?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
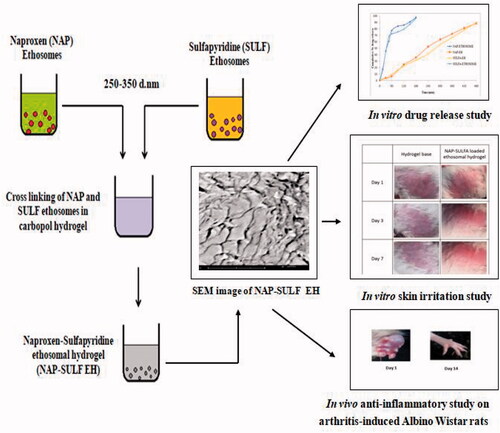
Current treatment for Rheumatoid arthritis (RA) utilizes Disease-modifying antirheumatic drugs, non-steroidal anti-inflammatory drugs or its combination, to decrease joint inflammation. In the present study, naproxen (NAP) and sulfapyridine (SULF) ethosomes were prepared by a thin-film hydration technique using PL90G and cholesterol, later crosslinked with carbopol®934. The ethosomes and ethosomal hydrogel were evaluated for rheological properties, physico-chemical analysis, in vitro and in vivo study. The results show, NAP and SULF ethosomes exhibited an average vesicle size between 251.1 ± 1.80–343.5 ± 3.23 nm and 269.0 ± 1.17–358.8 ± 1.22 nm, respectively, with good stability (zeta potential > 30 mV) and polydispersity index. Differential scanning calorimeter and Fourier transform infrared studies reveal no significant changes in the drug properties of ethosomes. Transmission electron microscopy analysis discloses spherical shape vesicles below 200 nm. The entrapment efficiency of NAP and SULF ethosomes was above 66%, and NAP-SULF ethosomes-hydrogel (EH) exhibited a sustained release effect (>8 h). In vivo studies on NAP-SULF EH shows significant inhibition of inflammation (84.63%), with less paw volume (0.1935 ± 0.08 ml) on induced arthritis Albino Wistar rats, (p < .01). NAP-SULF EH was stable at 25 °C ± 0.5 for 3-months. To conclude, a hybrid composite of NAP-SULF in hydrogel carrier prevents inflammation effectively, and could be novel for trans delivery of drugs in RA.
Introduction
Rheumatoid arthritis (RA) is a chronic, inflammatory, autoimmune disease that primarily affects the joints with varying severity among patients, later progresses and affects other organs [Citation1,Citation2]. RA is associated with autoantibodies that target various molecules including modified self-epitopes [Citation3]. Complications in RA include permanent joint damage requiring arthroplasty, rheumatoid vasculitis and Felty syndrome requiring splenectomy, and the treatment goal is to reduce pain and to stop/slow further joint damage [Citation4].
As per National Health Interview Survey (NHIS, USA), by 2040, 78 million (26%) adults will have doctor-diagnosed arthritis, and a predictable 35 million (44%) adults will report arthritis-attributable activity limitations, with total treatment expenditure exceeding $304 billion [Citation5].
Drug therapy for RA is predominantly the trial process and is often troublesome due to multifactorial conditions, including drug resistance [Citation6]. Recommendations include care by “treat” to “target” approaches (T to T), early diagnosis and treatment, use of biosimilars, withdrawal of Disease-modifying antirheumatic drugs (DMARDs) with cost-effective drugs [Citation7]. The increase jeopardy in treatment relates to poor understanding of characteristics, mechanisms and biological correlates of the factors, drug ineffectiveness, adverse drug reactions, comorbidities and negative disease outcomes [Citation8,Citation9]. RA is propagated and perpetuated by inflammatory cytokines (primarily IL-1, IL-6 and TNF-α) [Citation10].
Recently, monoclonal antibodies and immune-targeted therapies like hypomethylating agents as cytokine inhibitor, cell depleting agent and costimulation blocker are expansively used. The limitations include immune reactions, inadequate pharmacokinetics, tissue accessibility large size (150 kDa) and high cost [Citation11]. Treatment of RA utilizes combinative drug therapies (CDTs) such as NSAIDs (non-steroidal anti-inflammatory drugs) and analgesics with DMARDs [Citation12]. CDTs relieve symptoms and prevent long-term joint damage and disability, also they achieve therapeutic synergy, or a medicinal effect that is greater than the sum of each drug treatment alone [Citation13].
Long-term use of NSAIDs and corticosteroids causes severe gastrointestinal bleeding, cardiovascular side effects and NSAID induced nephrotoxicity, and can be circumvented by topical preparations, but is limited by its bioavailability [Citation14]. The complications can be managed by novel therapeutics using ethosomes and emulgel (hydrogel). Ethosomes are non-invasive carriers (vesicular systems) for transdermal applications and the permeability and stability relies on lipid composition and vehicle (ethanol) used [Citation15]. Hydrogels are polymeric networks in which their hydrophilic functional groups provide swelling of the system by holding the water in their 3 D network while crosslinking of network chains resist to water dissolution [Citation16].
A novel means of drug delivery could be by combining ethosomes-hydrogel (EH). In transdermal ethosomal hydrogel (TD-EH), ethosomes potentiates cell therapeutics (target), wound healing, cartilage/bone regeneration and deliver sustained release of drug through skin, whereas the hydrogel improves bio-compatibility, comfort and better patient compliance [Citation17]. TD-EH carriers avoid side effects on long-term therapy and deliver lipophilic or hydrophilic drugs safely. Also, hydrogels act as an ideal base for ethosomes and turns as a matrix for controlling drug release from the formulation [Citation18].
For developing TD EH, a combination of naproxen (NAP; NSAID) and sulfapyridine (SULF; DMARDs) was selected, as NSAID could reduce pain and decrease the inflammation, while DMARDs could avoid progression of the disease [Citation19,Citation20]. This combination is expected to provide faster relief from pain and offer better patient compliance in comparison to individual drug therapy. NAP was an ideal choice as it is poorly water-soluble (Mol. wt = 230.26 g/mol), weakly acidic, Log p-value of 3.18 and t1/2 12–17 h [Citation21,Citation22]. Also, SULF is a short-acting sulphonamide, insoluble in water (Mol. wt = 249.29 g/mol, Log p-value = 0.35) and is used in dermatitis herpetiformis, benign mucous membrane pemphigoid and pyoderma gangrenosum treatment [Citation23,Citation24].
In our present study, NAP-SULF EH was developed by modified thin film hydration technique. The ethosomes and EH were evaluated for rheological properties, physico-chemical study, in vitro and stability analysis. The in vivo inflammatory inhibition of optimized NAP-SULF EH in complete Freund’s Adjuvant (CFA) induced arthritis rats was also assessed.
Materials and methods
Materials
NAP was procured from Yarrow Chem Mumbai, India. SULF, Freund’s adjuvant from Sigma Aldrich, Bengaluru, India. Cholesterol (CH), carbopol®934 and triethanolamine (TEA) were obtained from Karnataka Fine Chemicals Ltd, Bengaluru, India. Lipoid® S PC-3(Hydrogenated-soya-Phosphatidylcholine PL90G) was a gift sample from Lipoid® GmbH (Ludwigshafen, Germany). All the other reagents and chemicals used were of analytical grade.
Development of NAP-SULF EH
NAP-SULF EH was developed by a step-by-step process using the modified thin-film hydration method [Citation25]. Initially, NAP and SULF ethosomes were developed, followed by preparation of carbopol®934 hydrogels, and later optimized NAP and SULF ethosomes were crosslinked in hydrogel.
Formulation and optimisation of NAP and SULF ethosomes
Initially, specified amounts of PL90G and CH (variable ratios) were accurately weighed and transferred separately to a round bottom flask (50 ml) each containing 10 ml of chloroform. The mixtures were stirred continuously at 45 °C using a magnetic stirrer (IKA®C-MAG, HS4 Digital, India) at 800 rpm for 30 min, and was continued for an additional 10 min to evaporate the solvents and to obtain a thin lipid film. For complete drying, the samples were kept overnight in a vacuum oven (HMG-HV90-S, India) at 25 °C. Later, each flask was hydrated using ethanolic solution (10%-50 v/v). The final volume was adjusted using distilled water (100 ml). The ethosomes were stored in glass vials at room temperature (24 °C) for further analysis and drug loading purpose. The compositions of ethosomes are given in .
Table 1. Formulation and optimisation of ethosomes by DLS measurement.
For incorporation of NAP in ethosomes, from the above lots, suitable batches viz. F1A, F2B, F3C, F4C and F5C with better vesicle characteristics were selected. Furthermore, these ethosomes alone were prepared in duplicate to incorporate SULF. The initial five batches containing ethosomal lipids were hydrated separately using 1% v/v ethanolic solution of NAP, and the remaining five with 1% v/v ethanolic solution of SULF (). Additionally, the volume was adjusted to 100 ml using distilled water. The above mixtures were stirred using a magnetic stirrer at 200 rpm for 15 min at 45 °C. The NAP and SULF ethosomes were labelled and kept overnight in a beaker for swelling and to form multilamellar vesicles. The resulting ethosomes were stored in glass vials in a refrigerator (2 °C–4 °C) for further analysis and for crosslinking with hydrogels [Citation26].
Table 2. Formulation of NAP and SULF loaded ethosomes.
Preparation of hydrogel
Hydrogels were prepared by (cold method) simple mixing using a mechanical stirrer (RQ-140/D, Remi-India). For the preparation, carbopol-934 (0.25%–1.5% w/v) were accurately weighed and transferred to a beaker containing distilled water (10 ml) and stirred slowly for 5 min. Later, the volume was made up to 100 ml with distilled water. The contents were mechanically stirred at 400 rpm for 1 h at 25 °C and kept overnight for swelling. Further, TEA-1% v/v was added dropwise for neutralisation (pH-7) and the mixture was stirred to form a translucent gel. The hydrogel was kept in a vacuum oven (HMG-HV90-S, India) overnight to remove air bubbles. The samples were labelled and stored in an airtight plastic container for further processing and evaluation [Citation27]. The batches with good rheological properties were further chosen for loading of NAP and SULF ethosomes.
Cross linking of NAP and SULF ethosomes
For incorporation of ethosomes in hydrogels, F4NE30 and F4SE30 were chosen. To the carbopol hydrogel (100 ml), NAP (100 ml) and SULF (100 ml) ethosomal suspensions were simultaneously added and gradually mixed (cross-linked) in a 500 ml beaker. The contents were mechanically stirred (RQ-140/D, Remi-India) at 200 rpm to obtain a cloudy homogenous mixture. Further, TEA-1 ml was added dropwise to complete the neutralisation and was confirmed by measuring the pH (CpH-101, Contech-India). The optimized NAP-SULF EH was kept in a vacuum oven at 25 °C and 350 mbar pressure overnight to remove air bubbles [Citation28]. NAP-SULF EH was weighed, stored in glass vials and kept in refrigerated condition (2 °C–4 °C) for further analysis.
Characterisation of ethosomes
Dynamic light scattering (DLS)
Measurement of average vesicle size, zeta potential (ZP) and polydispersity index (PDI) were performed using Malvern Zetasizer Nanoseries (Nano-ZS-90, UK). For analysis, 1 ml of suspension was diluted with 10 ml of deionized water and sonicated for 30 min using bath sonicator (Ultra Sonic Bath, Bio Techno Lab, India). The samples were pipetted into a disposable polystyrene cuvette. The mean vesicle size (d.nm) and PDI were measured at a fixed angle of 90° at 25 °C after five consecutive runs. The refractive index for NAP, SULF and deionized water were kept at 1.61, 1.67 and 1.33, respectively. All the measurements were performed in triplicate (n = 3) and the average was taken. For ZP (mV), samples were dispersed in deionized water and kept aside for 24 h and later injected into a clear disposable zeta cell after suitable dilution. The samples were measured after three consecutive scans [Citation29].
Determination of entrapment efficiency (%EE)
The NAP and SULF ethosomal suspensions (10 ml) were centrifuged at 4500 rpm for 30 min at 4 °C using a centrifuge (REMI-C24BL, India). For estimation of free drug, ∼4 ml of the supernatant liquid was taken, filtered employing disc filter (0.45 mm, Millipore, India) and later simultaneously estimated. The absorbances were measured using UV visible spectrophotometer (Agilent Technologies Cary 60 UV–Vis, India) at 233 nm (NAP) and 275 nm (SULF) respectively against a blank (ethanol). The free drug was calculated from the standard curve [Y = mX] (R2 = 0.998 (NAP) and [Y = mX] (R2 = 0.99 (SULF). The average of three readings (n = 3) was measured and the % EE was calculated [Citation30]:
Determination of rheological properties of hydrogel
Evaluation of viscosity (Pascal/s)
Hydrogel (5 g) was weighed accurately and transferred to a beaker; spindle (S93) was submerged and allowed to rotate at 10 rpm at 25 °C. The average of three readings (n = 3) was recorded in Brookfield viscometer (DV-II Pro Extra) [Citation31].
Spreadability coefficient
The spreadability coefficient was determined by earlier mentioned reports using two sets of glass slides of standard dimension [Citation32]. The average of three readings was taken (n = 3) and the spreadability coefficient “S” was calculated.
“m” represents weight tied to upper plate, “l” is the length of glass plate and “t” is time in taken in seconds.
In vitro drug release study on ethosomes and EH
In vitro drug release was performed using Franz diffusion cell (acceptor compartment of 125 ml and a diffusion area of 2.5 cm2). Approximately, 5 g of the sample was placed on cellophane membrane mounted in-between donor and receptor cells (PBS-pH 7.4). The sample was continuously stirred by a magnetic stirrer at 100 rpm, at 37 °C ± 0.5 °C. Samples were withdrawn, filtered by disc filter (Millipore, 0.5 µ) at predetermined time intervals (0–8 h) and sink conditions were maintained. The drug content was simultaneously analysed using UV-visible spectrophotometer (Agilent Technologies Cary 60 UV–Vis, India). All the experiments were performed in triplicate (n = 3) [Citation33].
The kinetics studies were determined using equations such as zero-order (%CDR vs. time), first-order (log % cumulative drug remaining vs. time), Higuchi’s (cumulative % drug release vs. square root of time), Hixson Crowell (cub. root of % drug remaining vs. time) and Koresmeyer-Peppas release model (log % cumulative drug release vs. log time). Mean values of R2 and n were calculated from the kinetic equations [Citation34].
Physico-chemical characterisation
Fourier transform infrared (FTIR) analysis
Infra-red spectrum of NAP, SULF, PM of NAP and SULF with PL90G and CH (1:1:1:1), and NAP-SULF EH were performed using a Bruker Tensor 27 Infrared spectrometer. Samples were placed on the ATR crystal and analysed using Opus software in the spectral region 4000–400 cm–1 [Citation35].
Differential scanning calorimetry (DSC) analysis
Samples (5 mg) were placed in an aluminium pan, crimped, sealed and heated at a constant rate of 10 °C/min under a dry nitrogen atmosphere (flow rate 100 ml/min) in room temperature. Samples were scanned in the range of 25 °C–250 °C in a differential scanning calorimeter (DSC60, Shimadzu, Japan). High purity indium was used to regulate the heat flow and heat capacity of the instruments [Citation36].
Electron microscopy analysis (Scanning electron microscopy (SEM)/Transmission electron microscopy (TEM))
For SEM analysis, samples were mounted on clear glass stub, vacuum freeze dried and coated with Inca 250 EDS Sputter coater (Oxford Instruments, UK) and visualized under Scanning Electron Microscope Carl Zeiss MA15/EVO 18. For TEM, samples were analysed using HR TEM-Jeol/JEM 2100-Japan.
In vivo studies
Animal study protocol
The protocol was approved by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Chennai (Reg:997/C/06/CPCSEA). The study was performed in accordance with the IAEC Ref. IAEC/ABMRCP/2019-20/2. For this study, 36 healthy female Albino Wistar rats weighing 200–230 g were selected. All rats had free access to water and diet. The animals were divided into four groups (I-control, II-NAP, II SULF, IV – NAP-SULF EH Test), each containing six rats. They were housed in a cage and a 12-h dark/light cycle was maintained (room temperature 21 °C–25 °C, RH 50%–70%). General and environmental conditions were monitored as per Organisation for Economic Cooperation and Development guideline [Citation37].
Skin irritation studies
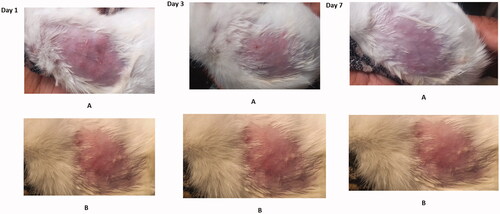
In this study, six female Albino Wistar rats, 7–8 weeks old, (mean weight 200–250 g) were taken. Approximately 0.25 g NAP-SULF EH was applied using cotton on the shaved abdominal dorsal region (2 × 2 cm2) of each rat. The skin was covered with square gauze and was fixed with tape to prevent leakage and evaporation. After 24 h, the gauze was removed carefully and observed for Draize skin reaction such as skin erythema, crust formation and oedema. The test subjects were observed for any skin irritation or rashes for 7 days and based on the occurrence of irritation on the skin, the mean scores were given [Citation38].
Induction and evaluation of arthritis
For induction of arthritis, rats were administered with CFA (Sigma Chemicals, St. Louis, MO, USA). Approximately, 0.2 ml of Freund’s adjuvant was admixed with PBS, pH-7.4 at ratio 1:1 and administered subcutaneously into the right-hind paw and fore paw (test group), keeping the left-hind paw and fore paw as controls. In addition, on day 4 a booster dose (0.1 ml-emulsion) was administered in the same manner to increase the severity of arthritis. The observations on skin surface were periodically done from both non-inflamed and inflamed paws of the rats. The progression of arthritis was assessed after 24 h and scoring was made semi-qualitatively and the average score of the six subjects were taken [Citation39].
Evaluation of anti-inflammatory activity
Paw oedema and limb circumference measurement
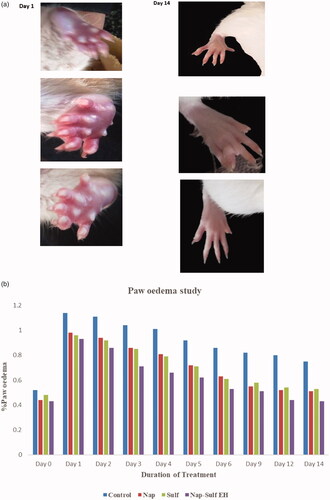
The paw oedema was measured using Plethysmometer (INCO, Haryana). The test sample (0.2 g) was applied twice daily (morning and evening) topically on the right hind limb after 1 h of induction of inflammation and was compared with control group. The paw oedema was measured on day 0 (before the onset of arthritis) and on days 1, 2, 3, 4, 5, 6, 7, 10, 13 and 14.
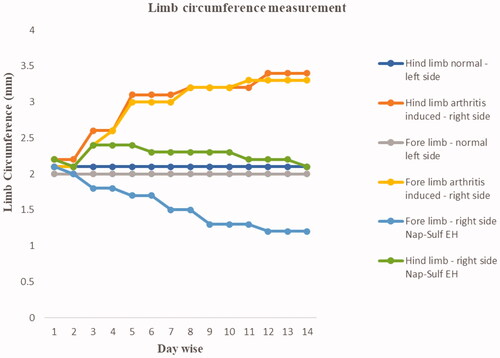
The circumference of the ankle of both forelimbs and hind limbs were measured using the digital calliper (Zhart, India) to a nearest of 0.01 mm prior to the onset of arthritis and on every consecutive day until the study was terminated [Citation40]. An average of three consecutive readings was considered in both the cases, and paw volume and percentage inhibition of inflammation was observed.
Stability studies
Stability studies were conducted as per International Council for Harmonisation guidelines at accelerated condition (40 °C ± 2 °C/75% ± 5% RH) and at 25 °C ± 2 °C/75% ± 5% RH for 90 days. Samples were kept in a stability chamber (Thermo lab, SGK325A501, India). The % drug content, pH and rheological properties were evaluated during the period [Citation41].
Statistical analysis
All data were analysed using Graph Pad Prism 5.0 software. The data for in vitro and in vivo evaluations of ethosomes, and hydrogels were performed using two-way analysis of variance, followed by Bonferroni post-test. Stability data were compared using paired t-test. For all analysis, the difference was considered significant when the p-value was <.05.
Results and discussion
The aim of the present research was to develop TD-EH containing NAP and SULF for effective management of RA. A substantial focus of the study was towards the selection of the right combination of lipids (PL90G:chol), and solvent (%v/v ethanol) for ethosome development by DLS measurement, as these may alter the vesicle size (d.nm), ZP and PDI of ethosomes. These critical parameters may in turn affect the drug loading process, tissue permeability and stability of the formulation leading to ineffective delivery and altered drug pharmacokinetics [Citation42]. Ethosomes were considered in formulation, as these vesicular carriers have cross-linked polymers and possess efficient drug loading, low toxicity and desirable swelling behaviour. The polymeric network controls the drug release and offers a sustained release effect, thereby offering better therapeutic activity. The optimisation steps and the formulation of NAP and SULF ethosomes is given in and , respectively.
Vesicle size, ZP and PDI
DLS measurements for NAP and SULF are given in and Citation3, respectively. In our investigation, all ethosomes exhibited a lower vesicle diameter (<350 nm) and confirmed their suitability for topical use. Precisely observing the impact of ethanol and PL90G on vesicle size in ethosomes, on increasing the ethanol and PL90G concentration by 35% (v/v) and 200 mg (w/v), the vesicle size increased drastically and exhibited narrow distribution (low PDI). Similar reports were also highlighted by few, where higher % of ethanol and PL90G can have a reversible effect on vesicle size [Citation43]. The PDI of both NAP and SULF ethosomes were between 0.40 and 0.60. In our study, all the ethosomal systems exhibited an increased negative charge, indicating higher stability. Further, as the ethanol concentration was increased, the ZP was also found to increase and may be associated with the polarity of ethanol [Citation44].
Entrapment efficiency (%EE)
The %EE and drug loading (%DLE) was used as an evaluation index for drug entrapment measurement. The %EE of NAP and SULF ethosomes were in the range of 39.8%–67.1% and 38.40% and 67.14%, respectively. A higher %EE was observed in F4NE30 (66.51%) and F4SE30 (67.14%) (). A low %EE (˂70%) in NAP and SULF ethosomes could be due to the variable concentration of ethanol used in the formulation. Increasing ethanol concentration beyond the optimum level would cause the ethosomal bilayer to be leaky and lead to a minor increase in vesicular size and can cause a severe decrease in %EE, and by further increasing percentage of ethanol it leads to solubilisation of vesicles [Citation45]. Based upon vesicle size, ZP, PDI and %EE, the ideal batch chosen for incorporation in the hydrogel was F4NE30 and F4SE30.
Table 3. DLS measurement and % EE of NAP and SULF loaded ethosomes.
Effect of lipid ratio (PL90G:Chol) and ethanol concentration on %EE
In our study, the %EE was found to increase in the order of 50:50 to 80:20, and observed to decrease when lipid ratio is 90:10 (). The %EE was also observed to increase up to 30% of alcohol usage, later decreased when ethanol exceeded beyond 40%, and this might be due to drug leakage from the lipid layer. Ethanol percentage is usually 10%–50% in ethosomes and the same was also considered in our formulation [Citation46].
Preparation of carbopol-934 hydrogel
For incorporating drugs, carbopol®934 hydrogels were prepared by polymerisation process. The hydrogels exhibited good spreadability and were further evaluated for rheological properties so as to be cast-off for loading NAP-SULF ethosomes. Hydrogels were considered, as it is composed of a large amount of water (70%–99%), possess high swelling due to the cross-linked polymeric network and holds ethosomal drug(s) effectively [Citation47].
Evaluation of rheological properties of hydrogel
Viscosity and spreadability
The viscosity was found to lower with higher stirring speed (>200 rpm). This could be correlated with the shear rate, and the flow behaviour was typical of non-Newtonian fluids. The results show that the viscosity is directly proportional to concentration of carbopol (%w/v) used and inversely proportional to the spreadability. Spreadability of the ideal batch was 7.7 g.cm/s and was found to decrease from 0.25% to 1.75% w/v. The results () disclose, carbopol with 1% w/v exhibited suitable viscosity (50–70 Pascal/s), good plastic flow and mechanical strength for transdermal application, and was further chosen for the incorporation of F4NE30 and F4SE30 ethosomes.
Table 4. Evaluation of carbopol hydrogel.
Evaluation of optimized-cross linked NAP-SULF EH
The NAP-SULF EH exhibited an average viscosity of 65.76 ± 32 Pascal, pH-7.15 ± 0.15 and a spreadability coefficient of 8.4 ± 0.61 g.cm/s. The % drug content in NAP-SULF EH was 95.18 ± 1.09. The change in hydrogel properties as a result of the addition of ethosome was also assessed.
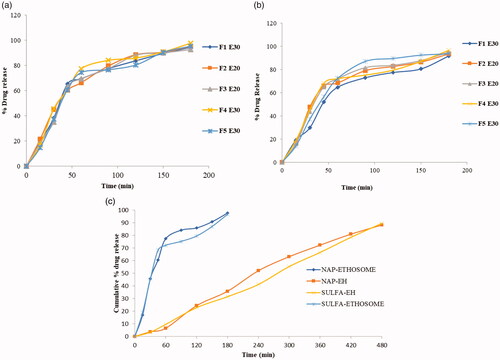
In vitro release study (%DR)
The %DR in NAP and SULF ethosomes was between 90% and 95% (3 h), while in NAP-SULF EH was 80%–90% within 8 h, and executed greater SRE. F4NE30 and F4SE30 exemplified higher release (>92%) and were further chosen for incorporation in hydrogel. The porosity in hydrogel permits drug loading into the gel matrix and subsequent drug release occurs at a rate dependent on the diffusion coefficient of small and macromolecules through the gel network [Citation48]. The results reveal that the release mechanism of NAP-SULF EH is controlled by diffusion, swelling, chemical or based on some environmental stimuli [Citation49]. The statistical result discloses that the drug release from EH was more effective as compared with NAP and SULF ethosomes and a significant difference (p < .05) was observed in all groups. The drug release profile of ethosomes and its comparison is given in , respectively.
Kinetic studies reveal that the release was more linear towards the Higuchi model with correlation coefficient R2 value of 0.786 (NAP) and 0.8793 (SULF) and describes the drug release as a diffusion process based on the Fick’s law. The second-best fit model was found to be first-order kinetics, R2 = 0.553 for NAP and 0.437 for SULF, followed by zero-order kinetics R2 = 0.9764 for NAP and 0.992 for SULF.
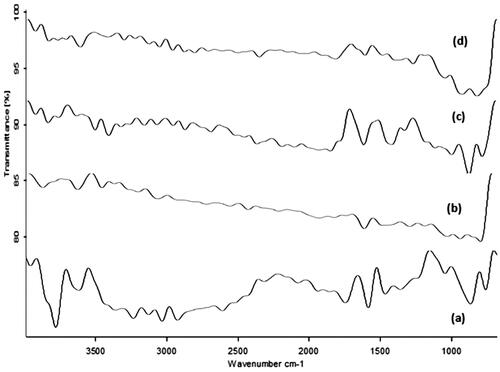
FTIR analysis
The FTIR data is represented in . NAP exhibited typical bands at 3740, 3092, 1719, 1569 and 1337 cm−1, assigned to O–H bending vibration, C–H vibrations of the aliphatic chain, C=O, C=C and C–O stretching vibrations, respectively [Citation50]. For SULF, typical bands were observed at 3429 cm−1(–N–H stretching), 3027 (C–H stretching), 1519 (C=C stretching) and 1406.25 cm−1 (–S = O stretching vibration) [Citation51]. All the prominent peaks of NAP and SULF were observed in the physical mixtures and in the formulation. The results demonstrated no chemical interactions and the formulations were stable.
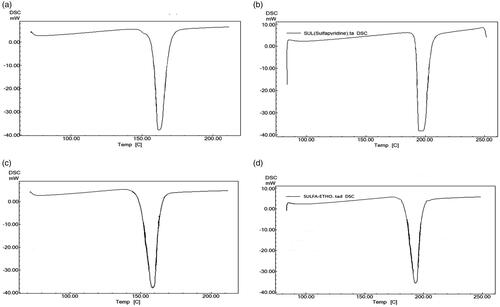
DSC analysis
DSC thermogram of NAP exhibited an onset of the peak at 151.74 °C (ΔΗ = −1.06 J/g) and a single sharp characteristic, endothermic melting peak at 162.32 °C (ΔΗ = −1.06 J/g (). The onset of the peak for SULF was observed at 192.02 °C (ΔΗ = −1.28 J/g) and a single sharp characteristic, endothermic melting peak at 197.51 °C (ΔΗ = −1.06 J/g) (). The onset of peak and endothermic peak for NAP ethosomes were observed at 151.74 °C (ΔΗ = −1.06 J/g and 158.76 °C (ΔΗ = −1.06 J/g), respectively (), while for SULF ethosomes were observed at 186.23 °C (ΔΗ= −1.26 J/g) and 193.34 °C (ΔΗ = −1.26 J/g (). The melting point of NAP and SULF ethosomes were slightly decreased and it may be due to the presence of excipients used in ethosomes. DSC studies confirm no chemical interaction between drugs and excipients and the ethosomes are stable.
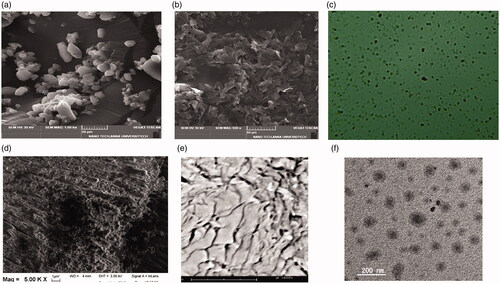
Electron microscopy analysis
SEM images of pure NAP and SULF treated with lipids, NAP-SULF ethosomes and NAP-SULF ethosomes in carbopol-934 are given in ). TEM images of optimized NAP-SULF EH (<200 nm) is illustrated in .
In-vivo study
Skin irritation studies
The irritation score index for hydrogel and NAP-SULF EH were between 0.0 and 0.5 for 7 days indicating non-irritancy (>1.2 indicates-severe irritating). The samples were devoid of any oedema and were considered safe and acceptable for topical administration ().
Anti-inflammatory activity
Induction of arthritis and evaluation in animals
The mean score value (severity of arthritis) after administration of Freund’s adjuvant (0.2 ml) IM to induce arthritis, were 1.8 ± 0.4 and 3.4 ± 0.5 from right forelimb and hind limb, respectively.
Paw oedema
No major change in inflammation was observed on day 1 and 2, and the highest reduction (>30%) of the paw oedema was observed on day 3. The inflammation and swelling were gradually decreasing and were normal by day 14 (). The paw volume in control (carbopol) and test (NAP-SULF EH) were 0.9486 ± 0.17 and 0.1935 ± 0.08 ml, respectively. The % inhibition of inflammation in NAP-SULF EH was 84.63%. The enhanced activity of NAP-SULF-EH could be due to higher %EE of ethosomes, and better ethosomal loading in hydrogels. Additionally, the ethosomes have superior stability when placed in hydrogel. This offers improved rigidity to ethosomes with minimal drug leakage. The greater therapeutic action could be also due to the presence of deformable lipid vesicular carriers embodying higher percentage ethanol (20%–45%). Due to their high deformability and smaller size (<270 nm), the vesicles permeate intact through the stratum corneum to the deeper skin layers and enhances the drug delivery. Also, the enhanced diffusion owes to molecular weight of drugs utilized in hydrogels and the results were comparable with the study performed by Rajendiran et al. [Citation52]. A statistical study shows significant results for test formulation (p < .01) compared to the control group.
Limb circumference measurement
The reduction of limb circumference depicts the decrease in swelling on the limbs of the rats. A noticeable reduction was observed on day 3 in NAP-SULF EH, and inflammation progressively declined by the 14th day and attained stability and normalcy (). The mean circumference of the fore limbs and hind limbs in the (right) inflamed limbs and the (left) control limbs measured 23.5 ± 0.32 mm and 19.4 ± 0.21 mm, 28.3 ± 0.31 mm and 20.7 ± 0.15 mm, respectively, and were statistically significant (p < .01).
Stability study
Stability studies results show that the NAP-SULF EH was stable at 25 °C ± 2 °C/75% ±5% RH with no change in drug content and rheological properties. However, at accelerated conditions, the drug content of NAP-SULF EH slightly decreased () and the rheological properties were also affected.
Table 5. Stability studies of optimized NAP-SUL EH.
Conclusion
In the present study, the NAP-SULF EH was developed and evaluated for trans delivery of drugs in the treatment of RA. A comparative study on rheological and physico-chemical properties, in vitro and in vivo analysis of NAP and SULF ethosomes and NAP-SULF EH were investigated. Based on the results, NAP-SULF EH exhibited appropriate rheological properties, displayed sustained release effect and provided effective skin permeation. In vivo studies depict that NAP-SULF EH avoids any skin irritation and was successful in decreasing the inflammation and swelling. Our findings reveal that NAP-SULF EH is a safe and effective alternative drug delivery system to oral NSAIDs and DMARDs. A combination of NAP-SULF EH reduces pain and inhibits inflammation effectively, and could be novel for trans delivery of drugs in RA.
Author statement
Not applicable as no human volunteers are involved in the study.
Acknowledgement
The authors acknowledge Acharya & BM Reddy College of Pharmacy, Bengaluru, SAIF-STIC Cochin and CNST-ANNA University, Chennai, India for providing laboratory and instrumentation analysis support. Our earnest recognition for Lipoid® GmbH, Germany in providing gift samples of phospholipids.
Disclosure statement
The authors declare that there is no conflict of interest in this work.
Data availability statement
Data generated at a central, large-scale facility, available upon request. Raw data were generated at Acharya & BM Reddy College of Pharmacy. Derived data supporting the findings of this study are available from the corresponding author Sajeev Kumar B on request.
Additional information
Funding
References
- Qiang G, Yuxiang W, Dan X, et al. Bone rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018;6(15):1–14.
- Arthritis foundation. [homepage on the Internet]. About arthritis. 2020 [Updated 2020 Feb 18]. https://www.arthritis.org/about-arthritis/understanding-arthritis
- Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet 2016;388(10055):2023–2038.
- Jacqueline B, Syed AAR, Ayman MS, et al. Rheumatoid arthritis: a brief overview of the treatment. Med Princ Pract. 2019;27(6):501–507.
- National Health Interview Survey. [homepage on the Internet]. About arthritis. 2020 [Updated 2020 Feb 25]. Available from: https://www.cdc.gov/nchs/nhis/index.htm
- Johan J, Anna KB, Rikard H. The genetics of rheumatoid arthritis and the need for animal models to find and understand the underlying genes. Arthritis Res. 2001;3(2):87–97.
- David SP. Advances in the treatment of rheumatoid arthritis: costs and challenges. N C Med J. 2017;78(5):337–340.
- Maria JH, Johannes WGJ, Jan LMS, et al. Difficult-to-treat rheumatoid arthritis: an area of unmet clinical need. Rheumatology 2018;57(7):1135–1144.
- Łukasz K, Małgorzata W. Comorbidities in rheumatic arthritis. Reumatologia 2018;56(4):228–233.
- Iain BM, Georg S. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–2219.
- Shinwan K, Jan TV, Borna R. Cytokines in inflammatory disease. Int J Mol Sci. 2019;20(23):6008.
- Tom DW, Catherine LH. Managing the drug treatment of rheumatoid arthritis. Aust Prescr. 2017;40(2):51–58.
- Liang M, Manish K, Andrew S. Nanoparticles for combination drug therapy. ACS Nano. 2013;7(11):9518–9525.
- Yuxiu Z, Dongmei C, Xin K, et al. Design and evaluation of a novel transdermal patch containing diclofenac and teriflunomide for rheumatoid arthritis therapy. Asian J Pharm Sci. 2014;9(5):251–259.
- Danaei M, Dehghankhold M, Ataei S, et al. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018;10(2):57.
- Ali D, Anuj K, Vijay SM, et al. New horizons in hydrogels for methotrexate delivery. Gels. 2021;7(2):1–20.
- Flavia L, Valérie K. Advances in drug delivery systems: Work in progress still needed? Int J Pharm. 2020;2:100050.
- Koushlesh M, Chanchal K, Shekhar V, et al. Transethosomes and nanoethosomes: recent approach on transdermal drug delivery system. Nanomedicine. 2019;2:33–54.
- Jafar A, Majid S, Katayoun M, et al. The design of naproxen solid lipid nanoparticles to target skin layers. Colloids Surf B Biointer. 2016;145:626–633.
- Robert JG, Liya S, Azra S, et al. On the action of 5-amino-salicylic acid and sulfapyridine on M. avium including subspecies paratuberculosis. PLoS One. 2007;2(6):e516.
- Kathleen K, Leonel L, Travis ML, et al. Treatment of a massive naproxen overdose with therapeutic plasma exchange in a dog. Clin Case Rep. 2019;7(8):1529–1533.
- Dominick J, Steven MW. Clinical pharmacology and cardiovascular safety of naproxen. Am J Cardiovasc Drugs. 2017;17(2):97–107.
- Jamal P, Janna H, Cheryl LKS. Sulfapyridine (polymorph III), sulfapyridine dioxane solvate, sulfapyridine tetra-hydro-furan solvate and sulfapyridine piperi-dine solvate, all at 173 K. Acta Crystallogr C. 2011;67(Pt 12):487–491.
- Perrin HL. The clinical use of sulfanilamide, sulfapyridine, sulfathiazole, sulfaguanidine, and sulfadiazine in the prophylaxis and treatment of infections. Can Med Assoc J. 1941;44(3):217–227.
- Vivek D, Dhirendra K, Shaila L, et al. Ethosome for enhanced transdermal drug delivery of aceclofenac. Int J Drug Deliv. 2010;2:81–92.
- Manish KC, Lifeng K, Sui YC. Nanosized ethosomes bearing ketoprofen for improved transdermal delivery. Results Pharma Sci. 2011;1(1):60–67.
- Tunyaluk L, Prapaporn B, Pasarat K, et al. Ethosomes of phenylethyl resorcinol as vesicular delivery system for skin lightening applications. Biomed Res Int. 2017;2017:1–12.
- Hawna JG, Neeraj KG, Sarwar B, et al. Nanosized ethosomes-based hydrogel formulations of methoxsalen for enhanced topical delivery against vitiligo: formulation optimization, in vitro evaluation and preclinical assessment. J Drug Target. 2016;24(3):1–14.
- Richa S, Gaurav T, Ruchi T, et al. Formulation and evaluation of the topical ethosomal gel of melatonin to prevent UV radiation. J Cosmet Dermatol. 2019;19( 8):1–12.
- Ping IL. Kinetics of drug release from hydrogel matrices. J Control Rel. 1985;2:277–288.
- Jiří S, Sabína J, Tomáš V, et al. Compositional and temperature effects on the rheological properties of polyelectrolyte–surfactant hydrogels. Polymers (Basel). 2019;11(5):927.
- Harish NM, Prabhu P, Charyulu RN, et al. Formulation and evaluation of in situ gels containing clotrimazole for oral candidiasis. Indian J Pharm Sci. 2009;71(4):421–427.
- Syed AI, Ismail AS, Rukhsana S, et al. In vitro assessment of pharmaceutical potential of ethosomes entrapped with terbinafine hydrochloride. J Adv Res. 2016;7(3):453–461.
- Rakesh R, Anoop KR. Formulation and optimization of nano-sized ethosomes for enhanced transdermal delivery of cromolyn sodium. J Pharm Bioallied Sci. 2012;4(4):333–340.
- Hajra AH, Shahzeb K, Muhammad S, et al. Engineering of naproxen loaded polymer hybrid enteric microspheres for modified release tablets: development, characterization, in silico modelling and in vivo evaluation. Drug Des Dev Ther. 2020;14:27–41.
- Maghsoodi M. Physicomechanical properties of naproxen-loaded microparticles prepared from eudragit L100. AAPS Pharm Sci Tech. 2009;10(1):120.
- Combes RD, Gaunt I, Balls M. A scientific and animal welfare assessment of the OECD health effects test guidelines for the safety testing of chemicals under the European Union REACH system. Altern Lab Anim. 2004;32(3):163–208.
- Sang-Han L. Evaluation of acute skin irritation and phototoxicity by aqueous and ethanol fractions of angelica keiskei. Exp Ther Med. 2013;5(1):45–50.
- Snekhalatha U, Anburajan M, Venkatraman B, et al. Evaluation of complete freund’s adjuvant-induced arthritis in a wistar rat model. Z Rheumatol. 2013;72(4):1–7.
- Cong HH, Khaziakhmetova VN, Zigashina LE. Rat paws oedema modeling and NSAIDs: Timing of effects. JRS. 2015;27(s1):S76–S77.
- Mohammad SA, Mohammad S, Nawazish A, et al. Preparation, characterization and stability study of dutasteride loaded nanoemulsion for treatment of benign prostatic hypertrophy. Iran J Pharm Res. 2014;13(4):1125–1140.
- Radhika N, Vladimir PT. Hydrogels and their applications in targeted drug delivery. Molecules. 2019;24(3):603.
- Ankush P, Sunthar P, Khakhar DV. Effects of ethanol addition on the size distribution of liposome suspensions in water. Ind Eng Chem Res. 2019;58(18):7511–7519.
- Yuangang Z, Ying Z, Xiuhua Z, et al. Preparation and characterization of chitosan–polyvinyl alcohol blend hydrogels for the controlled release of nano-insulin. Int J Biol Macromol. 2012;52:82–87.
- Chukwuemeka C, Philip FB, Chukwuma OA, et al. Development of ethosomal vesicular carrier for topical application of griseofulvin: effect of ethanol concentration. J Pharma Invest. 2019;49:27–36.
- Soheyla H, Foruhe Z. Effect of zeta potential on the properties of nano-drug delivery systems - a review. Trop J Pharma Res. 2013;12(2):255–264.
- Ibrahim MA, Yusrida D, Reem AA, et al. Transethosomal gels as carriers for the transdermal delivery of colchicine: statistical optimization, characterization, and ex vivo evaluation. Drug Des Dev Ther. 2018;12:795–813.
- Jianyu L, Mooney DJ. Designing hydrogels for controlled drug delivery. Nat Rev Mater. 2016;1(12):16071.
- Baljit S, Manu V. Development of novel hydrogels by modification of sterculia gum through radiation cross-linking polymerization for use in drug delivery. Nucl Instrum Methods Phys Res B. 2008;266:2009–2020.
- Viviane AG, Lígia NMR, Ana CSA, et al. Improved efficacy of naproxen-loaded NLC for temporomandibular joint administration. Sci Rep. 2019;9:11160.
- Rajendiran N, Siva S, Saravanan J. Inclusion complexation of sulfapyridine with α- and β-cyclodextrins: spectral and molecular modelling study. J Mol Struc. 2013;1054-1055:215–222.
- Rui S, Wufa F, Qin Y, et al. Size-dependent penetration of nanoemulsions into epidermis and hair follicles: implications for transdermal delivery and immunization. Oncotarget. 2017;8(24):38214–38226.