?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Recent evidence has implicated microRNA-219 (miR-219) in regulation of gene contributed in glioblastoma (GBM) pathogenesis. This study aimed to prepare miR-219 in chitosan (CS) nanoparticles (NPs), characterize and investigate their efficacy on human GBM cell line (U87 MG). NPs were prepared using ionic gelation method. The influence of process parameters on physicochemical characteristics of NPs was investigated. Apoptotic effect of miR-219 was examined on U87 MG cells. Formulated NPs showed particle size of 109 ± 2.18 nm, with poly dispersity index equal to 0.2 ± 0.05, and zeta potential of +20.5 ± 0.7 mV. Entrapment efficiency of miR-219 in loaded NP has reached 95%. The in vitro release study demonstrated sustained release pattern of miR-219 from CS-NPs. Gel retardation assay has confirmed the integrity of miR-219 after production process. The fabricated NPs reduced the survival of U87 MG cells to 78% after 24 h of post-transfection, and into 67.5% after 48 h. However, fibroblasts were not affected by the NPs, revealing their specificity for GBM cells. Given the tumour suppressing function of miR-219, and advantage of CS-NPs for gene delivery to the central nervous system, the presented NPs have a great potential for treatment of GBM.
Introduction
Glioblastoma (GBM) is the most common type of brain tumours. It had been classified by World Health Organisation as high-grade glioma, indicating its aggressive metastasis and invasiveness [Citation1,Citation2]. In this type of tumours, cells showing anaplasia, mitotic activity, microvascular proliferation and/or necrosis with poor prognosis [Citation3].
The present standard of care depends on surgery, radiotherapy (RT) and chemotherapy (CT) [Citation4]. This combination therapy fails to extend the survival more than 15 months [Citation5]. The molecular diversity of the disease, along with the presence of cancerous stem cells, lead to the tumour recurrence and poor prognosis [Citation6,Citation7]. Consequently, extensive research efforts have focussed on advanced strategies to target the disease that could significantly improve outcomes of GBM patients [Citation8]. In this regard, microRNAs (miRNAs) have drawn much attention owing to their essential role in biological characteristics of GBM [Citation9,Citation10]. They are 19–24 nucleotides of non-coding RNAs that negatively regulate gene expression post-transcriptionally through binding to complementary sequences on the 3 prime untranslated regions (3'UTR) of target mRNAs and typically silence genes [Citation11]. Through deregulation of their target genes, miRNAs paly crucial roles in GBM through several pathways involved in cell proliferation, resistance to apoptosis, autophagy, invasion, metastasis, angiogenesis and drug resistance [Citation3,Citation12].
Based on their biological context, microRNAs have been reported to function as oncogenes or tumour suppressors in GBM [Citation13–15]. In this aspect, it has been found that miRNA-219-5p (miR-219) is one of the downregulated miRNAs in GBM [Citation16,Citation17]. Microarray analysis of brain tissues collected from GBM patients from all stages of the disease revealed a substantial decrease in miR-219 expression compared to normal astrocytes. The study was also carried out on GBM cell lines and revealed reduced expression of miR-219 [Citation16]. Cell proliferation, migration and invasion were all reduced when human GBM cells (U87 MG) were transfected with miR-219 [Citation18–20]. The tumour suppression effect of miR-219 is found to be mediated through epidermal growth factor receptor (EGFR), Sal-like protein 4 (SALL4) and roundabout homolog 1 (ROBO1) proteins [Citation21–23]. In vivo, miRNA-219-5p inhibited tumour growth in nude mouse xenograft models [Citation18]. The findings of these studies suggest the therapeutic potential of miRNA-219-5p in treatment of GBM.
In animal studies convention enhanced delivery (CED), which is a technique based on catheters stereotactically implanted to infuse the therapeutics directly through the interstitial spaces of the central nervous system (CNS) to the tumour site, was utilized to deliver anti-miRNA let-7a into the brain of mice xenografted with an aggressive GBM [Citation24]. Administration of anti-miR let-7a by this strategy was efficient to significantly reduce the expression of target gene, a high-mobility group at-hook 2, which is one of the target genes of let-7a [Citation24].
Although direct delivery of miRNA into the brain appears to be effective, non- or less invasive delivery methods would be preferable. However, there are many limitations for administration of miRNAs in vivo because of their susceptibility to RNase degradation, poor stability and poor cellular uptake. Therefore, nanoparticles (NPs) seem a very promising strategy that has been utilized for gene delivery including miRNAs in cancer including GBM [Citation25–30]. Polymeric NPs fabricated from natural polymer such as chitosan (CS), have been utilized to deliver therapeutics including oligonucleotides to the CNS. CS is a naturally derived, biodegradable and biocompatible polymer. It is composed of N-acetyl-d-glucosamine and β-(1,4)-linked d-glucosamine linked by glycosidic bonds. It is polycationic in nature, which allows binding with negatively charged therapeutics, such as miRNA [Citation31,Citation32]. This polycationic nature of CS-NPs will stabilize the genetic material protect it from degradation, and promote its uptake into target cells [Citation31]. In recent study, CS-NPs were prepared and utilized to deliver small interfering RNA (siRNA) intranasally against galectin-1 to GBM GL261 cells, as well as to primary tumour cultures. These NPS resulted in significant reduction in galectin-1 expression in both cell lines [Citation33]. In other in vitro study, CS-tripolyphosphate (TPP) NPs have been fabricated to deliver anti heat shock protein 70 (HSP70) in the GBM U251N cell line, leading to 40% decrease in HSP70 levels with minor toxicity [Citation34]. These studies revealed successful implication of CS NPs as gene delivery in GBM treatment. According to our knowledge, there are no studies yet have developed CS NPs loaded with miR-219. Given the tumour suppressing function of miRNA-219-5-p, and advantage of CS NPs for gene delivery to the CNS, therefore, this study aimed to prepare CS NPs carrier for miR-219 as potential therapeutics for GBM.
Materials and methods
Materials
Low-molecular weight CS (LMW, 50 − 190 kDa, Degree of deacetylation; DD = 75%−85%), sodium TPP and acetic acid were purchased from Sigma-Aldrich (St Louis, USA). miR-219 mimic (UGAUUGUCCAAACGCAAUUCU,) with a molecular weight of 13989.51 g/mol (7281.4 Dalton) and negative control (NC) miRNA were obtained from RiboBio (Guangzhou, China). Quant-iT RiboGreen RNA Assay Kit was purchased from Invitrogen (Carlsbad, CA, USA). Human GBM cell line (U87 MG) was purchased from American Type Culture Collection (ATCC HTB-14, VA, USA). Normal human fibroblast was purchased from ATCC (CRL-1634, VA, USA). Dulbecco’s Modified Eagle Medium (DMEM), foetal bovine serum (FBS), penicillin-streptomycin solution and trypsin were obtained from (Gibco, UK). GenMute™ siRNA Transfection Reagent (TR) was obtained from SignaGen Laboratories (Gaithersburg, MD, USA). PrestoBlue™ Cell Viability Reagent was obtained from Invitrogen (Carlsbad, CA, USA). Agarose were obtained from MilliporeSigma (Burlington, MA, USA). OmniPurR Ethidium Bromide and OmniPurR DNA Gel Loading Dye 6×, and GeneRuler (50 bp) DNA Ladder were purchased from Thermo Scientific (Waltham, MA, USA).
Methods
Preparation of CNPs
CNP-miR-219 was generated by ionic gelation method as described previously [Citation35]. Solutions of LMW CS was prepared at different concentrations ranging from 0.025% to 0.5% (w/v), dissolved in 1% (v/v) of acetic acid. Then, pH of CS solution was adjusted into five using 10 N NaOH. For addition of miRNA, TPP was pre-incubated with 24 µg of miR-219 or miR-NC for each 2 ml of NPs at different N/P ratio of (50, 100, 150, 200 and 250) before the addition to CS solutions. Then, TPP containing miRNA was added dropwise to the CS solution at optimized parameters under stirring for 1 h at room temperature. After an incubation time of 40 min at 4 °C, NPs were collected by ultracentrifugation using 40,000 ×g, at 4 °C for 50 min. The supernatant was conserved for EE% determination. The collected NPs were reconstituted with 400 μl of 12% sucrose, lyophilized and stored for further use.
Entrapment efficiency (EE%)
The miRNA EE% in prepared NPs was determined as previously described using Quant-iT RiboGreen RNA Assay Kit [Citation36]. Briefly, NPs loaded with miRNA were centrifuged at 40,000 xg for 50 min and then, the amount of unentrapped miRNA in the supernatant was quantified using RiboGreen® assay. EE% was calculated by following equation:
Determination of PS and ZP
Particle size (PS), polydispersity index (PDI) and zeta potential (ZP) of the formed NPs were measured in triplicate, without further dilutions, at 25 °C using Zetasizer Nano ZS90 (Malvern Instruments Ltd, UK). The samples were measured in phosphate saline buffer (pH 7.4) and each measurement was done in triplicate
Characterisation of NPs morphology
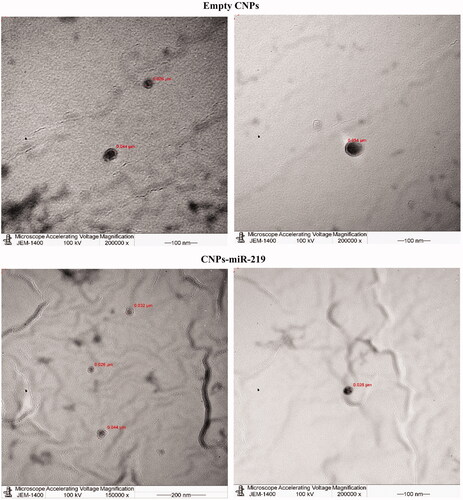
The morphology of prepared NPs was visualized by transmission electron microscope (TEM) (JEM-1230EX; Tokyo, Japan). One drop of diluted CNPs solution was loaded on a carbon film 300 mesh copper grid, allowing to sit until air-dried. The sample was stained with 1 M uranyl acetate solution for 5 s at 7 °C before viewing on the TEM.
In vitro release study of miR-219 from NPs formulation
CNPs-miR-219 were suspended in 1 ml of Tris-EDTA buffer (TE buffer) in RNase free Eppendorf tubes and shaken in water bath (60 rpm) at 37 °C as described previously [Citation37]. At pre-determined time intervals, the supernatant was collected for analysis and replaced with fresh buffer. The amount of miR-219 released was determined using Quant-iT RiboGreen RNA Assay Kit.
Gel retardation assay
miR-219 integrity
CNPs were loaded onto a 2% agarose gel which was prepared with Tris/borate/EDTA buffer. The integrity of miR-219 was analysed by gel electrophoresis.
Enzyme degradation study
CNPs were incubated with RNase at 37 °C for different time intervals. NPs were loaded onto a 2% agarose gel. Free miR-219 was also incubated with equivalent concentration of RNase and loaded onto the gel.
Storage stability
CNPs were resuspended in ultrapure water and stored at 4 °C and 25 °C temperatures. PS, PDI and surface charge were measured every month.
Transfection/treatment of cell lines
Cell line culturing
Human GBM cell line (U87 MG) was cultured in DMEM (1 g glucose) medium supplemented with 10% FBS and 1% Antibiotic-Antimycotic solution. The cells maintained in an incubator containing 5% CO2 at 37 °C.
In vitro transfection
Transfection was performed as previously described [Citation38]. U87 MG cells were seeded (5k per well) onto 96 well tissue culture plates in culture media and left overnight at 37 °C. After reaching 75% confluency, the media was replaced with starvation culture media containing 1% of FBS and kept overnight at 37 °C. In the next day, the cells were treated with CNPs-miR-219 and NPs loaded with negative control miRNA (CNPs-NC). In addition, different concentrations of miR-219 were transfected using GenMute™ TR according to manufacturer’s instructions to compare it with NPs system. The delivery systems and controls were incubated with the cells for 5 h to mediate transfection. After the 5-h treatment time, the media was changed into culture media containing 10% FBS and placed in the incubator for the following 24 h. After that, the cells were pelleted for further analysis.
Effect of miRNA loaded NPs on cells proliferation
Cells proliferation was detected using Presto-Blue reagent. CNPs-miR-219 and CNPs-NC were added to GBM cells for 24 and 48 h of post-transfection. After experimental treatment, the reagent was mixed to the media in 1/10 ratio and incubated at 37 °C for 1 h. The fluorescence of transformed Presto-Blue solution in the wells was measured at 570 and 590 nm using microplate reader. Percentage of cell viability was calculated using equation:
where “F of sample” is the fluorescence of the transformed Presto-Blue in cells incubated with the formula and “F of control” is the fluorescence of transformed Presto-Blue in cells incubated with the CNPs-NC.
Statistical analysis
The difference between experimental groups was analysed using Prism 8 (GraphPad), taking p < .05 as the lowest acceptable threshold for significance. For two groups, unpaired two-tailed Student’s t-test was applied to analyse the difference significance. One-way analysis of variance was used for the comparison between more than two groups followed by Tukey’s test post-hoc test. The results were expressed as averages ± SD.
Results
The study was aiming to generate CNPs that able to entrap, protect and deliver miR-219 for GBM treatment. The optimal pharmaceutical goal was to produce NPs with the smallest particle size and overall positive surface charge. Accordingly, the influence of pre-determined parameters that contribute to size and charge of produced NPs were investigated and optimized. These parameters include CS concentration, its pH, its ratio to TPP and weight ratio of NPs to loaded gene.
Optimisation of prepared CNPs
Optimisation of CS concentration
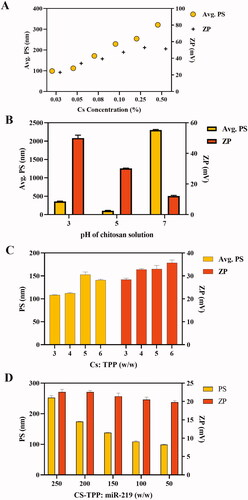
The concentration of CS played a significant role in the resultant PS and ZP. It is clear from that the PS and ZP were increasing with increasing of CS concentration. The selected concentration was 0.05% (0.5 mg/mL), in where PS was of 112.2 ± 2.27 nm, and surface charge equal to 33.9 ± 0.9 mV have been obtained.
Figure 1. Optimization of CNPs formulation. (A) Influence of CS concentration on PS and ZP of NPs. Different CS concentrations were prepared in 1% acetic acid. Particle size and zeta potential were measured without dilution at room temperature. Results indicate mean ± SD. (B) Influence of CS solution pH on PS and ZP of NPs. Using 0.05% of CS. pH was adjusted with 10 N of NaOH or HCl. PS and ZP were measured at room temperature. Results indicate mean ± SD, n = 3. (C) Influence of CS: TPP on PZ and ZP of NPs. The pH was adjusted into 5. PS and ZP were measured at room temperature. Results indicate mean ± SD, n = 3. (D) Influence of CS-TPP: miR-219 ratio on PZ and ZP of NPs. 24 µg of miR-219 were loaded in CNPs at different N/P ratio. PZ and ZP were measured at room temperature. Results indicate mean ± SD, n = 3.
Optimisation of CS solution pH
The pH of CS had influenced the resultant size and charge of produced NPs as shown in . CS solution with pH of 5 was chosen fairly to be the optimal value.
Optimisation of CS: TPP ratio (w/w)
The increasing of CS:TPP ratio had shifted the formulation into higher particle size and surface charge (). Obviously, 3:1 ratio has exhibited the minimum PS with optimal positive charge.
Quantification of EE%
Optimisation of Cs-TPP:miR-219 ratio
Optimal ratio selection depended on size, charge and EE%. EE% had varied correspondingly with the ratio modification. The statistical analysis among all formulations had resulted in no significant difference (p = .99). However, PS and surface charge were also criteria for selection of the optimal condition. shows formulations characteristics that referred to ratio manipulation. The effect of manipulation on NPs size and charge is illustrated in . It exhibited that the nanosized particles of miR-219 loaded in NPs were enlarged subsequently with greater N/P ratio. Subsequently, 100:1 ratio has been selected based on the minimum particles size and the highest EE%.
Table 1. Influence of CS-TPP:miR-219 ratio on NPs physicochemical properties.
Transmission electron microscope
The formulations were visualized under TEM. In , both miR-219-loaded and empty CNPs showed spherical shapes.
In vitro release profile of miR-219 from NPs formulation
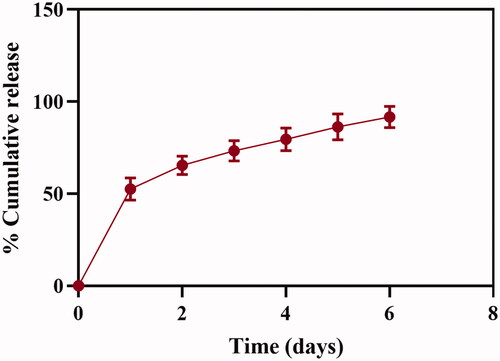
Cumulative percentage of released miR-219 was calculated. The entrapped drug had been released from NPs at two stages. In the first day, a rapid release of ∼50% of the drug was detected. In the following 6 days, drug release showed a constant and sustained release for more than 90% of the loaded miR-219. illustrates drug release profile of miR-219 from the fabricated NPs.
Figure 3. In vitro release profile of miR-219 from chitosan NPs. NPs were suspended in 1 mL of TE buffer and shaken in water bath at 37 °C. Percentage of release expressed as mean ± SD, n = 3. Naked miR-219 was analyzed during the study as a control.
Mir-219 integrity
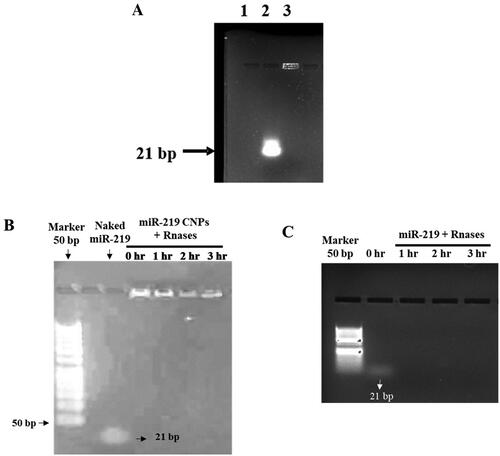
To demonstrate integrity of entrapped miR-219, NPs were loaded on gel electrophoresis and compared to equal amount of naked miR-219. Gel retardation assay, which is presented in , shows retardation of miR-219 in the third lane, indicating stability of the gene after production process.
Figure 4. Integrity of miR-219 in CNPs. (A) Gel retardation assay of NPs loaded with miR-219. Using 2% agarose gel. Lane 2 was loaded with naked miR-219, Lane 3 displays retarded miR-219. Gel was stained by ethidium bromide. (B and C) Protection of miR-219 from RNase. (B) miR-219 loaded CS NPs incubated with RNase at time (0, 1, 2, and 3 hrs). (C) miR-219 incubated with RNase at time (0, 1, 2, and 3 hrs).
Enzyme degradation study
Gel assay showed a stability of miR-219 in the established NPs. Furthermore, non-formulated miR-219 was incubated with equivalent concentration to ensure enzyme degradation of the nucleic acids ().
Storage stability
The formulation was stable for up to one month at 4 °C. Conversely, ambient temperature resulted in size and charge reduction after one month. summarizes the consequent effects of different storage conditions on NPs stability for three months.
Table 2. Storage stability of miR-219 CNPs.
Apoptotic effect of CS NPs loaded with miR-219 on GBM cells
U87 MG GBM cell lines were treated by NPs loaded with miR-219, beside other NPs that were loaded with NC, which is non-functional miRNA. In addition, miR-219 and NC were transfected separately into the cells through Genemute™ TR. Cell proliferation was detected by Presto-blue kit at three concentrations of the miR-219. After 24 h of post-transfection, NPs that were loaded with miR-219 at all doses had reduced cell viability into 78% (p < .05; ). Another experiment was run for 48 h of post-transfection using a dose of 10 picomole (pmol) from CNPs-miR-219, and CNPs-NC as a control. In , the results showed a further reduction on the percentage of cell viability into 67.5%.
Figure 5. Cell proliferation assay. (A) Cells viability was assessed on U87 cells using Presto-blue kit 24 hr after transfection, the fluorescence was detected excitation 570 nm and emission 590 nm, cell viability was reduced to 78% (p < .05). CNPs: chitosan nanoparticles, NC: negative control miRNA, TR: transfection reagent. (B) Cell proliferation assay on U87 cells after 48 hours of post-transfection. The cells were treated with a dose of 10 picomoL from CNPs-miR-219, and CNPs-NC as a control. Cell viability was assessed 48 hr post transfection and found to be reduced into 67.5%, (*p < .05). CNPs: chitosan nanoparticles, NC: negative control miRNA. (C) Investigation of CNPs-miR-219 toxicity on fibroblasts. Using presto-blue kit. Fibroblasts viability were not changed, p = .3.
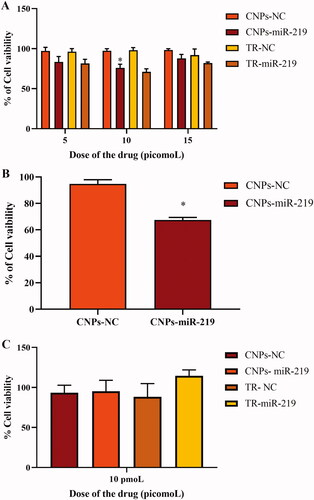
Cell proliferation assay was also performed on fibroblast cells. Cell viability was not affected after miR-219 treatment when compared with the control. MiR-219 in NPs as well as in TR had not suppressed viability of fibroblasts, representing normal cells, which indicates formulation safety and specificity for GBM cells ().
Survival data
The Cancer Genome Atlas (TCGA) data was extracted from over 200 patients suffering from GBM disease. The analysis was made through OncoLnc on 4 April 2020 [Citation39]. The OncoLnc database includes survival data for 8647 patients from 21 cancer examination completed by TCGA. Looking at TCGA database, significance was found between miR-219, ROBO1 and SALI4 expressions relevant to GBM. In , expression of miR-219 in GBMs for both live and dead populations is presented. However, high expression for ROBO1 and SALL4 significantly (p-value .0286 and .0498, respectively) corresponded to worse prognosis than patients with low expression of these genes which corresponded to better prognosis ().
Figure 6. miR-219, ROBO1, and SALL4 expression in GBM patients. (A) Expression status of miR-219 among dead and alive GBM patients. The red rectangle represents Low expression population of the deceased patients, obtained from TCGA survival database [Citation35]. (B) ROBO1 low expressing patients live longer. Kaplan–Meier curve classifying survival of glioblastoma (GBM) patients based on ROBO1 expression. Low expression cut off is equal or less than 20% of expression while High expression is above 20%. Significantly, obtained from TCGA survival database [Citation35]. (C) SALL4 low expressing patients live longer. Kaplan–Meier curve classifying survival of glioblastoma (GBM) patients based on Sall4 expression. Low expression cut off is equal or less than 20% of expression while High expression is above 20%. Significantly, obtained from TCGA survival database [Citation35].
![Figure 6. miR-219, ROBO1, and SALL4 expression in GBM patients. (A) Expression status of miR-219 among dead and alive GBM patients. The red rectangle represents Low expression population of the deceased patients, obtained from TCGA survival database [Citation35]. (B) ROBO1 low expressing patients live longer. Kaplan–Meier curve classifying survival of glioblastoma (GBM) patients based on ROBO1 expression. Low expression cut off is equal or less than 20% of expression while High expression is above 20%. Significantly, obtained from TCGA survival database [Citation35]. (C) SALL4 low expressing patients live longer. Kaplan–Meier curve classifying survival of glioblastoma (GBM) patients based on Sall4 expression. Low expression cut off is equal or less than 20% of expression while High expression is above 20%. Significantly, obtained from TCGA survival database [Citation35].](/cms/asset/75afa57f-692b-4eea-9bc5-b16306aa641a/ianb_a_2092123_f0006_c.jpg)
Discussion
Considering poor survival of GBM and complexity nature of the disease, implication of new therapeutics such as miRNAs is required [Citation40]. Lately, miR-219 was proposed to be a tumour suppressor for GBM, in which several glioma tissues exhibit low levels of miR-219 expression. Ectopic expression of miR-219 has decreased the proliferation, migration and invasion of human GBM cell lines U87 MG, and promotes apoptosis. Such effect mediated through targeting of miR-219 to EGFR, ROBO1 and SALL4 that are involved in the pathogenesis of GBM. Therefore, miR-219 has great potential as therapeutic agent for GBM [Citation21–23]. Many limitations should be treated for successful clinical application of miRNAs such as susceptibility to RNase degradation, stability and poor cellular uptake. To overcome these barriers, polymeric NPs such as CNPs have been used extensively for gene delivery involving miRNAs [Citation41,Citation42]. CS is a natural cationic polymer, which enhances its complexation with negatively charged miRNAs and improves the stability of genes. Superiority of CS is gained owing to its safety, biodegradability and biocompatibility features [Citation43]. Our fundamental criterion for selection of optimal formulation were the size and the surface charge of the fabricated NPs. Literature reports higher cellular uptake and transfection efficiency of the smaller sized particles [Citation41,Citation44,Citation45]. Furthermore, positive charge of the formulation has higher probability of internalisation as consequence of improved electrostatic binding with cell membrane [Citation42,Citation46,Citation47]. In addition, condensed positive charge could promote escape of NPs from endosomes, which promote intracellular release of drug, thus enhance targets genes silencing [Citation48–51].
Pre-determined parameters were investigated and optimized to qualify a formulation with the smallest PS and a considerable surface charge. CS concentration, its pH and its ratio to TPP were studied being critical process parameters that largely affect size and charge of produced particles. As shown in , PS was decreasing with decreasing of CS concentration. Lower concentration of CS reduces viscosity of the solution, hence higher solubility and better reaction of CS with TPP, leading to smaller PS [Citation52,Citation53]. Therefore, 0.05% CS concentration has been selected as an optimal solution that gave small PS and adequate positive surface which able to carry miRNAs.
Different pH values of CS solutions have observed a notable effect on PS and ZP. Slightly acidic solution of pH = 5 was fairly chosen as an ideal condition to generate the smallest PS. When pH of solution is under the pKa of CS, which is 6.5, amino groups in CS backbone are protonated into positive ions, promoting CS interaction with negatively charged nucleic acid in a stable complex. On the other hand, when pH of the medium is towards a neutral or alkali, the protonation of primary amines is decreased, hence the electrostatic binding between polymer and miR-219 turns into unstable polyplexes. Even though pH of 7 had generated a complex, as presented in , previous research has shown that the complexation between CS and TPP under neutral pH is referred to weaker links namely non-electrostatic, like hydrogen and hydrophobic bonds [Citation38,Citation54].
Weight ratio of CS to TPP was also optimized. demonstrates larger particles along with ratio increasing. Earlier research has endorsed the effect of CS:TPP ratio on PS [Citation35,Citation55]. Consequently, 3:1 ratio was selected to obtain the smallest particles. CNPs were loaded with miR-219 at different N:P ratio. The ratio was optimized to minimize PS and maximize EE%. Obviously, 100:1 ratio has generated NPs that achieved pre-determined goal, like prior reports [Citation38,Citation54]. Gel retardation assay confirms integrity of miR-219 after loading in CNPs (). Production process at optimized condition has insured the stability of miR-219. In addition, miR-219 loaded NPs were physically stable for one month at 4 °C. These results are in agreement with Raja et al. study in which CS NPs were formulated, using TPP as cross linker, not others including sodium sulphate and poly-d-glutamic acid sodium salt, and were stable for two weeks [Citation56]. Also, in agreements with previous studies [Citation56,Citation57], these results revealed that TPP’s cross-linking effect exerts its properties as a stabilizer and provokes polymer molecules to create stronger interactions and more stable structures, which are less prone to aggregation. Contrarily, particle size and ZP of our formulation, as well as for the mentioned groups, had been reduced after one month of storage at room temperature. Degradation of NPs is responsible for this reduction. In general, higher temperature possibly will accelerate kinetic motion of NPs [Citation58]. Subsequently, it is recommended to store the prepared NPs at 4 °C for one month along with further assessment of apoptotic and gene silencing effects.
In vitro release profile of the drug was also studied. Release of the drug from designed system has exhibited two distinct stages, started by a rapid release of drug in the first day, followed by a constant release at the rest of days. Obviously, kinetic of drug release is dependent on multiple factors, such as shape and size of NPs, amount of cross linker as well as other process and medium condition [Citation59]. Drug release from CS particles incorporates three different mechanisms: (I) release of drug entrapped in surface of particles, (II) diffusion of matrix and (III) release due to polymer erosion [Citation60]. In most cases, release of drug is subjected to more than one mechanism. Adsorbed drug will be released from particle surface leading to a burst effect, which agrees with the first day release in our formulation. On the other hand, entrapped miRNAs were released by diffusion and erosion mechanisms, assuring sustained release of drug. These finding are also supported by earlier studies [Citation56,Citation58,Citation61,Citation62]. Sustained release of miR-219 could promote a favourable prolonged gene silencing effect [Citation43].
U87 MG cells have been treated with 5, 10 and 15 pmol of the optimized formulation. The highest apoptotic effect is shown with 10 pmol, which reduced the survival to 78% when compared with the control after 24 h of post-transfection (). The cell viability was further reduced to 67.5% on average after 48 h (), indicating the sustained release characteristic of CNPs. Apoptosis of GBM cell line could be a result of high cellular uptake, successful intracellular release and effective gene silencing. A comprehensive understanding of NPs uptake process by targeted cells is an essential consideration for maximising the effectiveness of delivery. Even though CS-based delivery systems have been broadly studied, the definite pathway of cellular uptake has not been fully understood [Citation63,Citation64]. Many considerations involve in adequate internalisation of NPs, such as physicochemical properties of the system and type of targeted cells [Citation65].
To demonstrate the toxicity and specificity of miR-219, normal cells (fibroblast cells) were treated by the generated NPs. Fibroblasts cells viability has not diminished after treatment with miR-219 through both NPs and TR (). Importantly, the used CT and RT show no specificity for GBM tissue. Generalized treatments impair defective and healthy cells at the same time, reducing the tolerance of the therapy. Consequently, the provided treatment approach illustrates more affordable and safe therapy. In addition, presented the deceased population of low expression population miR-219 which are the target population that could have been saved by increasing miR-219 expression in order to delay or prevent death. Increasing miR-219 expression will decreases both expression of both ROBO1 and SALL4. This decrease are depicted in the population with prolonged survival () when both genes are reduced. This suggests that reversing miR-219 expression from low to high is potentially good for prognosis.
Conclusion
In the current study, CNPs loaded with miR-219 were formulated successfully by the ion gelation method and presented optimal physicochemical characteristics after optimisation of Cs concentrations, Cs to TPP ratio, pH of Cs and N to P ratio. The mean diameter of CNPs containing miR-219 was below 109 nm, with positive ZP and optimal PDI. The encapsulation efficiency was high, integrity of miR-219 was maintained after formulation process and sustained release pattern of miR-219 from CNPS was shown. Moreover, the NPs maintained their stability at 4 °C for at least one month. The produced miR-219 loaded CNPs reduced cell viability of human GBM cells more prominently after 48 h, revealing sustained release properties of the CNPs. Such decrease in cell viability was not observed on normal fibroblast revealing the specificity of miR-219 loaded CNPs to cancerous cells. Therefore, the developed CNPs loaded with miR-219 have promising potential for GBM treatment.
Author contributions
FYA and FSA: Conceptualisation, design of study, acquisition of data, validation and writing original draft. RA: Acquisition of data, analysis of data, visualisation, interpretation of data and writing first draft preparation. BA, MB, QA and HA: Acquisition of data, analysis of data, visualisation and interpretation of data. IA: Resources, visualisation, interpretation of data, writing- reviewing and editing. All authors have read and agreed to the published version of the manuscript.
Disclosure statement
The authors declare no conflict of interest.
Data availability statement
Data available on request from the authors.
Additional information
Funding
References
- Clarke J, Butowski N, Chang S. Recent advances in therapy for glioblastoma. Arch Neurol. 2010;67(3):279–283.
- Meyer MA. Malignant gliomas in adults. N Engl J Med. 2008;359(17):1850.
- Gupta A, Dwivedi T. A simplified overview of world health organization classification update of Central nervous system tumors 2016. J Neurosci Rural Pract. 2017;8(4):629–641.
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996.
- Miller TE, Liau BB, Wallace LC, et al. Transcription elongation factors represent in vivo cancer dependencies in glioblastoma. Nature. 2017;547(7663):355–359.
- Wainwright DA, Nigam P, Thaci B, et al. Recent developments on immunotherapy for brain cancer. Expert Opin Emerg Drugs. 2012;17(2):181–202.
- Cai X, Sughrue ME. Glioblastoma: new therapeutic strategies to address cellular and genomic complexity. Oncotarget. 2018;9(10):9540–9554.
- Chen R, Cohen AL, Colman H. Targeted therapeutics in patients with high-grade gliomas: past, present, and future. Curr Treat Options Oncol. 2016;17:1–11.
- Surgeons A. Brain tumors. Available from: https://www.aans.org/en/Patients/Neurosurgical-Conditions-and-Treatments/Brain-Tumors.
- Sciences F. Nerves: neural supporting cells. Available from: https://www.histology.leeds.ac.uk/tissue_types/nerves/Nerve_support_cell.php.
- Wesseling P, Capper D. WHO 2016 classification of gliomas. Neuropathol Appl Neurobiol. 2018;44(2):139–150.
- Howlader N, Krapcho NA, Miller M, et al. SEER Cancer Statistics Review, 1975. 2016. Available from: https://seer.cancer.gov/csr/1975_2016/.
- Ahir BK, Ozer H, Engelhard HH, et al. MicroRNAs in glioblastoma pathogenesis and therapy: a comprehensive review. Crit Rev Oncol Hematol. 2017;120:22–33.
- Banelli B, Forlani A, Allemanni G, et al. MicroRNA in glioblastoma: an overview. Int J Genomics. 2017;2017:7639084.
- MicroRNA Targeted Cancer Therapy. 2014.
- Cancer Genome Atlas Research. N. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068.
- Hsu SD, Chu CH, Tsou AP, et al. miRNAMap 2.0: genomic maps of microRNAs in metazoan genomes. Nucleic Acids Res. 2008;36(Database issue):D165–169.
- Ellor SV, Pagano-Young TA, Avgeropoulos NG. Glioblastoma: background, standard treatment paradigms, and supportive care considerations. J Law Med Ethics. 2014;42(2):171–182.
- Perry J, Zinman L, Chambers A, on behalf of the Neuro-oncology Disease Site Group of Cancer Care Ontario’s Program in Evidence-Based Care, et al. Neuro-oncology disease site, G.; cancer care ontario's program in Evidence-Based, C. The use of prophylactic anticonvulsants in patients with brain tumours-a systematic review. Curr Oncol. 2006;13(6):222–229.
- Blissitt PA. American Association of Neuroscience Nurses. N. Clinical practice guideline series update: care of the adult patient with a brain tumor. J Neurosci Nurs. 2014;46(6):367–368.
- Rao SA, Arimappamagan A, Pandey P, et al. miR-219-5p inhibits receptor tyrosine kinase pathway by targeting EGFR in glioblastoma. PLoS One. 2013;8(5):e63164.
- Jiang B, Li M, Ji F, et al. MicroRNA-219 exerts a tumor suppressive role in glioma via targeting Sal-like protein 4. Exp Ther Med. 2017;14(6):6213–6221.
- Jiang Y, Yin L, Jing H, et al. MicroRNA-219-5p exerts tumor suppressor function by targeting ROBO1 in glioblastoma. Tumour Biol. 2015;36(11):8943–8951.
- Davis ME. Glioblastoma: overview of disease and treatment. Clin J Oncol Nurs. 2016;20(5 Suppl):S2–S8.
- Toh CH, Castillo M. Early-Stage glioblastomas: MR imaging-Based classification and imaging evidence of progressive growth. AJNR Am J Neuroradiol. 2017;38(2):288–293.
- Zhang H, Pan X, Wu Q, et al. Manganese carbonate nanoparticles-mediated mitochondrial dysfunction for enhanced sonodynamic therapy. Exploration. 2021;1(2):20210010.
- Zheng M, Du Q, Wang X, et al. Tuning the elasticity of polymersomes for brain tumor targeting. Adv Sci. 2021;8(20):2102001.
- Huang H, Dong C, Chang M, et al. Mitochondria-specific nanocatalysts for chemotherapy-augmented sequential chemoreactive tumor therapy. Exploration. 2021;1(1):50–60.
- Jiang T, Qiao Y, Ruan W, et al. Cation-free siRNA micelles as effective drug delivery platform and potent RNAi nanomedicines for glioblastoma therapy. Adv Mater. 2021;33(45):2104779.
- Guo S, Li K, Hu B, et al. Membrane-destabilizing ionizable lipid empowered imaging-guided siRNA delivery and cancer treatment. Exploration. 2021;1(1):35–49.
- Wilson TA, Karajannis MA, Harter DH. Glioblastoma multiforme: state of the art and future therapeutics. Surg Neurol Int. 2014;5:64.
- Perry J, Okamoto M, Guiou M, et al. Novel therapies in glioblastoma. Neurol Res Int. 2012;2012:428565.
- Hoover JM, Chang SM, Parney IF. Clinical trials in brain tumor surgery. Neuroimaging Clin N Am. 2010;20(3):409–424.
- Aguilar LK, Arvizu M, Aguilar-Cordova E, et al. The spectrum of vaccine therapies for patients with glioblastoma multiforme. Curr Treat Options Oncol. 2012;13(4):437–450.
- Malhotra M, Kulamarva A, Sebak S, et al. Ultrafine chitosan nanoparticles as an efficient nucleic acid delivery system targeting neuronal cells. Drug Dev Ind Pharm. 2009;35(6):719–726.
- Wu G, Feng C, Hui G, et al. Improving the osteogenesis of rat mesenchymal stem cells by chitosan-based-microRNA nanoparticles. Carbohydr Polym. 2016;138:49–58.
- Katas H, Raja MA, Lam KL. Development of chitosan nanoparticles as a stable drug delivery system for protein/siRNA. Int J Biomater. 2013;2013:146320.
- Van Woensel M, Wauthoz N, Rosiere R, et al. Development of siRNA-loaded chitosan nanoparticles targeting galectin-1 for the treatment of glioblastoma multiforme via intranasal administration. J Control Release. 2016;227:71–81.
- Anaya J. OncoLnc: linking TCGA survival data to mRNAs, miRNAs, and lncRNAs. PeerJ Comput Sci. 2016;2:e67. Available from: https://peerj.com/articles/cs-67/#.
- Delgado-Lopez PD, Corrales-Garcia EM. Survival in glioblastoma: a review on the impact of treatment modalities. Clin Transl Oncol. 2016;18:1062–1071.
- Mao S, Sun W, Kissel T. Chitosan-based formulations for delivery of DNA and siRNA. Adv Drug Deliv Rev. 2010;62(1):12–27.
- Buschmann MD, Merzouki A, Lavertu M, et al. Chitosans for delivery of nucleic acids. Adv Drug Deliv Rev. 2013;65(9):1234–1270.
- Cao Y, Tan YF, Wong YS, et al. Recent advances in chitosan-based carriers for gene delivery. Mar Drugs. 2019;17(6):381.
- Zhang H, Mardyani S, Chan WC, et al. Design of biocompatible chitosan microgels for targeted pH-mediated intracellular release of cancer therapeutics. Biomacromolecules. 2006;7(5):1568–1572.
- Gref R, Domb A, Quellec P, et al. The controlled intravenous delivery of drugs using PEG-coated sterically stabilized nanospheres. Adv Drug Deliv Rev. 1995;16(2-3):215–233.
- Thibault M, Nimesh S, Lavertu M, et al. Intracellular trafficking and decondensation kinetics of chitosan-pDNA polyplexes. Mol Ther. 2010;18(10):1787–1795.
- Yue ZG, Wei W, Lv PP, et al. Surface charge affects cellular uptake and intracellular trafficking of chitosan-based nanoparticles. Biomacromolecules. 2011;12(7):2440–2446.
- Gao Y, Zhang Z, Chen L, et al. Chitosan N-betainates/DNA self-assembly nanoparticles for gene delivery: in vitro uptake and transfection efficiency. Int J Pharm. 2009;371(1-2):156–162.
- Wagner E. Application of membrane-active peptides for nonviral gene delivery. Adv Drug Deliv Rev. 1999;38(3):279–289.
- Regberg J, Srimanee A, Langel U. Applications of cell-penetrating peptides for tumor targeting and future cancer therapies. Pharmaceuticals (Basel). 2012;5(9):991–1007.
- Malhotra M, Tomaro-Duchesneau C, Prakash S. Synthesis of TAT peptide-tagged PEGylated chitosan nanoparticles for siRNA delivery targeting neurodegenerative diseases. Biomaterials. 2013;34(4):1270–1280.
- Fan W, Yan W, Xu Z, et al. Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloids Surf B Biointerfaces. 2012;90:21–27.
- Sreekumar S, Goycoolea FM, Moerschbacher BM, et al. Parameters influencing the size of chitosan-TPP nano- and microparticles. Sci Rep. 2018;8(1):4695.
- Sharma K, Somavarapu S, Colombani A, et al. Nebulised siRNA encapsulated crosslinked chitosan nanoparticles for pulmonary delivery. Int J Pharm. 2013;455(1-2):241–247.
- Karimi M, Avci P, Ahi M, et al. Evaluation of chitosan-tripolyphosphate nanoparticles as a p-shRNA delivery vector: formulation, optimization and cellular uptake study. J Nanopharm Drug Deliv. 2013;1(3):266–278.
- Raja MA, Katas H, Jing Wen T. Stability, intracellular delivery, and release of siRNA from chitosan nanoparticles using different cross-linkers. PLoS One. 2015;10(6):e0128963.
- Rodrigues S, da Costa AM, Grenha A. Chitosan/carrageenan nanoparticles: effect of cross-linking with tripolyphosphate and charge ratios. Carbohydr Polym. 2012;89(1):282–289.
- Katas H, Alpar HO. Development and characterisation of chitosan nanoparticles for siRNA delivery. J Control Release. 2006;115(2):216–225.
- Dong Y, Ng WK, Shen S, et al. Scalable ionic gelation synthesis of chitosan nanoparticles for drug delivery in static mixers. Carbohydr Polym. 2013;94(2):940–945.
- Agnihotri SA, Mallikarjuna NN, Aminabhavi TM. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J Control Release. 2004;100(1):5–28.
- He P, Davis SS, Illum L. Sustained release chitosan microspheres prepared by novel spray drying methods. J Microencapsul. 1999;16(3):343–355.
- Loh JW, Schneider J, Carter M, et al. Spinning disc processing technology: potential for large-scale manufacture of chitosan nanoparticles. J Pharm Sci. 2010;99(10):4326–4336.
- Fonseca SB, Pereira MP, Kelley SO. Recent advances in the use of cell-penetrating peptides for medical and biological applications. Adv Drug Deliv Rev. 2009;61(11):953–964.
- Mae M, Langel U. Cell-penetrating peptides as vectors for peptide, protein and oligonucleotide delivery. Curr Opin Pharmacol. 2006;6(5):509–514.
- Nel AE, Madler L, Velegol D, et al. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009;8(7):543–557.