?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The management of bacterial infections, especially trains of methicillin-resistant Staphylococcus aureus observe in health care settings, has markedly improved with the introduction of established drugs but using newer nano-based formulations. This study investigates the effects of vancomycin-linoleic acid nanoparticles on testicular tissue in an experimental animal model. Twenty-five adult male Sprague–Dawley rats maintained at the Animal House of the Biomedical Resources Unit were assigned to five groups namely E – solid lipid nanoparticles; F – vancomycin solid lipid nanoparticle; G – linoleic acid nanoparticle; H – vancomycin linoleic acid; and A – control. Perturbations in seminal fluid parameters showed a reduced sperm count in groups F & G which was statistically significant (p < .05) but motility and morphology were not significant when compared to controls (A). Reduced testosterone levels were found in groups E, F and H but were not statistically significant (p > .05). There was also increased luteinizing hormone (LH) and decreased in follicular stimulating hormone (FSH) levels was statistically significant (p < .05). Hypoplasia, tubular atrophy and shrinkage were observed in histologic sections of the treated groups with basement membrane thickening. Vancomycin solid lipid nanoparticle and its constituents SLN and LA disrupted testicular morphometry and the hormonal milieu sufficient to potentially induce altered reproductive function.
Introduction
Africa as a continent is endowed with the most significant infectious disease burden and the weakest public health infrastructure amongst all regions of the world [Citation1]. The crisis in antimicrobial resistance is increasing the global incidence of infectious diseases [Citation2]. Drug-resistant bacterial strains continue to be a challenge for most countries, including South Africa [Citation3,Citation4]. The current global crisis of antimicrobial drug resistance further amplifies the threat of infectious diseases to public health [Citation5]. It is estimated that more than 90% of death and disability caused by neglected diseases occurs in Sub-Saharan Africa [Citation6].
The development of drug resistance, inadequate target drug concentration at sites of infection and poor patient compliance often related to high administration frequency are significant factors for suboptimal therapeutic outcomes with the current spectrum of conventional antimicrobial drugs [Citation7,Citation8]. Efforts have been made to develop advanced and novel pharmaceutic material with optimal formulation and performance to treat serious infectious diseases [Citation9]. Despite giant strides, vancomycin-resistant enterococcal infections are now perceived as a significant burden of global health concerns owing to limited therapeutic modalities [Citation10]. Recently, authors have described the surge in vancomycin-resistant infections as a grave public health issue as it may result in serious intractable systemic infections [Citation11]. Emerging shreds of evidence implicate urogenital bacterial infections as a key contributor to the disruption of normal reproductive function [Citation12]. The challenges in vancomycin resistance have been attributed to alterations in the peptidoglycan terminus, changing it from the normal d-ala-d-ala to d-ala-d-lac. This alteration results in a decrease in vancomycin binding, decreasing the peptidoglycan efficacy leading to reduced cell wall thickness and failure to prevent cell wall synthesis [Citation13]. To circumvent the challenge of antimicrobial (vancomycin) resistance stemming from inadequate drug concentration at the target tissues and various reproductive complications resulting from antimicrobial resistance, researchers are now considering loading antimicrobial agents into nano-size particles of similar size scale to the microorganisms themselves.
Nanoparticles are materials with two or more dimensions in the range of 1–100 nm. These nanometre-sized particles are in a similar size range to antibodies and proteins [Citation14]. The reason nanoparticles (NPs) have become so useful resulting in this remarkable paradigm shift in pharmaceutics that can be attributed to their unique aspect ratio resulting in a very high surface area to volume ratio. This seemingly unremarkable feature, however, dramatically modulates their physical and chemical properties and consequently alters their catalytic activity making them highly reactive when compared to the bulk material [Citation15]. Various classes of NPs such as polymeric micelles, metallic, ceramic, lipid-based, carbon-based, metal oxide, quantum dots, porous materials and semiconductors have been previously described [Citation16,Citation17].
These NPs have been employed in cancer treatment, wound dressing, gene delivery, hyperthermia therapy, antimicrobial agents and drug delivery [Citation18,Citation19]. More so, candidature and effectiveness of nanoparticles in diagnosing viral infections with high sensitivity and in a short time have been reported [Citation17]. Lipid-based nano-conjugates such as liposomes, nano-emulsions, nano-structured lipid carriers, nano-liposomes, nano-cubosomes and solid lipid nanoparticles have widespread application in biomedical research. These wide research attentions were attributed to their physicochemical properties, high loading ability, steady release, low toxicity potential and biodegradable material properties [Citation20]. In a recent study, porous materials such as metal-organic frameworks (MOFs) 1,2 have received research attention and application as antimicrobial agents. Abdelhamid and colleagues (2020) prepared cerium-based MOFzyme (Ce-MOF) and investigated their antimicrobial activities. Their findings show effective inhibition of different fungal species [Citation21]. Furthermore, two nanoparticles could be coupled together to ensure synergistic antimicrobial activities. Recently, silver–carbon nanoparticles (AgCNPs) were prepared and administered to Holstein Friesian bulls in a recent study. The result of this investigation showed that AgCNPs were effective as alternative antibiotic agents as bull semen extenders without any cytotoxicity to the spermatozoa [Citation22].
However, NPs can affect biological behaviour at the cellular, subcellular, protein synthesis and gene levels, which necessitate continued research to determine their toxicity potential [Citation23] and to establish safe dosing levels in humans.
NPs can often be identified as foreign substances by the host immune cells causing them to react inappropriately against surface receptors and mount an unwanted inflammatory response. This often involves the production of copious amounts of diverse cytokines which can severely disrupt the host–pathogen response [Citation24]. This response can trigger major oxidative tissue and DNA damage leading to potential mutagenesis and various other auto-immune tissue damages [Citation25]. For example, the reported spermicidal activity of silver nanoparticles has impeded its application in the field of artificial insemination [Citation22].
In vitro testing of ion paired fatty acid with vancomycin found it to be a superior antimicrobial to vancomycin alone, especially when incorporated in solid lipid nanoparticles [Citation15]. However, in vivo data are required to rule out potential limiting toxicity. A sensitive biological marker for tissue toxicity is the effect on reproductive tissue – notably the testis. Here the sensitivity of spermatozoa to toxicity and the high cell turnover rates enable the subtle impact of xenobiotics to be detected at a relatively early stage.
Still, an improved understanding of nanoparticle characteristics regarding testicular tissue anatomy will provide a better understanding of potential adverse effects and assist in designing nanoparticles for better targeted drug delivery. In many cases, a suitable surface modification such as pegylation may render toxic nanoparticles much less toxic. Our current research was designed to investigate the testicular morphological profile and the hormonal changes that follow a therapeutic dose equivalent of vancomycin nanoparticles administered to Sprague–Dawley rats. The aim was also to undertake a numerical stereological analysis to quantify the changes in the reproductive parameters following the administration of vancomycin-loaded NPs.
Materials and methods
Preparation of ion-paired vancomycin SLN
Triethylamine (TEA) (500 mg), linoleic acid (10 mg) and water (0.2mls) were added to vancomycin-HCL (5 mg) and heated to 80 °C until excess TEA evaporated. The vanco-LA conjugate formed was then characterized by Fourier transformed-infrared analysis using a Bruker Alpha spectrophotometer (Ettlinglen, Germany) and by molecular modelling to characterize the conjugate and its lipid solubility (n-octanol/water partition coefficient) to determine the influence of LA on the behaviour of vancomycin. The SLN was prepared by hot homogenization and ultrasonication methods. Compritol 888 ATO was used as the solid lipid and Lurol F68 (3% w/w) as the surfactant. Both were heated separately to 80 °C, added to the liquid phase and homogenized at 6000 rpm for 15 min. The resulting emulsion was immediately subjected to high-intensity probe sonication for 30 min and cooled to 20 °C in an ice bath. LA only, vanco-HCL only and SLN were similarly prepared. SLNs were characterized for size, polydispersity index (PI) and zeta potential (ZP) measured at 25 °C with values 102.7 ± 1.01, 0.225 ± 0.02 and −38.8 ± 2.1 mV, respectively. The entrapment efficiency (%EE) for VCM-HCL SLN and LA-VCM were 16.81 ± 3.68 and 70.73 ± 5.96, respectively. Surface morphology and physical stability were characterized by transmission electron microscopy (TEM) and showed spherical NPs in the size range of 95–100 nm. The particles were stable at 4 °C for 3 months.
Laboratory animals
Twenty-five pathogen-free adult male Sprague–Dawley rats maintained at the animal house of the Biomedical Resources Unit, University of KwaZulu-Natal, South Africa, were used in this study. They were randomly assigned to a control and four treatment groups with five animals per group. The controls were administered normal saline intra-peritoneally (IP). The treatment was conducted between 8:00 am and 10:00 am for 4 weeks and all administrations were done via the intraperitoneal route. Doses were calculated from human dose equivalents as follows:
Vancomycin dose 10 mg/kg 12 h ➔ 20–40 mg/day per 130–170 g rat ➔ 5 mg/d per rat. The 5 mg of vancomycin [Citation26] was used to prepare a 1.28 mg/ml [Citation27] vancomycin solution providing 10 mg of linoleic acid dispersed in 1 ml solution [Citation28].
SLN – no vancomycin (group E), vancomycin solid lipid nanoparticle VSLN (group F), linoleic acid nanoparticle LN (group G) and vancomycin linoleic acid VLA (group H) were administered to the treatment groups E, F, G and H. All animals received humane care following the Principle of Laboratory Animal Care of the National Medical Research Council and the Guide for the Care and Use of Laboratory Animals of the National Academy of Sciences (National Institute of Health Guide, 1985).
Ethical approval was obtained from the Animal Research Ethics Committee (Reference number AREC/010/016PP).
All the rats were housed in well-ventilated plastic cages (5 rats per cage) having dimensions of (52 cm long × 36 cm wide and 24 cm high) and softwood shavings were employed as bedding. They were maintained under standardized animal house conditions (temperature: 28–31 °C and light: approximately 12 h natural light per day), fed standard rat pellets (sourced from Meadow, a Division of Astral Operations Limited, Durban, South Africa) and given tap water ad libitum. The initial bodyweight of the animals was measured, and the animals randomly assigned with five rats per group.
Experimental design and treatment
Animal maintenance and weight measurement
Animals were weighed on the first day of the experiment, thereafter weekly and then on the last day of the study. Weights were taken in the morning between 8:00 am and 10:00 am using a calibrated electronic balance (Zeiss West Germany (Pty) Ltd, Jena, Germany).
Animal sacrifice and collection of samples
At the end of the experiment, the animals were euthanized by placing them in a flask with excess Halothane® following which, blood samples were immediately collected via trans-cardiac puncture. Five millilitres (5 ml) sample of blood was obtained from each animal and immediately transferred into plain tubes and allowed to clot. Serum was then obtained by centrifuging at 3000 rpm for 10 min.
The testes were excised following laparotomy and separated from the cauda epididymis. Each testis was weighed individually using an electronic balance (Mettler Toledo, Microstep (Pty) Ltd., Greifensee, Switzerland). The weight of the testis from each animal was individually measured and recorded. Each testis was then measured for length and maximum diameter for future stereological analysis. One testis was immediately fixed in Bouin’s solution for histological analysis while biopsies were taken from the second testis and fixed with glutaraldehyde for later electron microscopy (EM). Fresh biopsies were also taken, and the tissue immersed in liquid nitrogen for later tissue antioxidant assay.
Sperm motility, count and morphology
The cauda epididymis from each animal was minced in 5 ml of normal saline and used to determine sperm count, sperm motility and sperm morphology. For sperm motility, a drop of epididymal fluid was collected within 5 min following sacrifice of the animal and the sample was placed on a prepared glass slide and covered with a coverslip (22 × 22 mm). It was then immediately examined under low light microscopy at 400× magnification [Citation29]. Each field was systematically scanned, and the motility of spermatozoa was assessed and subjectively graded as progressive, non-progressive and dead by. At least, 10 fields per slide were observed and averaged. The relative percentage of motile spermatozoa of all assessed microscope fields was estimated and recorded to the nearest 5% using a standardized subjective method [Citation30]. Each step of the process was repeated by a second investigator for confirmation.
The epididymal fluid was thoroughly mixed and approximately 10 μl of this diluted specimen was transferred to each of the counting chambers of the haemocytometer (Bio-RadR, Hercules, CA). Both sides of the counting chamber were used for each specimen and the average recorded to the nearest million/millilitre [Citation31].
Sperm morphology was assessed by staining a dry spermatozoa smear on a glass slide and stained with eosin–nigrosine followed by observation under a light microscope (Leica DM 500) at 400× magnification. The number of normal spermatozoa, spermatozoa with abnormal heads, spermatozoa with abnormal tails and spermatozoa with abnormal mid-pieces were recorded as a percentage of normal [Citation31].
Measurement of relative testis weight
The relative testes weights were calculated for the testes using the following formula:
Histological, histochemical and morphometric analyses
Testicular tissue was fixed in Bouin’s fluid and transferred to 70% ethanol. They were then processed using a graded ethanol series and embedded in paraffin wax. The paraffin wax sections were cut at 5 µm-thickness using a rotary microtome (micron HM 315 microtome, Walldorf, Germany) and stained with haematoxylin and eosin (H&E) [Citation32].
Histochemical studies were also done using the periodic acid-Schiff (PAS) staining technique used in routine histopathological investigations. This stain detects the presence of polysaccharides (e.g. glycogen) and muco-substances (e.g. glycoproteins, glycolipids and mucins) in tissue. The PAS technique is most commonly used to visualize the basement membrane and other components containing abundant glycogen. Further staining with Masson trichrome (MT) enabled assessment of the fibrous architecture. Sections were viewed and photographed using an Olympus light microscope (Olympus BX51, Tokyo, Japan) with an attached camera (Olympus E-330, Olympus Optical Co. Ltd., Tokyo, Japan).
For histomorphometric analysis, seven vertical sections from the polar and equatorial regions of the testis were sampled as an unbiased numerical estimation of the diameter and cross-sectional area of the seminiferous tubule (ST) and seminiferous epithelial thickness. This ensured that the determination used a systematic random sampling fair distribution technique [Citation33]. Eighteen seminiferous tubules with round profiles were then randomly selected on each slide. The vertical and horizontal diameters of each seminiferous tubule were measured as d1 and d2, respectively, and the mean diameter (D) was recorded as an observation. This was done to minimize longitudinal profiles that exhibit different degrees of damage or irregular shrinkage [Citation34]. The height and the tubular diameter of the ST epithelium were scanned using Leica SCN 400 (Leica Microsystems GmbH, Wetzlar, Germany), and measurements taken using the image analyzer and Leica microsystems software. The cross-sectional areas of the ST were also measured using the image analyzer and Leica microsystems software.
Determination of reproductive hormones
Blood samples were collected via cardiac puncture after overnight fasting. Samples were transferred into serum bottles and allowed to stand for 30 min before being centrifuged at 3000 rpm for 15 min in a Beckman bench centrifuge. The serum levels of luteinizing hormone (LH) and testosterone were determined using Elabscience enzyme immunoassay (ELISA) rat specific kits with catalogue numbers: E-EL-R0026 and E-EL-R0389 following the manufacturer’s protocol.
Statistical analysis
Data were analyzed using a one-way ANOVA followed by the LSD post-hoc test using Graph pad version 6 (GraphPad Software, La Jolla, CA). A p < .05 was considered statistically significant.
Results
Body weight, testicular weight and hormonal data
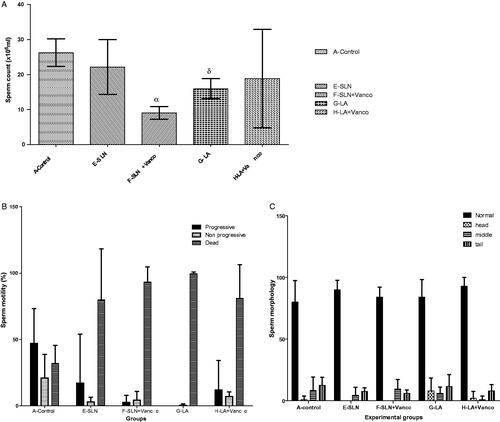
There was a reduction of body weight in animals treated with VSLN and LA-alone groups (3.4 ± 10 g and 7.0 ± 23 g, respectively) compared with weight gain in the control and other groups. The hormonal assay showed serum testosterone levels to be generally lower in groups E, F and H compared to controls, but this was not statistically significant (p > .05). LH levels were significantly elevated in groups E, F and G (p < .05), while values for group H were elevated but did not reach statistical significance (p > .05). There was a reduction in the levels of FSH (p < .05) in all groups when compared to controls ().
Table 1. Hormonal and weight changes in experimental groups.
Morphometry – seminiferous tubular diameter, cross-sectional area, membrane thickness
A significant decrease in the seminiferous tubular diameter was observed across the treated groups compared to the controls (p < .05). Similarly, the seminiferous tubular cross-sectional area in group E (SLN-treated rats) was 74,390 ± 9459 μm2 which was significantly lower than those of the controls (103,800 ± 13780 μm2) , and lower than those of groups F,G and H. On the contrary, the seminiferous tubular epithelial thickness showed a significant increase in groups E (SLN), F (VSLN) and H (VLA) compared to the control group (A). In contrast, only group G (LA) was significantly lower than group E (SLN) (p < .05) ).
Table 2. Morphometry of the seminiferous tubule – diameter, area, membrane thickness and cell count.
Seminal fluid analyses – sperm count, motility and morphology
A significant decrease in sperm count in the VSLN (F) and LA (G) groups was noted when compared with controls (p < .05). Data on sperm motility showed an overall variability that was not statistically significant (p > .05). Of note, the overall percentage of progressive motility was much lower than the control group with a correspondingly higher percentage of dead spermatozoa. Sperm morphology values showed little variation from normal morphology amongst all groups ().
Histology and histochemical staining of testicular sections – H&E, MT and PAS
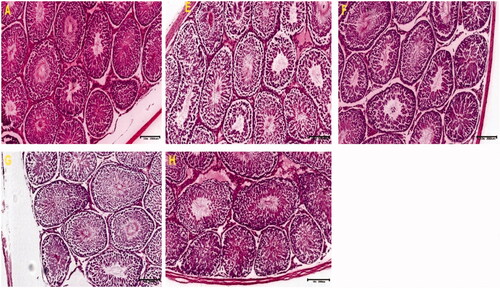
Testicular tissue stained with H&E from control animals showed well-preserved cyto-architecture with normal cellular composition in the seminiferous germinal epithelium and spermatozoa within the lumen. The interstitial spaces were normal with no observable infiltrations. The outlines of the seminiferous tubules in treated groups showed various levels of hypoplasia with tubular atrophy as well as thickening of the basement membrane ().
Figure 2. These photomicrographs depict the testicular sections of control and treated rats. The section of control shows well-preserved cytoarchitecture with normal cellular composition in the seminiferous germinal epithelium (GE). The interstitial (I) spaces filled with Leydig cells were normal. The lumen (L) is also populated by immotile spermatozoa (A). Notice thickened basement membrane (BM) with hypoplastic ST with loss of germ cells and hypocellular interstitium in other groups (H&E).
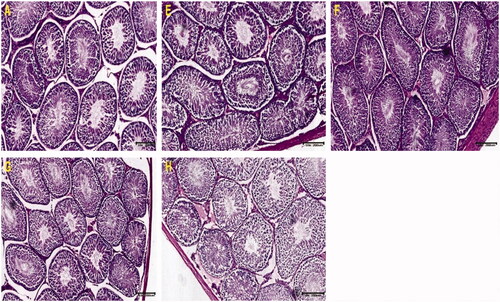
Masson’s trichrome showed fibrous tissue staining blue with the cytoplasm of tubular cells staining red with dark nuclei. Sections of testicular tissue in the control group showed normal, red-stained tubular cells and fibrous elements that were stained light blue especially around the lumen which was filled with spermatozoa. There was extensive and intense staining of seminiferous tubular basement membrane and interstitial spaces with hypoplasia in groups E, F and H compared with controls. Nuclei can be seen as dark red or almost black structures within the tubular cell population while fibrous elements are stained light blue. It is noted that sections in group H showed very poor fibrillary architecture coupled with generalized hypoplasia ().
Figure 3. Photomicrograph of testicular sections stained with Masson’s Trichrome for connective tissue. Note the extensive, intense staining of seminiferous tubular Basement membrane and interstitial spaces alongside hypoplasia in sections E, F and H compared with control (A). Nuclei can be seen as dark red or black structures within the tubular cell population, while fibrous elements are stained light blue.
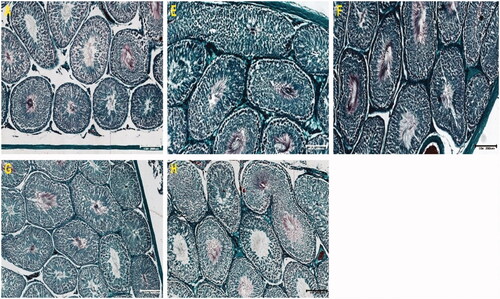
PAS-stained sections reveal the presence of glycogen in the tissues which stained pink. Control sections showed good staining and well-preserved architecture indicating seminiferous tubular integrity. Of note is the intense pink colour of PAS for tubular cells in groups E, F and G which showed hypoplasia with poor staining in some tubules (G and H). The extensive and generalized staining in slides E and F reveal large amounts of glycogen in tubules and interstitial spaces with pink-stained cells, neutral polysaccharides and basement membranes distinctly PAS-positive. While PAS staining demonstrates glycogen, indications of distortions in the seminiferous tubular cell architecture were also evident ().
Figure 4. Photomicrographs of testicular sections stained with PAS. Note the intense pink colour and PAS-intensity of tubular cells in groups E, F and G. Control sections showed well stained and preserved architectural layout with preserved seminiferous tubular integrity. Evidence of hypoplasia with poor PAS staining in some tubules (G & H).
Discussion
NPs are widely used as drug carriers in medical applications. However, the specific properties and safety profile of NPs are still unclear and there are also concerns about their potential negative environmental effects. While vancomycin has been available to clinicians for over 50 years, its use has increased dramatically in the past two decades in line with the rise in methicillin-resistant Staphylococcus aureus (MRSA) infections seen in both community and health care settings [Citation35]. The present study investigates the potential toxicity of linoleic acid vancomycin nanoparticles on testicular tissue in an experimental animal model using Sprague–Dawley rats.
We report a reduction in sperm count in the VSLN (F) and LA (G) groups when compared to controls. A recent study by Yousef et al. [Citation22] confirms that silver nanoparticles with sizes between 1 and 5 nm are spermicidal and, therefore, contraindicated for the purposes of artificial insemination. However, the indices in the LA group (G) were still within the normal range for sperm count with sperm motility and morphology not significantly different compared to controls.
Several factors are considered when evaluating male fertility, but sperm count and motility remain the most valuable indicators [Citation36, Citation37]. It has been reported that sperm count [Citation38] and motility [Citation39] correlates positively with pregnancy rates. In our study, VSLN reduced the sperm count and as such could potentially be related to male infertility which currently accounts for a staggering 50% of primary male infertility [Citation40].
Furthermore, we report perturbations in testis morphology with observable alterations in the ST of treatment groups. These perturbations were manifest as epithelial sloughing, tubular shrinkage and atrophy with loss of critical intracellular components. It is important to stress that the blood testes barrier (BTB) is critically important for maintaining the integrity of testicular tissue and, therefore, successful spermatogenesis. A reduction in tubular size is closely associated with epithelial detachment and germ cell loss which was seen histologically. These changes were more evident in the VSLN group (F). Reduction in Sertoli cell numbers causes a decrease in sperm count due to their crucial role in spermatogenesis i.e. providing cellular nutrition, support, immune protection and hormone signalling [Citation41,Citation42]. Collagen deposition (evident from the extensive MT-stained fibrillary components) in the VSLN group (F) was increased as demonstrated by PAS and MT staining. The presence of abnormal collagen tissue is a good indicator of fibrosis in the testis [Citation43]. This usually occurs as a result of tissue inflammation and cell damage [Citation43].
Stereological studies mirror the perturbations seen in morphology with a significant reduction in ST diameter, ST area and a significant increase in basement membrane thickness in all treatment groups. Other studies have shown that zinc oxide (ZnO) NPs can alter stereological parameters and the histomorphology of seminiferous tubules; it significantly reduces tubule diameter and the height of germinal epithelium, causes maturation arrest and triggers an increase in germ cell apoptosis [Citation44,Citation45]. Another study reported that specific nanoparticles could lead to direct DNA damage [Citation46]. Conventional vancomycin medication is associated with several toxic effects. Reports have suggested a higher incidence of nephrotoxicity with high-dose therapy [Citation47,Citation48] as well as being linked to ototoxicity [Citation49]. These are pointers that the effects of vancomycin with its already significant inherent toxicity may be exacerbated when combined with specific nanoparticle preparations which may cause it to reach toxic levels in the testis as seen from the effects reported in this study.
The reduction in testosterone levels although not statistically significant in the VSLN group (F) compared to control animals may yet be of significance as the concentration of testosterone in the ST is normally markedly higher that the plasma concentration would suggest owing to the presence of specific androgen binding proteins found in the ST. The LH level were also notably significantly higher in the treatment groups when compared to controls suggesting alterations in the hypophyseal-pituitary gonadal axis. In males, LH stimulates interstitial cells to produce testosterone. Also, of note are FSH level which were also significantly reduced when compared to the controls. FSH acts directly on Sertoli cells. An elevated FSH level indicates abnormal spermatogenesis and may suggest impending testicular failure [Citation50]. Azoospermia has been found in association with raised FSH and LH levels and a normal to low testosterone level [Citation51]. These changes are relevant to findings in this study which suggest that the use of the preparations under study could lead to subfertility or frank infertility.
Oxidative stress resulting from an increase in reactive oxygen species is firmly implicated in testicular dysfunction [Citation52, Citation53]. Oxidative stress results from an imbalance between the production of reactive oxygen species and their efficient degradation by available antioxidant mechanisms [Citation54]. The testis is protected by several antioxidant mechanisms including catalase and dismutase [Citation55]. It is possible that the vancomycin nanoparticles used may have compromised this system leading to some of the toxicities reported in this study, but this requires further studies to confirm.
Conclusion
Vancomycin solid lipid nanoparticle (VSLN) and its constituents SLN and LA are likely to cause a reduction in the various parameters related to the spermatozoa integrity, perturbations in hormonal levels and sperm morphology in the test groups as well as altered morphometric indices. These findings suggest that the use of vancomycin nanoparticles may contribute in some degree to male infertility. Further studies are warranted on the potential usefulness of nano-antibiotics while minimizing adverse effects such as male infertility.
Acknowledgements
The authors acknowledge the award of operational funds by the College of Health Science, University of KwaZulu-Natal, to Dr ECS Naidu and Dr AI Peter.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data are included in the submission/manuscript file.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Lederberg J, Davis JR. Emerging infectious diseases from the global to the local perspective: workshop summary. New York (NY): National Academies Press; 2001.
- Michael CA, Dominey-Howes D, Labbate M. The antimicrobial resistance crisis: causes, consequences, and management. Front Public Health. 2014;2:145.
- Ferraz V, Duse A, Kassel M, et al. Vancomycin-resistant Staphylococcus aureus occurs in South Africa. S Afr Med J. 2000;90(11):1113.
- Okeke IN, Sosa A. Antibiotic resistance in Africa-discerning the enemy and plotting a defence. Africa Health. 2003;25:10–15.
- Ferri M, Ranucci E, Romagnoli P, et al. Antimicrobial resistance: a global emerging threat to public health systems. Crit Rev Food Sci Nutr. 2017;57(13):2857–2876.
- Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379(9832):2151–2161.
- Lange C, Abubakar I, Alffenaar J-WC, TBNET, et al. Management of patients with multidrug-resistant/extensively drug-resistant tuberculosis in Europe: a TBNET consensus statement. Eur Respir J. 2014;44(1):23–63.
- Ayukekbong JA, Ntemgwa M, Atabe AN. The threat of antimicrobial resistance in developing countries: causes and control strategies. Antimicrob Resist Infect Control. 2017;6(1):47.
- Kalhapure RS, Sonawane SJ, Sikwal DR, et al. Solid lipid nanoparticles of clotrimazole silver complex: an efficient nano antibacterial against Staphylococcus aureus and MRSA. Colloids Surf B Biointerfaces. 2015;136:651–658.
- Ayobami O, Willrich N, Reuss A, et al. The ongoing challenge of vancomycin-resistant Enterococcus faecium and Enterococcus faecalis in Europe: an epidemiological analysis of bloodstream infections. Emerg Microbes Infect. 2020;9(1):1180–1193.
- McGuinness WA, Malachowa N, DeLeo FR. Vancomycin resistance in Staphylococcus aureus. Yale J Biol Med. 2017;90(2):269–281.
- Dutta S, Sengupta P, Izuka E, et al. Staphylococcal infections and infertility: mechanisms and management. Mol Cell Biochem. 2020;474(1–2):57–72.
- Kumar P. Pharmacology of specific drug groups. Pharmacology and therapeutics for dentistry. 7th ed. Amsterdam: Elsevier; 2017. p. 457–487.
- Peters A, Ruckerl R, Cyrys J. Lessons from air pollution epidemiology for studies of engineered nanomaterials. J Occup Environ Med. 2011;53(6 Suppl):S8–S13.
- Kalhapure RS, Mocktar C, Sikwal DR, et al. Ion pairing with linoleic acid simultaneously enhances encapsulation efficiency and antibacterial activity of vancomycin in solid lipid nanoparticles. Colloids Surf B Biointerfaces. 2014;117:303–311.
- Naidu ECS, Olojede SO, Lawal SK, et al. Nanoparticle delivery system, highly active antiretroviral therapy, and testicular morphology: the role of stereology. Pharmacol Res Perspect. 2021;9(3):e00776.
- Abdellah AR, Abdelhamid HN, El-Adasy A-B, et al. One-pot synthesis of hierarchical porous covalent organic frameworks and two-dimensional nanomaterials for selective removal of anionic dyes. J Environ Chem Eng. 2020;8(5):104054.
- Long NV, Yang Y, Teranishi T, et al. Biomedical applications of advanced multifunctional magnetic nanoparticles. J Nanosci Nanotechnol. 2015;15(12):10091–e10107.
- Vallabani NVS, Singh S. Recent advances and future prospects of iron oxide nanoparticles in biomedicine and diagnostics. 3 Biotech. 2018;8(6):279.
- Hashemi FS, Farzadnia F, Aghajani A, et al. Conjugated linoleic acid loaded nanostructured lipid carrier as a potential antioxidant nanocarrier for food applications. Food Sci Nutr. 2020;8(8):4185–4195.
- Abdelhamid HN, Mahmoud GA, Sharmoukh W. Correction: a cerium-based MOFzyme with multi-enzyme-like activity for the disruption and inhibition of fungal recolonisation. J Mater Chem B. 2020;8(33):7557–7557.
- Yousef MS, Abdelhamid HN, Hidalgo M, et al. Antimicrobial activity of silver-carbon nanoparticles on the bacterial flora of bull semen. Theriogenology. 2021;161:219–227.
- Sanvicens N, Marco MP. Multifunctional nanoparticles-properties and prospects for their use in human medicine. Trends Biotechnol. 2008;26(8):425–433.
- Dobrovolskaia MA, McNeil SE. Immunological properties of engineered nanomaterials. Nat Nanotechnol. 2007;2(8):469–478.
- Rim K-T, Song S-W, Kim H-Y. Oxidative DNA damage from nanoparticle exposure and its application to workers' health: a literature review. Saf Health Work. 2013;4(4):177–186.
- Kajita M, Morishita M, Takayama K, et al. Enhanced enteral bioavailability of vancomycin using water‐in‐oil‐in‐water multiple emulsion incorporating highly purified unsaturated fatty acid. J Pharm Sci. 2000;89(10):1243–1252.
- Mühlberg E, Umstätter F, Domhan C, et al. Vancomycin-lipopeptide conjugates with high antimicrobial activity on Vancomycin-resistant enterococci. Pharmaceuticals. 2020;13(6):110.
- Seedat N, Kalhapure RS, Mocktar C, et al. Co-encapsulation of multi-lipids and polymers enhances the performance of vancomycin in lipid–polymer hybrid nanoparticles: in vitro and in silico studies. Mater Sci Eng: C. 2016;61:616–630.
- Organisation WHO. WHO laboratory manual for the examination and processing of human semen. Switzerland: WHO Press; 2010.
- Ogedengbe OO, Jegede AI, Onanuga IO, et al. Coconut oil extract mitigates testicular injury following adjuvant treatment with antiretroviral drugs. Toxicol Res. 2016;32(4):317–325.
- Jegede AI, Offor U, Onanuga IO, et al. Effect of co-administration of Hypoxis hemerocallidea extract and antiretroviral therapy (HAART) on the histomorphology and seminal parameters in Sprague Dawley rats. And Andrologia. 2017;49(2):e12640.
- Latendresse JR, Warbrittion AR, Jonassen H, et al. Fixation of testes and eyes using a modified Davidson's fluid: comparison with Bouin's fluid and conventional Davidson's fluid. Toxicol Pathol. 2002;30(4):524–533.
- Gundersen H, Jensen E. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147(Pt 3):229–263.
- Christensen A, Peacock K. Increase in Leydig cell number in testes of adult rats treated chronically with an excess of human chorionic gonadotropin. Biol Reprod. 1980;22(2):383–391.
- Levine DP. Vancomycin: a history. Clin Infect Dis. 2006;42(Suppl 1):S5–S12.
- Deveneau NE, Sinno O, Krause M, et al. Impact of sperm morphology on the likelihood of pregnancy after intrauterine insemination. Fertil Steril. 2014;102(6):1584–1590. e2.
- Hamilton J, Cissen M, Brandes M, et al. Total motile sperm count: a better indicator for the severity of male factor infertility than the WHO sperm classification system. Hum Reprod. 2015;30(5):1110–1121.
- Silber SJ. OPINION: the relationship of abnormal semen parameters to male fertility. Hum Reprod. 1989;4(8):947–953.
- Zinaman M, Brown C, Selevan S, et al. Semen quality and human fertility: a prospective study with healthy couples. J Androl. 2000;21(1):145–153.
- Agarwal A, Mulgund A, Hamada A, et al. A unique view on male infertility around the globe. Reprod Biol Endocrinol. 2015;13:37.
- M Escott G, A da Rosa L, da Silveira Loss E. Mechanisms of hormonal regulation of sertoli cell development and proliferation: a key process for spermatogenesis. Curr Mol Pharmacol. 2014;7(2):96–108.
- Oliveira PF, Alves MG. The Sertoli cell at a glance. In: OliveiraPF, Alves MG (Eds.), Sertoli cell metabolism and spermatogenesis. Cham, Switzerland: Springer International Publishing; 2015. p. 3–13.
- Suskind A, Hayner‐Buchan A, Feustel PJ, et al. Fibrosis correlates with detailed histological analysis of human undescended testes. BJU Int. 2008;101(11):1441–1445.
- Talebi AR, Khorsandi L, Moridian M. The effect of zinc oxide nanoparticles on mouse spermatogenesis. J Assist Reprod Genet. 2013;30(9):1203–1209.
- Moridian M, Khorsandi L, Talebi A. Morphometric and stereological assessment of the effects of zinc oxide nanoparticles on the mouse testicular tissue. BLL. 2015;116(05):321–325.
- Han Z, Yan Q, Ge W, et al. Cytotoxic effects of ZnO nanoparticles on mouse testicular cells. Int J Nanomedicine. 2016;11:5187–5203.
- Jeffres MN, Isakow W, Doherty JA, et al. A retrospective analysis of possible renal toxicity associated with vancomycin in patients with health care-associated methicillin-resistant Staphylococcus aureus pneumonia. Clin Ther. 2007;29(6):1107–1115.
- Lodise TP, Lomaestro B, Graves J, et al. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother. 2008;52(4):1330–1336.
- Forouzesh A, Moise PA, Sakoulas G. Vancomycin ototoxicity: a reevaluation in an era of increasing doses. Antimicrob Agents Chemother. 2009;53(2):483–486.
- Gordetsky J, van Wijngaarden E, O'Brien J. Redefining abnormal follicle‐stimulating hormone in the male infertility population. BJU Int. 2012;110(4):568–572.
- Esteves SC, Miyaoka R, Agarwal A. An update on the clinical assessment of the infertile male. Clinics (Sao Paulo). 2011;66(4):691–700.
- Aprioku JS. Pharmacology of free radicals and the impact of reactive oxygen species on the testis. J Reprod Infertil. 2013;14(4):158–172.
- Turner TT, Lysiak JJ. Oxidative stress: a common factor in testicular dysfunction. J Androl. 2008;29(5):488–498.
- Birben E, Sahiner UM, Sackesen C, et al. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5(1):9–19.
- Nordberg GF, Fowler B, Nordberg M, et al. Handbook on the toxicology of metals. Amsterdam, The Netherlands: Academic Press; 2007.