2017;6(7):307–311.
- Ashtaputrey SD, Ashtaputrey PD, Yelane N. Green synthesis and characterization of copper nanoparticles derived from Murraya koenigii leaves extract. Int J Chem Pharm Sci. 2017;10(3):1288–1291.
- Chung I, Rahuman AA, Marimuthu S, et al. Green synthesis of copper nanoparticles using Eclipta prostrata leaves extract and their antioxidant and cytotoxic activities. Exp Ther Med. 2017;14(1):18–24.
- Batoool M, Masood B. Green synthesis of copper nanoparticles using Solanum lycopersicum (tomato aqueous extract) and study characterization. J Nanosci Nanotechnol Res. 2017;1:1–5.
- Kaur P, Thakur R, Chaudhury A. Biogenesis of copper nanoparticles using peel extract of Punica granatum and their antimicrobial activity against opportunistic pathogens. Green Chem Lett Rev. 2016;9(1):33–38.
- Kulkarni V, Kulkarni P. Synthesis of copper nanoparticles with Aegle marmelos leaf extract. J Nanosci Nanotechnol. 2014;8(10):401–404.
- Wen J, Nie Z‐L, Soejima A, et al. Phylogeny of Vitaceae based on the nuclear GAI1 gene sequences. Can J Bot. 2007;85(8):731–745.
- Huang WY, Cai YZ, Zhang Y. Natural phenolic compounds from medicinal herbs and dietary plants: potential use for cancer prevention. Nutr Cancer. 2010;62(1):1–20.
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999;269(2):337–341.
- Niki E. Assessment of antioxidant capacity in vitro and in vivo. Free Radic Biol Med. 2010;49(4):503–515.
- Harborne JB. Phytochemical methods. 2 ed. New York: Chapman and Hall; 1984.
- Liyana-Pathirana CM, Shahidi F. Antioxidant activity of commercial soft and hard wheat (Triticum aestivum L.) as affected by gastric pH conditions. J Agric Food Chem. 2005;53(7):2433–2440.
- Ramyadevi J, Jeyasubramanian K, Marikani A, et al. Synthesis and antimicrobial activity of copper nanoparticles. Mater Lett. 2012;71:114–116.
- Nasrollahzadeh M, Sajadi SM, Khalaj M. Green synthesis of copper nanoparticles using aqueous extract of the leaves of Euphorbia esula L and their catalytic activity for ligand-free Ullmann-coupling reaction and reduction of 4-nitrophenol. RSC Adv. 2014;4(88):47313–47318.
- Song Y, Cho D, Venkateswarlu S, et al. Systematic study on preparation of copper nanoparticle embedded porous carbon by carbonization of metal–organic framework for enzymatic glucose sensor. RSC Adv. 2017;7(17):10592–10600.
- Suárez-Cerda J, Espinoza-Gómez H, Alonso-Núñez G, et al. A green synthesis of copper nanoparticles using native cyclodextrins as stabilizing agents. J Saudi Chem Soc. 2017;21(3):341–348.
- Ruparelia JP, Chatterjee AK, Duttagupta SP, et al. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008;4(3):707–716.
- Sivaraj R, Rahman PK, Rajiv P, et al. Biogenic copper oxide nanoparticles synthesis using Tabernaemontana divaricate leaf extract and its antibacterial activity against urinary tract pathogen. Spectrochim Acta A Mol Biomol Spectrosc. 2014;133:178–181.