Abstract
Mesenchymal stromal cells (MSCs) show immunosuppressive effects both via cell-to-cell contact (direct) with immune cells and by producing paracrine factors and extracellular vesicles (indirect). A key challenge in delivering this therapeutic effect in vivo is retaining the MSCs at the site of injection. One way to address this is by encapsulating the MSCs within suitable biomaterial scaffolds. Here, we assess the immunosuppressive effect of alginate-encapsulated murine MSCs on proliferating murine splenocytes. Our results show that MSCs are able to significantly suppress splenocyte proliferation by ∼50% via the indirect mechanism and almost completely (∼98%) via the direct mechanism. We also show for the first time that MSCs as monolayers on tissue culture plastic or encapsulated within alginate, when physically isolated from the splenocytes via transwells, are able to sustain immunosuppressive activity with repeated exposure to fresh splenocytes, for as long as 9 days. These results indicate the need to identify design strategies to simultaneously deliver both modes of MSC immunosuppression. By designing cell-biomaterial constructs with tailored degradation profiles, we can achieve a more sustained (avoiding MSCs migration and apoptosis) and controlled release of both the paracrine signals and eventually the cells themselves enabling efficient MSC-based immunosuppressive therapies for wound healing.
Introduction
Mesenchymal stromal cells (MCSs) have been shown to have immunosuppressive activity both in vitro and in vivo [Citation1–4]. They have been tested in addressing various immune conditions such as graft-vs-host disease [Citation5,Citation6], inflammatory bowel disease [Citation7,Citation8] and osteoarthritis [Citation9,Citation10]. MSCs interact with immune cells, including T-cells and macrophages, either by direct cell-to-cell contact [Citation2,Citation11] or indirectly via paracrine signalling [Citation12,Citation13]. In the direct mechanism, in vivo, cytotoxic T-cells induce MSCs to undergo perforin-dependent apoptosis which is a key step in initiating MSC-induced immunosuppression [Citation2]. Additionally, MSCs are phagocytosed by monocytes and through the latter influence the behaviour of other immune cells [Citation2,Citation11]. In the paracrine mechanism, the MSCs need to be ‘licensed’ by the inflammatory cells before they respond to the inflammatory environment by secreting immunosuppressive factors [Citation14]. This licencing happens when the MSCs encounter inflammatory molecules such as tumour necrosis factor-α (TNF-α) and interferon γ (INFγ) [Citation15–17]. In response, the MSCs secrete anti-inflammatory factors such as nitric oxide synthase 2 (NOS2) [Citation16], tumour growth factor β (TGFβ) [Citation18] and immunosuppressive extracellular vesicles [Citation13,Citation19,Citation20] to attenuate the inflammation.
When delivering MSCs for suppressing inflammation in vivo, these cells are typically injected intravenously or directly to the wound site. The limitation of this approach is the lack of targeted delivery (intravenous) and hence the potential need for a higher dosage – MSCs typically localise in the lungs and the liver [Citation21–23]. Similarly, for the direct injection approach there is a risk of the therapeutic cells migrating away from the wound site [Citation24], reducing their impact where needed. One route to reduce MSC migration is to exploit chemokine signalling that promotes both exogenous and endogenous stem cells recruitment to the site of injury [Citation25]. Another approach to circumvent these problems is to encapsulate the MSCs within a material such as a hydrogel and inject them directly into the wound site. In essence, the cell-biomaterial construct will be a controlled-release implant for sustained delivery of the MSCs’ immunosuppressive activity. In addition, MSC encapsulation in alginate has shown to reduce the secretion of pro-inflammatory cytokines such as TNF-α whilst enhancing the secretion of anti-inflammatory cytokines such as PGE-2 [Citation26].
Various hydrogel materials have been studied for cellular encapsulation such as fibrin [Citation27–30], alginate [Citation20,Citation28,Citation31–33] Pullulan [Citation34] and collagen [Citation35,Citation36]. Alginate is a polysaccharide derived from seaweed and crosslinks in the presence of divalent cations. It is an FDA-approved material and, when ultrapure, is highly biocompatible and has a low immunogenic response [Citation37] making it a promising biomaterial for cellular delivery. Alginate microspheres have been explored for loading and delivering immunomodulatory biomolecules [Citation13,Citation38,Citation39] for various in vivo applications [Citation33]. In addition, alginate encapsulation of MSCs has been shown to prolong MSC survival and retention in vivo [Citation26,Citation40] and more recently to enhance the therapeutic immunomodulatory effect of MSCs in treating osteoarthritis [Citation41].
In this study, we assess the immunosuppressive activity of MSCs against proliferating mouse splenocytes activated with the inflammatory mitogen Concanavalin A (ConA). We compared the cell-to-cell contact mode (direct) of immunosuppression of MSCs against a purely paracrine mode (indirect). Furthermore, we assessed the immunosuppressive activity of MSCs when encapsulated within alginate. In particular, we performed a longitudinal study evaluating the duration of the immunosuppressive activity of alginate-encapsulated MSCs with repeated exposure to fresh batches of activated splenocytes. Our study, for the first time, investigates the long-term immunosuppressive response of alginate-encapsulated MSCs when repeatedly exposed to fresh batches of splenocytes, comparing it to MSC monolayers on tissue culture plastic.
Materials and methods
Murine MSCs
Balb/c mouse were bred in-house with tissues obtained following approved Schedule 1 methods from the UK Home Office Animals (Scientific Procedures) Act of 1986 and authorisation from King’s College London local ethics committee. Bone marrow aspirates were obtained from Balb/c mice and the MSC was isolated from them. The isolated cells were sub-cultured in 1 mg/ml DMEM (ThermoFisher Scientific, UK) supplemented with 1% (v/v) Glutamine (ThermoFisher Scientific, UK) and 1% (v/v) Penicillin/Streptomycin solution (ThermoFisher Scientific, UK) and 15% (v/v) foetal bovine serum (ThermoFisher Scientific, UK). Cells were regularly sub-cultured every 72 h following trypsinization and grown as monolayers in T-flasks.
Murine splenocyte
Freshly culled Balb/c mice were dissected to isolate the spleen. The spleen was mechanically dissociated in RPMI-1640 (ThermoFisher Scientific, UK) supplemented with 5% foetal bovine serum (splenocyte proliferation media) to release all the cells and the solution was centrifuged to isolate the cells. The pellet was incubated with ACK lysing buffer (ThermoFisher Scientific, UK) for 5 min at room temperature to lyse the RBC fraction of the extract. Following incubation, the lysing buffer was neutralised with 20 ml 5% splenocyte proliferation media, cells pelleted via centrifugation and resuspended in phosphate saline buffer (ThermoFisher Scientific, UK). The suspension was filtered via a 70 µm cell strainer and the filtrate containing the splenocytes, was washed twice with PBS to remove any excess proteins.
Splenocyte staining
Splenocytes were stained with the CellTrace™ cell proliferation kit (Life Technologies, UK). Briefly, the isolated splenocyte suspension was incubated with 5 µm CellTrace™ Violet dye at 37 °C for 20 min in the dark. Following incubation, excess media was added and cells were pelleted via centrifugation to remove excess dye. The pellet was resuspended, cells counted and used for further analysis.
Alginate bead preparation and depolymerisation
Similar to the encapsulation of cells with alginate-based hydrogel as described by Fathi et al., MSCs were encapsulated within pure alginate microbeads [Citation42]. Sterile sodium alginate (Pronova™ SLG20, Novamatrix®) powder was dissolved in 0.9% (w/v) NaCl (saline). The molecular weight of alginate is in the 75000–220000 g/mol range with a G/M ratio of ≥1.5. The polymerisation solution used was a 1.2% (w/v) CaCl2 solution in saline. MSCs were trypsinized using 0.05% trypsin (ThermoFisher Scientific, UK), pelleted via centrifugation and resuspended in saline. The cells were counted using a haemacytometer and cell suspension was prepared by mixing the cells in saline with the alginate solution. The MSC-alginate solution was dropped via a 27 G needle into the CaCl2 bath with a magnetic stirrer to enhance gelation. The alginate beads were stirred in the CaCl2 bath for 10 min to ensure complete polymerisation. Following polymerisation, they were transferred to fresh saline solution and stirred for 5 min twice and a further 5 min in splenocyte proliferation media to remove any excess CaCl2. The polymerised MSC-loaded alginate beads were counted to determine cells/bead and an appropriate number of the beads was transferred to each well of a well plate to obtain the desired number of MSCs/well. A similar procedure was followed for preparing the empty alginate beads, but the cell addition step was absent. The size of the beads, as measured using the ImageJ software on bright field micrographs of the beads was found to be 1.57 mm ± 0.5 mm (SD; n = 5).
MSCs loaded within the alginate beads were obtained for further analysis post-experiments. This was performed by collecting the beads and washing them with 1.2% (w/v) CaCl2 solution in saline. Following the wash, the alginate was depolymerised by incubating with 50 mM Ethylenediaminetetraacetic acid (EDTA) in 10 mM HEPES buffer at 37 °C for 5 min. Once the beads had completely depolymerised, the cells were pelleted via centrifugation, washed once with 2% FBS (v/v) in PBS and finally resuspended in PBS for further analysis.
Direct versus indirect mode of MSC immunosuppression
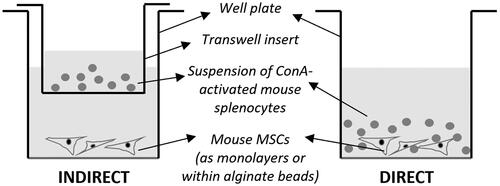
To compare the direct versus indirect mode of immunosuppression by MSCs of splenocyte proliferation, the MSCs in splenocyte proliferation media were added to the wells of 6-well plates. For the direct mode ( right), the CellTrace™-dye stained splenocytes, in ConA (Sigma, UK)-supplemented proliferation media, were also added directly to the wells. ConA is a T-cell mitogen that can trigger the proliferation of T-cells. In contrast, for the indirect mode ( left), the CellTrace™ dye-stained splenocytes, in ConA-supplemented proliferation media, were added to the Corning™ Transwell™ inserts (0.4 µm pore size; FisherScientific, UK) placed within the wells. The Transwells physically separate the two cell types whilst allowing soluble factors and cell-secreted vesicles to pass across the pores. Two controls were set up, both with only the splenocytes without any MSCs. One set of controls had just the splenocyte proliferation media whilst the other had ConA supplemented splenocyte proliferation media. The results were all normalised to the proliferation index (PI) of the latter. All samples were incubated for 72 h following which the splenocytes (or in the case of the direct mode, all cells) were collected for flow cytometric analysis. The MSCs were unstained and hence did not interfere with the flow cytometric measurements.
Long-term immunosuppressive activity of MSCs
To determine the long-term immunosuppressive effect of MSCs on splenocyte proliferation, the experiment was set up in the following way. The alginate-encapsulated MSCs were added to 6-well plates with splenocyte proliferation media. In a setup similar to (left), the CellTrace™ dye-stained splenocytes in ConA-supplement proliferation media were added to the Transwells. Every 72 h, the Transwells were removed and splenocytes were collected for flow cytometric analysis. A fresh batch of splenocytes in Transwells was added to the MSC wells and the media was replaced with fresh ConA-containing media. This was repeated once more resulting in the MSCs being exposed to 3 batches of freshly activated splenocytes in total.
Evaluating immunosuppression: flow cytometry and analysis
CellTrace™ dye-stained splenocytes, following the experiments, were analysed for proliferation via flow cytometric analysis. The cells were recovered from the well plates or Transwells, washed and resuspended in PBS. The cells were analysed on an LSR Fortessa flow cytometer (BD Biosciences, UK) and data acquisition was performed using the BD FACS Diva software (BD Biosciences, UK). The PI was calculated using the FlowJo software (BD Biosciences, UK) by curve fitting. The PI was normalised by setting the PI of ConA-activated splenocytes not exposed to any MSCs or alginate to 100%.
Gene expression analysis
MSCs were analysed for the expression profiles of key immunomodulatory genes following stimulation. The cells were grown as monolayers on tissue culture plastic and where either (i) untreated or exposed to (ii) 10 ng/ml recombinant murine interferon-γ (IFN-γ; PeproTech, UK) which has a molecular weight of 15.6 kDa (iii) 10 ng/ml recombinant murine tumour necrosis factor-α (TNF-α; PeproTech, UK) which has a molecular weight of 17.3 kDa or (iv) 10 ng/ml IFN-γ + 10 nm/ml TNF-α. The expression of indoleamine 2,3-dioxygenase (IDO), arginase 1 (ARG1), inducible nitric oxide synthase (iNOS2) and prostaglandin E2 (PTGS2) were measured using quantitative real-time polymerase chain reaction (qRT-PCR). RNA was extracted using miRneasy mini kit (Qiagen, UK) and PCR was performed using TaqMan® RNA-to-CT™ 1-step kit (Applied Biosystems, UK) and TaqMan probes as primers (Applied Biosystems, UK). Gene expression was calculated with respect to untreated MSCs and normalised to 2 housekeeping genes, HPRT1 (hypoxanthine phosphoribosyltransferase 1) and β-actin, using the ΔΔCT method. Data was collected and analysed using the StepOne™ software (Applied Biosystems, UK).
Statistical analysis
All experiments were performed as 3 biological repeats. Data are presented as mean ± standard error of the means (SEM). The significance of differences between groups was assessed using the independent t-test or the one-way ANOVA as appropriate. As the samples had an equal number of repeats, the Tukey’s test for post-hoc analysis (p < .05) was performed for all data.
Results
Direct vs. indirect suppression of splenocyte proliferation by MSCs
Prior to testing the suitability of alginate for the sustained delivery of MSCs’ immunosuppressive activity, we compared the immunosuppressive potency of MSCs via the two modes: direct cell-to-cell contact with proliferating splenocytes (direct) versus with no contact but via only paracrine factors and/or extracellular vesicles (indirect). We co-cultured MSCs and ConA activated splenocytes (labelled with CellTracker™ dye) either together (direct) or across a Transwell (indirect) () in well plates. The splenocytes were analysed for proliferation via flow cytometry following a 72 h incubation with the MSCs. shows the PI of splenocytes normalised to ConA-activated splenocytes (100%).
Figure 2. Direct versus indirect immunosuppression: Splenocytes were stained with CellTracker™ Violet dye and their proliferation measured using flow cytometry following 72h in culture. Data was normalized by setting the proliferative index of ConA-treated splenocytes (ConA + SPL) to 100%. There is no proliferation observed for the unstimulated splenocytes (SPL). >90% suppression is observed for ConA-treated splenocytes co-cultured with MSCs in direct contact at a ratio of 1 MSC for every 50 splenocyte (DIRECT MSC:SPL::50:1). ∼50% suppression is observed when ConA treated-splenocytes were co-cultured with MSCs separated using a Transwell at a ratio of 1 MSC for every 50 splenocytes (INDIRECT MSC:SPL::50:1). Error bars represent standard error for N = 3 and line over columns indicate groups that were significantly different from each other (One-way ANOVA using Tukey’s HSD post-hoc, p < 0.05).
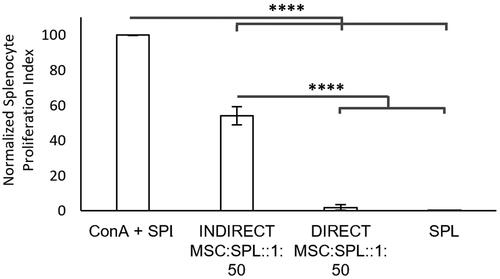
After 72 h, MSCs almost completely suppress splenocyte proliferation (98% ± 2%; N = 3; ) when in direct contact, whilst we observe 46% ± 4% (N = 3; ) suppression through the indirect mode. These results show that with direct cell-to-cell contact combined with the secretion of paracrine signalling molecules, the MSCs immunosuppressive activity is the most potent at the dosage studied (1 MSC for every 50 splenocytes). In contrast, MSCs are able to significantly suppress splenocyte proliferation via the paracrine mechanism, albeit to a lesser extent.
2. Effect of alginate encapsulation on the expression of key immunosuppressive genes
When MSCs are exposed to molecules typical of an inflammatory environment, such as interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α) [Citation18,Citation43,Citation44], they secrete immunosuppressive paracrine factors [Citation10] including indoleamine 2,3-dioxygenase (gene: IDO) [Citation45], arginase 1 (gene: ARG1) [Citation46], inducible nitric oxide synthase 2 (gene: iNOS2) and prostaglandin E2 (gene: PTGS2) [Citation18]. To determine whether alginate as a carrier material impacts the expression of key immunosuppressive genes on MSCs, we measured the levels of expression of IDO, NOS2, ARG1 and PTGS2 genes. We compared their expression between MSC monolayers on tissue culture plastic (TCP) () against MSCs encapsulated within alginate beads after a 24 h exposure to the inflammatory cytokines TNF-α and INF-γ. The MSCs as monolayers and within alginate beads are shown in , respectively.
Figure 3. Effect of alginate encapsulation on MSC immunosuppressive genes: Gene expression (ΔΔCt) of key immunosuppressive genes IDO (a), NOS2 (b), ARG1 (c) and PTGS2 (d) in MSCs as measured using qRT-PCR. MSC as monolayers on tissue culture plastic or encapsulated within alginate beads were either left untreated or exposed to 10 mg/ml TNF-α, 10 ng/ml INFγ or both (10 ng/ml TNF-α + 10 ng/ml INFγ) for 24h. Both β-Actin and HPRT-1 were used as the housekeeping genes. Error bars represent standard error for N = 3 and line over columns indicate groups that were significantly different from each other (Tukey’s HSD, p < 0.05). Bright field micrographs of monolayer of MSCs on tissue culture plastic (e-h) and encapsulation with alginate beads (i - l). Scale bar = 200 μm.
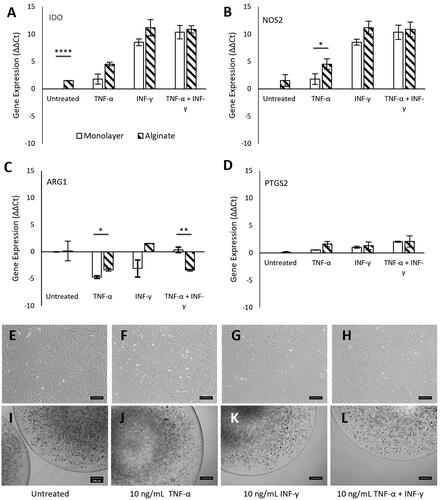
Without any immune stimulation (untreated), there was a significant upregulation (p < 0.0001; N = 3) of IDO expression in MSCs encapsulated within alginate compared to MSC monolayer. However, NOS2, ARG1 and PTGS2 gene expression levels were unaffected in the untreated condition. With TNF-α (10 ng/ml) stimulation, the expression of the NOS2 was significantly upregulated (p < 0.5; N = 3) and ARG1 significantly downregulated (p < 0.5; N = 3) while there was no difference in the expression levels of the IDO and PTGS2 genes. For cells stimulated with INF-γ only (10 ng/ml), no significant difference was observed between the MSC monolayer and alginate-encapsulated MSCs (p > 0.5, N = 3). In the case of the combined 10 ng/ml of INF-γ and 10 ng/ml of TNF-α stimulation, IDO, NOS2 and PTGS2 levels were unaffected whilst ARG1 expression was significantly downregulated (p < 0.01; N = 3). The encapsulation procedure and alginate itself, albeit being ultrapure, do have a weak but significant effect on the expression of immunomodulatory genes when exposed to a minimal immunostimulatory environment (untreated or TNF-α only). However, under highly immunostimulatory conditions (INF-γ + TNF-α) the much stronger profiles of the immunomodulatory gene expression are not affected by alginate encapsulation except in the case of the ARG1 gene (where the expression was downregulated). Careful choice of the type of encapsulation and purity of the encapsulation material can reduce non-specific MSC responses.
3. Long-term immunosuppressive activity of alginate-encapsulated MSCs
The same batch of MSCs was repeatedly exposed to fresh batches of activated splenocytes to measure how sustained the immunosuppressive activity of the MSC could be (). Splenocytes in Transwells were placed in empty wells or in wells containing MSC monolayers or wells containing MSCs encapsulated in alginate. The Transwells containing the splenocytes were removed following 72 h in co-culture for further analysis and a fresh batch of ConA-activated splenocytes in Transwells was inserted into the wells with the same batch of MSCs. The PI of a monoculture of ConA-activated splenocytes (; Cell and Alginate free – ConA), the positive control, was set at 100% and all other samples normalised to this value. As expected, unstimulated splenocyte monocultures did not show any proliferation (; Cell and Alginate free – No ConA). Splenocytes exposed to empty alginate beads (; Cell-free Alginate beads – ConA) also did not significantly affect proliferation compared to the positive control (denoted by groups a1, a2 and a3 in ) indicating that the material did not induce any significant responses from the splenocytes. The same batch of MSCs, grown as monolayers (; MSC Monolayer MSC: SPL: 1: 50 – ConA) on tissue culture plastic, was consistently able to suppress splenocyte proliferation by ∼50% (as was the case in ; indirect) over the 3 successive exposures (exposure 1, Mean PI = 52.7 ± 3.5 SEM, N = 3; exposure 2, Mean PI = 44.3 ± 12.8 SEM, N = 3; exposure 3, Mean PI = 59.3 ± 14.5 SEM, N = 3; ) to fresh batches of actively proliferating splenocytes each kept in place for 72 h. This indicates that MSCs retain their indirect immunosuppressive activity with repeated exposure to actively proliferating splenocytes for a minimum of at least ∼9 days. However, due to the large error, a significance in suppression of splenocyte proliferation is only observed for the first and second exposures (p < 0.001, N = 3; ) compared to the positive control.
Figure 4. Effect of alginate encapsulation on the long-term immunosuppressive potential of MSCs: Unstimulated (No ConA) and ConA stimulated (ConA) splenocytes were stained with CellTracker™ Green dye and their proliferation measured using flow cytometry following 72h in culture. Data was normalized by setting the proliferative index of ConA-treated splenocytes (Cell and Alginate free ConA) was set as 100%. The proliferative index of unstimulated splenocytes (Cell and Alginate free No ConA) and ConA treated splenocytes in coculture with cell-free alginate beads (Cell-free Alginate beads ConA), MSC monolayer (MSC Monolayer MSC : SPL :: 1 : 50 ConA) and alginate-encapsulated MSCs (MSC-Alginate beads MSC : SPL :: 1 : 7.5 ConA) are shown. Each exposure was for 72 h. Error bars represent standard error for N = 3 and line over columns indicate groups that were NOT significantly different from each other (One-way ANOVA using Tukey’s post-hoc, p < 0.05 comparing between different treatments within the same exposure regime).
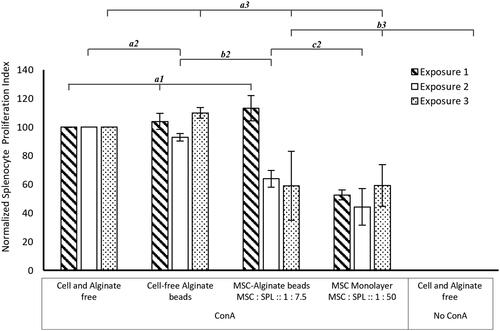
Interestingly, MSCs encapsulated within alginate (; MSC-Alginate beads MSC: SPL: 1: 7.5) do not show immunosuppression upon the first exposure. In contrast, with the second (exposure 2, Mean PI = 64 ± 5.9 SEM, N = 3; ) and third exposure (exposure 1, Mean PI = 59 ± 24.1 SEM, N = 3; ) to fresh batches of splenocytes, a comparable level of suppression of splenocyte proliferation, to that by the MSC monolayer is observed. It is important to note that the ratio of MSCs to splenocytes is much higher in the alginate-encapsulated MSC sample (1 MSC for every 7.5 splenocytes) to obtain a similar level of suppression as that achieved for the MSC monolayer (1 MSC for every 50 splenocytes). Although the trend is quite clear, once again due to the large error, significant suppression is only observed between the positive control and the alginate-encapsulated MSCs in the second exposure (p < 0.05, N = 3; ). The suppression levels of the alginate-encapsulated MSCs are comparable to the MSC monolayers in the second exposure (denoted by group c2 in ). The delayed onset of the immunosuppressive response of the alginate-encapsulated MSCs could be due to a slower licencing of MSCs within the alginate beads or due to diffusion-related limitations or both.
Discussion
In this study, we demonstrate that (1) murine MSCs are capable of suppressing actively proliferating murine splenocytes, albeit to different degrees (at identical MSC to immune cell ratios) via both direct and indirect modes; (2) alginate does not significantly interfere with the immunosuppressive gene expression of MSCs when treated with high doses of a combination of INF-γ and TNF-α simulating a highly inflammatory niche; (3) alginate-encapsulated MSCs are capable of sustaining their immunosuppressive activity via an indirect mode with repeated exposure to fresh batches of proliferating splenocytes for at least 9 days.
In addition to the strong immunosuppressive activity of MSCs in the direct mode, these cells can also have a significant immunosuppressive effect purely through the paracrine mechanism as demonstrated in our study and elsewhere [Citation9,Citation19,Citation47]. To the author’s knowledge no other studies have directly compared the potency of the two modes of suppression in murine cells in vitro. In the work by Di Nicola et al. [Citation47], human bone marrow-derived stromal cells (BMSCs) strongly suppressed T-cell proliferation (>90%) when in direct contact with each other whilst in a Transwell set-up similar to our study, there was a 70% ± 13% reduction after a 5-day incubation period. While the degree of suppression, via the direct mode, is comparable to our study, in the indirect mode the suppression is stronger in the work by Di Nicola et al. This can be explained by the fact that this study was looking at human MSCs and T-cells and using a significantly higher ratio of 10 MSCs for every T-cell (compared to 1 MSC for 50 splenocytes in our case) for a longer duration (for 5 days compared to 3 days in our study).
Studies have shown that MSC can undergo apoptosis in the direct mode [Citation2,Citation48] and encapsulating them within a biomaterial is a potential strategy to temporarily circumvent this [Citation32,Citation39]. In our study, we demonstrate that MSCs, when encapsulated within alginate beads, survive for a period of at least 9 days (the duration of our study) and can actively immunosuppress fresh batches of proliferating splenocytes during this period. Mohammadi et al., demonstrated that alginate microcapsules loaded with human umbilical cord MSC-derived exosomes suppressed the proliferation of CD3/CD28 activated murine splenocytes in vitro [Citation20]. The PI of CFSE-labelled splenocytes co-cultured with anti-CD3 and soluble CD28 in the presence or absence of 20 and 200 μg/mL of exosome-loaded alginate microcapsules was measured after a 96 h incubation. Compared to the untreated controls which had 9603 ± 871 cells (100%), the addition of 20 and 200 μg/mL exosome-loaded alginate microcapsules reduced splenocyte numbers to 1253 ± 1038 (13%) and 1570 ± 1010 (16%), respectively. Since we have used MSC-encapsulated alginate as opposed to MSC-derived exosome-loaded alginate, a direct comparison is challenging between the two studies. However, these results demonstrate that by tailoring the dosage of the therapeutic, whether exosomes or the MSCs themselves, it is possible to exert a stronger immunosuppressive response through the indirect mode.
Our results suggest that alginate is a potential biomaterial for delivering the immunosuppressive activity of MSCs. Our data suggest that MSC when encapsulated required >72 h to be ‘licensed’ and to start immunosuppressing by 144 h. This could be because of the dimensions of our alginate beads whose diameter was in the millimetre scale. Also, a higher dose of the MSCs was required to achieve a similar level of immunosuppression when compared to MSC monolayers on tissue culture plastic. This again could be due to slower rates of diffusion, across the alginate matrix, of larger signalling molecules, especially extracellular vesicles whose diffusion across matrices has been shown to depend on matrix mechanics [Citation49]. Smaller beads such as microspheres generated via electrospraying [Citation50], using modified alginate or other biomaterials that are more permeable to diffusion of biomolecules/vesicles and/or which promote MSC attachment might improve their response time and viability to an inflammatory environment. For example, using Triazole containing modifications [Citation51] or sulphated alginate microbeads [Citation52] has been shown to have reduced pericapsular fibrotic overgrowth and hence can promote improved diffusion of signalling molecules across the implanted cell-encapsulated beads. In the work by Mao et al. [Citation53], encapsulating MSCs within poly-D-lysine-modified alginate microspheres was shown to increase their half-life in vivo in mouse by more than an order of magnitude compared to injection of naked MSCs. However, they did not compare the immunosuppressive activity between the two modes. From our results, considering that one mechanism (direct) is more potent than the other (indirect), but also potentially short-lived, as the therapeutic cells undergo apoptosis, there is a need to design MSC-biomaterial constructs that facilitate a hybrid mode of immunosuppression. For instance, we could encapsulate the MSCs in biodegradable biomaterials, so the encapsulated cells release paracrine immunosuppressive molecules, while, as the material degrades, the released cells can act through direct contact with immune cells to deliver their anti-inflammatory effect. In addition, by tailoring the degradation rate, we can control the rate of release of the MSCs to achieve a more sustained and strong response, which will be especially key to efficiently treat chronic inflammations.
Conclusion
The therapeutic benefit of MSCs is highly promising for future healthcare, although the delivery of such a treatment is complicated due to the various cell and material parameters that must be optimised. The current study demonstrates that MSCs can suppress the proliferation of activated splenocytes purely through an indirect mode and this effect is sustained for over a week with repeated exposure to new batches of immune cells. This provides a strong foundation for designing biomaterial-encapsulated MSCs to deliver their immunosuppressive activity. Specifically, by engineering the biomaterial composition, architecture, and encapsulation route, it will be possible to facilitate sustained and controlled immunosuppression. The biomaterial can be designed to degrade at a predetermined rate to initially enable the paracrine mode of MSCs’ immunosuppression followed by a slow release of the cells into the milieu to achieve the more potent direct mode of immunosuppression. Further studies are required to assess the potential of combined modes of action. They will occur at different rates, and potentially circumvent an immediate loss of the MSCs due to migration and/or apoptosis whilst enabling a stronger as well as a more sustained response for efficient long-term immunosuppression.
Author contributions
Sandhya Moise: Conception and design, financial support, collection of data, data analysis and interpretation, manuscript writing, final approval of manuscript. Luigi Dolcetti: Conception and design, collection of data, data analysis and interpretation. Francesco Dazzi: Conception and design, financial support, provision of study material, data analysis and interpretation, Paul Roach: Conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript. Lee Buttery, Sheila MacNeil, Nick Medcalf: Conception and design, data interpretation.
Acknowledgement
The authors would also like to thank David Farrar (Biomaterials Manager) at Xiros Ltd, UK for his valuable guidance and advice in steering the research project.
Disclosure statement
The authors report there are no competing interests to declare.
Data availability statement
All data created during this research is openly available from the University of Bath Research Data Archive at [https://doi.org/10.15125/BATH-01069] [Citation49].
Additional information
Funding
References
- Rashidghamat E, Kadiyirire T, Ayis S, et al. Phase I/II open-label trial of intravenous allogeneic mesenchymal stromal cell therapy in adults with recessive dystrophic epidermolysis bullosa. J Am Acad Dermatol. 2020;83(2):447–454.
- Galleu A, Riffo-Vasquez Y, Trento C, et al. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci Transl Med. 2017;9(416):eaam7828.
- Muller L, Tunger A, Wobus M, et al. Immunomodulatory properties of mesenchymal stromal cells: an update. Front Cell Dev Biol. 2021;9(637725):637725.
- Wu X, Jiang J, Gu Z, et al. Mesenchymal stromal cell therapies: immunomodulatory properties and clinical progress. Stem Cell Res Ther. 2020;11(1):345.
- Burnham AJ, Foppiani EM, Horwitz EM. Key metabolic pathways in MSC-Mediated immunomodulation: Implications for the prophylaxis and treatment of graft versus host disease. Front Immunol. 2020;11:609277.
- Dotoli GM, De Santis GC, Orellana MD, et al. Mesenchymal stromal cell infusion to treat steroid-refractory acute GvHD III/IV after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2017;52(6):859–862.
- Mao F, Tu Q, Wang L, et al. Mesenchymal stem cells and their therapeutic applications in inflammatory bowel disease. Oncotarget. 2017;8(23):38008–38021.
- Khan S, Khan RS, Newsome PN. Cellular therapies for the treatment of immune-mediated GI and liver disease. Br Med Bull. 2020;136(1):127–141.
- Zhao X, Zhao Y, Sun X, et al. Immunomodulation of MSCs and MSC-derived extracellular vesicles in osteoarthritis. Front Bioeng Biotechnol. 2020;8(575057):575057.
- Harrell CR, Markovic BS, Fellabaum C, et al. Mesenchymal stem cell-based therapy of osteoarthritis: current knowledge and future perspectives. Biomed Pharmacother. 2019;109:2318–2326.
- de Witte SFH, Luk F, Sierra Parraga JM, et al. Immunomodulation by therapeutic mesenchymal stromal cells (MSC) is triggered through phagocytosis of MSC by monocytic cells. Stem Cells. 2018;36(4):602–615.
- Riazifar M, Mohammadi MR, Pone EJ, et al. Stem Cell-Derived exosomes as nanotherapeutics for autoimmune and neurodegenerative disorders. ACS Nano. 2019;13(6):6670–6688.
- Mohammadi M, Luong JC, Rodriguez SM, et al. Controlled release of stem cell secretome attenuates inflammatory response against implanted biomaterials. Adv Healthcare Mater. 2020;9(12):e1901874.
- Cheung TS, Bertolino GM, Giacomini C, et al. Mesenchymal stromal cells for graft versus host disease: Mechanism-Based biomarkers. Front Immunol. 2020;11:1338.
- Prasanna SJ, Gopalakrishnan D, Shankar SR, et al. Pro-inflammatory cytokines, IFNgamma and TNFalpha, influence immune properties of human bone marrow and Wharton jelly mesenchymal stem cells differentially. PLOS One. 2010;5(2):e9016.
- Ren G, Zhang L, Zhao X, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2(2):141–150.
- Poggi A, Varesano S, Zocchi MR. How to hit mesenchymal stromal cells and make the tumor microenvironment immunostimulant rather than immunosuppressive. Front Immunol. 2018;9:262.
- English K, Ryan JM, Tobin L, et al. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4 + CD25(high) forkhead box P3+ regulatory T cells. Clin Exp Immunol. 2009;156(1):149–160.
- Bazzoni R, Kamga T, Tanasi P, et al. Extracellular vesicle-dependent communication between mesenchymal stromal cells and immune effector cells. Front Cell Dev Biol. 2020;8:596079.
- Mohammadi MR, Rodriguez SM, Luong JC, et al. Exosome loaded immunomodulatory biomaterials alleviate local immune response in immunocompetent diabetic mice post islet xenotransplantation. Commun Biol. 2021;4(1):685.
- Kraitchman DL, Tatsumi M, Gilson WD, et al. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation. 2005;112(10):1451–1461.
- Eggenhofer E, Benseler V, Kroemer A, et al. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol. 2012;3:297.
- Eggenhofer E, Luk F, Dahlke MH, et al. The life and fate of mesenchymal stem cells. Front Immunol. 2014;5(148):148.
- Li L, Chen X, Wang WE, et al. How to improve the survival of transplanted mesenchymal stem cell in ischemic heart? Stem Cells Int. 2016;2016:9682757.
- Hocking AM. The role of chemokines in mesenchymal stem cell homing to wounds. Adv Wound Care. 2015;4(11):623–630.
- Stucky EC, Schloss RS, Yarmush ML, et al. Alginate micro-encapsulation of mesenchymal stromal cells enhances modulation of the neuro-inflammatory response. Cytotherapy. 2015;17(10):1353–1364.
- Bujoli B, Scimeca JC, Verron E. Fibrin as a multipurpose physiological platform for bone tissue engineering and targeted delivery of bioactive compounds. Pharmaceutics. 2019;11(11):556.
- Gomez-Florit M, Pardo A, Domingues RMA, et al. Natural-Based hydrogels for tissue engineering applications. Molecules. 2020;25(24):5858.
- Heher P, Mühleder S, Mittermayr R, et al. Fibrin-based delivery strategies for acute and chronic wound healing. Adv Drug Deliv Rev. 2018;129:134–147.
- Heo DN, Hospodiuk M, Ozbolat IT. Synergistic interplay between human MSCs and HUVECs in 3D spheroids laden in collagen/fibrin hydrogels for bone tissue engineering. Acta Biomater. 2019;95:348–356.
- Zhang F, King MW. Biodegradable polymers as the pivotal player in the design of tissue engineering scaffolds. Adv Healthc Mater. 2020;9(13):e1901358.
- Ho SS, Murphy KC, Binder BY, et al. Increased survival and function of mesenchymal stem cell spheroids entrapped in instructive alginate hydrogels. Stem Cells Transl Med. 2016;5(6):773–781.
- Liu ZC, Chang TMS. Artificial cell microencapsulated stem cells in regenerative medicine, tissue engineering and cell therapy. Adv Exp Med Biol. 2010;670:68–79.
- Bulman SE, Coleman CM, Murphy JM, et al. Pullulan: a new cytoadhesive for cell-mediated cartilage repair. Stem Cell Res Ther. 2015;6:34.
- Gao Y, Kong W, Li B, et al. Fabrication and characterization of collagen-based injectable and self-crosslinkable hydrogels for cell encapsulation. Colloids Surf B Biointerfaces. 2018;167:448–456.
- Lam D, Enright HA, Peters SKG, et al. Optimizing cell encapsulation condition in ECM-Collagen I hydrogels to support 3D neuronal cultures. J Neurosci Methods. 2020;329:108460.
- Orive G, Ponce S, Hernández RM, et al. Biocompatibility of microcapsules for cell immobilization elaborated with different type of alginates. Biomaterials. 2002;23(18):3825–3831.
- Farah S, Doloff JC, Müller P, et al. Long-term implant fibrosis prevention in rodents and non-human primates using crystallized drug formulations. Nat Mater. 2019;18(8):892–904.
- Chen T, Yuan J, Duncanson S, et al. Alginate encapsulant incorporating CXCL12 supports long-term allo- and xenoislet transplantation without systemic immune suppression. Am J Transplant. 2015;15(3):618–627.
- Khatab S, Leijs MJ, van Buul G, et al. MSC encapsulation in alginate microcapsules prolongs survival after intra-articular injection, a longitudinal in vivo cell and bead integrity tracking study. Cell Biol Toxicol. 2020;36(6):553–570.
- McKinney JM, Pucha KA, Doan TN, et al. Sodium alginate microencapsulation of human mesenchymal stromal cells modulates paracrine signaling response and enhances efficacy for treatment of established osteoarthritis. Acta Biomater. 2021;15(141):315–332.
- Fathi E, Farahzadi R, Valipour B. Alginate/gelatin encapsulation promotes NK cells differentiation potential of bone marrow resident C-kit + hematopoietic stem cells. Int J Biol Macromol. 2021;177:317–327.
- Putra A, Ridwan FB, Putridewi AI, et al. The role of TNF-α induced MSCs on suppressive inflammation by increasing TGF-Î2 and IL-10. Open Access Maced J Med Sci. 2018;6(10):1779–1783.
- Polchert D, Sobinsky J, Douglas G, et al. IFN-gamma activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur J Immunol. 2008;38(6):1745–1755.
- Meisel R, Zibert A, Laryea M, et al. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103(12):4619–4621.
- Vladimirovna IL, Sosunova E, Nikolaev A, et al. Mesenchymal stem cells and myeloid derived suppressor cells: common traits in immune regulation. J Immunol Res. 2016;2016:7121580.
- Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–3843.
- Weiss ARR, Dahlke MH. Immunomodulation by mesenchymal stem cells (MSCs): mechanisms of action of living, apoptotic, and dead MSCs. Front Immunol. 2019;10(1191):1191.
- Lenzini S, Bargi R, Chung G, et al. Matrix mechanics and water permeation regulate extracellular vesicle transport. Nat Nanotechnol. 2020;15(3):217–223.
- Iansante V, Dhawan A, Masmoudi F, et al. A new high throughput screening platform for cell encapsulation in alginate hydrogel shows improved hepatocyte functions by mesenchymal stromal cells co-encapsulation. Front Med. 2018;5:216.
- Syanda AM, Kringstad VI, Blackford SJI, et al. Sulfated alginate reduces pericapsular fibrotic overgrowth on encapsulated cGMP-Compliant hPSC-Hepatocytes in mice. Front Bioeng Biotechnol. 2022;9:816542.
- Vegas AJ, Veiseh O, Doloff JC, et al. Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates. Nat Biotechnol. 2016;34(3):345–352.
- Mao AS, Ozkale B, Shah NJ, et al. Programmable microencapsulation for enhanced mesenchymal stem cell persistence and immunomodulation. Proc Natl Acad Sci USA. 2019;116(31):15392–15397.