?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Genistein (GEN), a natural isoflavone possesses a wide range of pharmacological properties and nutraceutical applications. GEN has been studied for its anticancer activity against different types of cancers, but its use in clinical practice is limited due to its low water solubility, rapid metabolism and excretion, lack of cancer cell targeting and poor bioavailability. In the present study, we investigated folate receptor-targeted and PEGylated poly(lactide-co-glycolide) nanoparticles (PLGA-PEG-FA NPs) containing GEN for targeted delivery to ovarian cancer cells. PLGA-PEG and PLGA-PEG-FA polymer conjugates were synthesized and characterized. Nano-precipitation method was employed for the fabrication of NPs of PLGA, PLGA-PEG and PLGA-PEG-FA containing GEN. GEN containing PLGA-PEG and PLGA-PEG-FA NPs prepared were small (104.17 ± 1.61 and 125.41 ± 3.11 nm, respectively) and exhibited sustained release of GEN for around six days. Folate-decorated PLGA-PEG NPs showed increased cellular uptake in comparison to non-targeted PLGA-PEG NPs. The GEN containing PLGA-PEG-FA NPs showed superior anticancer activity than non-targeted PLGA and PLGA-PEG NPs in folate receptor-overexpressing ovarian cancer cell line, SKOV-3. The IC50 of GEN, GEN encapsulated NPs of PLGA, PLGA-PEG and PLGA-PEG-FA were 51.48, 26.70, 23.43 and 11.98 µg/ml, respectively. Folate-targeted PLGA nanoparticles could be developed for potential target-specific delivery of GEN in the treatment of ovarian cancer.
Introduction
Cancer has high morbidity and mortality rate worldwide, and World Health Organization (WHO) assumed that the number of new cancer cases would be around 22 million in the next two decades compared to 14 million in 2012. Various therapeutic strategies for cancer management include surgery, radiation, biological therapy and chemotherapy. Clinically used chemotherapeutic agents have toxic effects, erratic bioavailability, rapid metabolism, unwanted effects, non-selective distribution, etc. One of the approaches to overcome the above limitations is targeted drug delivery to the affected site. This approach can be achieved by developing poly(D,L-lactide-co-glyco-lide) (PLGA) nanoparticles (NPs) conjugated with poly(ethylene glycol) (PEGylation) and/or a targeting ligand such as folic acid (FA). PEGylation and FA conjugation enhances the efficacy and targeting features of the formulation [Citation1]. PLGA is an FDA approved polymer due to its exceptional biocompatibility, biodegradability and mechanical strength [Citation2]. PLGA-based NPs have been employed to improve the antitumour efficacy of drug molecules [Citation3–5]. PEG is a highly biocompatible non-ionic hydrophilic polyether. Conjugation of PEG with PLGA NPs has numerous advantages such as enhancement of the half-life, aqueous solubility and stability, and reduction in aggregation, opsonization and immunogenicity of the NPs. FA, a water-soluble vitamin B, is required in the production of new cells and is essential for both tumour and normal cells. There are two types of FA uptake pathways: membrane-bound folate receptor (FR) and transmembrane reduced folate carrier. Normal cells utilize the later as the principal mode for FA uptake. FR, a glycosylphosphatidylinositol anchored membrane receptor, overexpressed on many cancer cells, including ovarian cancer cells, while it is scantly expressed or absent in normal tissues [Citation6–9]. FR is overexpressed in more than 90% of ovarian cancer cells [Citation10]. FA, as a targeting ligand, is largely applied on the surface of polymeric NPs to distribute these NPs into cells via receptor-mediated endocytosis [Citation11]. FA is non-immunogenic, small size, low cost and highly specific ligand for FR-α, which is about 100–300 times overexpressed in the cell membrane of ovarian cancer cells over normal cells [Citation12,Citation13]. These characteristics make FA a suitable ligand to encourage active targeting. Some of the drug molecules explored for targeted delivery to cancer cells are capecitabine [Citation14], paclitaxel [Citation15], saquinavir [Citation16], 5-flurouracil [Citation1], vincristine [Citation11], 17-allylamino-17-demethoxygeldanamycin [Citation6], etc. Docetaxel, gemcitabine and paclitaxel have been delivered with FR-targeted nano-formulations for the treatment of ovarian cancer [Citation10,Citation12,Citation17].
Genistein or 4′,5,7-trihydroxyisoflavone (GEN, ) is a natural isoflavone and abundantly found in Soy products, having a wide range of pharmacological and nutraceutical applications. It shows anticancer, antidiabetic, antiosteoporotic, immunomodulatory, antiobesity, cardioprotective and neuroprotective properties [Citation18]. GEN has been studied for its anticancer activity against different cell lines such as breast, ovarian, prostate, head, neck, lung, lymphoma and leukaemia. The molecular mechanism of anticancer activity of GEN includes inhibition of protein-tyrosine kinase (PTK)-mediated signalling pathway inhibition, genes that regulate apoptosis and cell cycle and signalling pathways of carcinogenesis, metastasis and angiogenesis [Citation19–21]. GEN produces anticancer effect against ovarian cancer and its growth via pleiotropic mechanisms against oestrogen receptors, apoptosis, cell propagation, metastasis, angiogenesis and oxidation [Citation22–30]. It also inhibits the formation of reactive oxygen species, which normally increases during oxidative stress through the modulation of NF-κB [Citation31]. Despite promising anticancer properties, use in clinical practice of GEN is limited due to its low water solubility, rapid metabolism and excretion, lack of cancer cell targeting and poor bioavailability. In an earlier study, folic acid-conjugated chitosan NPs enhanced cellular internalization of the NPs and increased cytotoxicity of GEN in cervical cancer cells, HeLa [Citation32].
In the present study, we planned to functionalize PLGA polymer with PEG and FA (PLGA-PEG-FA conjugate) and use it to formulate NPs loaded with GEN for targeted therapy of ovarian cancer. We fabricated GEN-loaded PLGA, PLGA-PEG and PLGA-PEG-FA NPs by the nano-precipitation method. The NPs were characterized for different physicochemical characters, in vitro cytotoxicity and cellular internalization. Furthermore, the effect of cryoprotectants on controlling the increase in particle size during lyophilization was also analysed.
Materials and methods
Materials
The following items were purchased from commercial sources: Polylactide-co-glycolide, PLGA (50/50, MW 43,900) from Durect Corporation, Birmingham, AL; N,N-dicyclohexylcarbodiimide (DCC) and triethylamine (TEA) from Alfa Aesar (Heysham, Lancashire, UK); N-hydroxysuccinimide (NHS) from Fluka (FlukaChemie, GmbH, Germany); Genistein from TCI America (OR, USA); Folic acid (FA), Tween-80 and 3-(4,5-dimethylthiazol-2-yl)-3,5-diphenyl tetrazolium bromide (MTT) from Sigma-Aldrich (St. Louis, MO); Mannitol from Spectrum Pharmaceuticals (New Brunswick, NJ) and Sucrose and Coumarin-6 from MP Biomedical (Solon, OH). DMEM media, trypsin, DMSO and PBS were procured from Mediatech Inc. (Manassas, VA, USA). Polyether diamine with a predominant polyethylene glycol (PEG) backbone (Jeffamine ED-2003 XTJ-502, MW 2000) was gifted by Huntsman International LLC (Austin, TX) and is referred in this article as PEG-bis-amine. Kolliphor P 407 (P407) was obtained as gift from BASF Corporation (Florham Park, NJ). All other solvents and reagents used were of analytical grade. Ultrapure water (Millipore water purification system, Burlington, MA) was used in all the experiments.
Synthesis of PLGA-PEG-FA conjugate
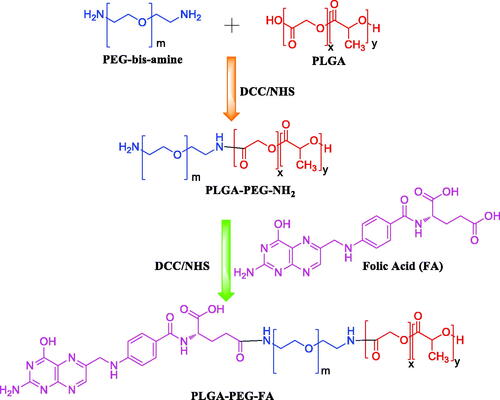
The conjugate PLGA-PEG-FA was synthesized in a 4-step process as reported earlier [Citation1,Citation6,Citation11,Citation33–36] with some modifications.
Activation of PLGA
PLGA (2195 mg; 0.05 mmol), DCC (41.27 mg; 0.2 mmol) and NHS (23.02 mg; 0.2 mmol) were dissolved in 10 ml of anhydrous dichloromethane (DCM) in a 50 ml round bottom flask and left to stir (400 rpm) at room temperature under nitrogen atmosphere for 24 h (PLGA/DCC/NHS stoichiometric molar ratio: 1/4/4). The resultant solution was then filtered through a 0.45 µm PTFE syringe filter (VWR International, USA) to remove the by-product dicyclohexylurea and added drop-wise to 20 ml of cold anhydrous diethyl ether to precipitate the activated PLGA. The liquid layer was decanted, and the residual mass was repeatedly washed (3 × 5 ml) in an ice-cold mixture of diethyl ether and methanol (1:1) to remove the remaining NHS. In each washing after the addition of an ice-cold mixture of diethyl ether and methanol, the contents were mixed by shaking and left for 5 min and decanted. Finally, the product was washed with 10 ml of diethyl ether and completely dried under vacuum to obtain the solid activated PLGA.
PEGylation of PLGA
The activated PLGA (1317 mg; 0.03 mmol) was dissolved in 5 ml of anhydrous DCM in a 50 ml round bottom flask by stirring. PEG-bis-amine (360 mg; 0.18 mmol) dissolved in 2 ml of anhydrous DCM was added drop-wise to the PLGA solution. PEG-bis-amine was used in excess (PLGA/PEG-bis-amine stoichiometric molar ratio: 1/6) to prevent the formation of PLGA-PEG-PLGA triblock copolymers [Citation1,Citation6]. The reaction was carried out with gentle stirring (400 rpm) for 24 h in a dark condition under a nitrogen atmosphere. The reaction mixture was then precipitated with cold methanol and washed with the same solvent (3 × 5 ml) to remove unreacted PEG-bis-amine. Then 5 ml of cold ether was added, stirred with a spatula for washing and then decanted. The precipitated product, amine-terminated diblock copolymer (PLGA-PEG-NH2), was filtered and dried under vacuum.
Activation of folic acid (formation of NHS-folate)
Folic acid (110.35 mg; 0.25 mmol) was first activated with DCC (103.15 mg; 0.5 mmol) and NHS (57.54 mg; 0.5 mmol) in 10 ml of anhydrous DMSO in presence of 0.1 ml triethylamine as a catalyst, under light-protected nitrogen atmosphere overnight. The solution was filtered to get rid of the dicyclohexylurea by-product and then precipitated in 15 ml of cold anhydrous diethyl ether. The product was washed several times with ether. To get rid of DMSO, an excess of acetone was added, mixed and left aside. Then decanted carefully to get the product. The product was dried completely under vacuum.
Conjugation of folic acid to PLGA-PEG
Activated folic acid (26.5 mg; 0.06 mmol) and PLGA-PEG (688.5 mg; 0.015 mmol) were co-dissolved in 4 ml anhydrous dimethyl sulphoxide (DMSO) in a 50 ml round bottom flask in light-protected conditions under nitrogen atmosphere at room temperature for 24 h. An excess amount of activated FA was used to get maximum conjugation of folic acid to PLGA-PEG. The reaction mixture was then added to cold methanol, left for 15–20 min, then decanted and dried under vacuum. The dried mass was dissolved in 15 ml DCM (to precipitate free folic acid in DCM but conjugated folic acid was dissolved). Then the mixture containing PLGA-PEG-FA was filtered through a 0.45 µm PTFE syringe filter (VWR International, USA) and the filtrate was dried under vacuum.
Determination of folate content of the PLGA-PEG-FA conjugate
The FA content in the PEG-PLGA-FA conjugate was determined by dissolving a pre-weighed amount of PEG-PLGA-FA in DMSO and measuring the absorbance at 364 nm (Varioskan Flash, Thermo Scientific, USA). The amount of FA was calculated through a calibration curve constructed using serially diluted concentrations of free FA in DMSO [Citation1,Citation35].
Chemical characterization of PLGA-PEG and PLGA-PEG-FA conjugates
The synthesized PLGA-PEG and PLGA-PEG-FA conjugates were characterized by proton nuclear magnetic resonance (1H NMR, Bruker 400 MHz, Billerica, MA) analysis using DMSO-d6 as solvent.
Preparation of nanoparticles
Nanoparticles (NPs) were prepared using the nano-precipitation method [Citation6]. After an initial trial with PVA or P407 as stabilizer with varying concentration of drug and polymer, and ratio of acetone and water, it was observed that P407 concentration of 0.5% (w/v), drug and polymer ratio of 1.0:9.0 (w/w), and 1:4 ratio of organic to aqueous phase (v/v) were the optimum conditions for preparing PLGA NPs. Similar conditions were applied for the formulation of PLGA-PEG and PLGA-PEG-FA NPs. Briefly, 13.5 mg of polymer (PLGA or PLGA-PEG or PLGA-PEG-FA) and 1.5 mg of GEN were co-dissolved in 2.5 ml of acetone. This solution was added dropwise to 10 ml of a stabilizer solution [0.5% (w/v) P407 in ultrapure water] [Citation37,Citation38], stirred at 500 rpm with a magnetic stirrer. Stirring was continued for 4 h to evaporate acetone and to form the NP suspension where genistein is incorporated into the polymer matrix and some molecules may also remain attached on the surface and then, the NP suspension was centrifuged at 25,000 g (Allegra™ 25 R Centrifuge, Beckman Coulter, Inc, Brea, CA, USA) for 45 min at 4 °C. The supernatant was discarded carefully, and then the pellet was suspended in 3.0 ml of deionized (DI) water. The contents were transferred to a clean and dry amber-coloured bottle, kept at −80 °C for 2 h and then lyophilized overnight (FreeZone® Plus™, Labconco Corporation, MO, USA). All the batches were prepared in triplicates. All experiments were carried out in light-protected conditions. Blank NPs were prepared by adding 15 mg of polymer into 2.5 ml of acetone without the drug. Coumarin-6 loaded NPs were also prepared similarly, but 5 µl of 1.0 mg/ml coumarin-6 solution in acetone was used instead of genistein.
Characterization of nanoparticles
Particle size and zeta potential
Average particle size and size distribution of the nanoparticles was determined using NanoBrook 90 Plus PALS (Brookhaven Instruments, Holtsville, NY) by diluting 25 µl of freshly prepared nanoparticle suspension with 3 ml of ultrapure water. Nanoparticle suspension (75 µl) was diluted with 2 ml of DI water and the zeta potential was determined using the same instrument. The temperature of the suspension in the measuring cell was maintained at 25 °C with a detection angle of 90°. All measurements were performed in triplicates.
Drug loading and encapsulation efficiency
Genistein being an isoflavone is soluble in ethanol on sonication or warming. PLGA is soluble in most chlorinated solvents. Hence, DCM and ethanol mixture was used. Sonication was used to extract the drug from the nanoparticles to the organic solvent mixture. After lyophilization, 1.0 mg of NPs was weighed directly into an amber-coloured glass vial. To the vial, 1.0 ml of solvent mixture containing DCM and ethanol (3:7) was added, sonicated for 5 min and left for 1 h at room temperature. The mixture was filtered through a 0.22 µ PVDF membrane filter (Millex®-GV Syringe driven filter unit, Millipore corporation, Bedford, MA,USA), and the absorbance of the prepared solution was measured by UV-spectrophotometry (Varioskan Flash, Thermo Scientific, USA) at maximum absorbance of 262 nm. Blank nanoparticle solution, prepared similarly in the same concentration, was used as blank for spectrophotometric measurement. The amount of genistein in the NPs was determined through the calibration curve (y = 0.028x − 0.024, R2 = 0.999 and the concentration range used was 10–50 µg/ml). Percentage encapsulation efficiency (% EE) and drug loading (% DL) were calculated using the following formula after determining the weight of drug in the produced NPs.
In vitro drug release study
Nanoparticle suspension (1 ml PBS, pH 7.4, containing 175 µg of GEN equivalent) was placed in a dialysis bag (Spectra/Por molecular porous membrane, MWCO 6-8000, Spectrum Laboratories Inc., CA, USA). GEN dissolved in DMSO was placed in the dialysis bag and used as the control. The bag was tied with threads on both sides and immersed in an amber glass vial containing 35 ml of PBS, pH 7.4 with 0.5% Tween 80. The vial was placed in an orbital shaking incubator (VWR International, West Chester, PA, USA) at 37 °C and 100 rpm. One (1.0) ml of release medium was withdrawn at predetermined time intervals for determination of the concentration of GEN and replaced with fresh media. The concentration of GEN in the collected release media was determined using UV-spectrophotometry at 262 nm. The results were expressed as percentage of drug released calculated by using the following formula. The experiments were performed in triplicates.
Effect of cryoprotectants on particle size of nanoparticles after lyophilization
To study the effect of freeze-drying (with or without a cryoprotectant) on the size of the prepared nanoparticles, a small volume nanoparticle suspension was transferred to an amber vial, to which an equal volume of either sucrose or mannitol solution was added to make the final sugar concentration of 5% or 10%, respectively. The cryoprotectants and the concentration were selected from the results of our previous optimization studies of lyophilization with cryoprotectants [Citation6]. The suspensions were frozen at −80 °C for 2 h and freeze-dried overnight (FreeZone® Plus™, Labconco Corporation, MO, USA). The particle size of the lyophilized NPs was measured following reconstitution in 3 ml of deionized water and sonication for few seconds.
Cell lines and culture
Ovarian cancer cell line (SKOV-3) was obtained from ATCC (Manassas, VA). The cells were grown in T75 flasks containing Dulbecco’s modified Eagle’s medium (DMEM) (Mediatech, Manassas, VA) supplemented with 10% foetal bovine serum (Mediatech, Manassas, VA) and 1% penicillin/streptomycin (Penicillin Streptomycin Solution 100X with 10,000 IU/ml penicillin and 10,000 µg/ml streptomycin, Mediatech, Manassas, VA). The cells were incubated at 37 °C in a humidified atmosphere having 5% carbon dioxide. Cells were trypsinized and passaged after confluence.
Cellular uptake study
SKOV-3 cells were grown on a cover slip placed inside a twenty-four well plate (LabTek, USA) at a density of 50,000 cells/well and incubated for 24 h. The media was then replaced with 0.5 ml of media containing coumarin-6 loaded PLGA-PEG or PLGA-PEG-FA NPs at a concentration of 0.25 µg/ml. After incubation for 4 h, cells were washed three times with ice-cold PBS, fixed with 4% paraformaldehyde in PBS. Coverslip was mounted on the slide and photographed using Nikon Eclipse 80i fluorescence microscopy (Nikon Instruments Inc., NY, USA).
In vitro cytotoxicity study
SKOV-3 cells were seeded into 96-well tissue culture plates at a density of 3000 cells/well except the outer peripheral wells. Only plain fresh media without any cells were added to these peripheral wells. The plates were placed in the incubator for 24 h before treatments. After 24 h of incubation, the culture medium of the wells containing the cells was replaced with fresh medium containing the pure drug or drug-loaded NPs at different concentrations (1.71 to 27.24 µg/ml). The media of a set of wells containing the cells were replaced by plain fresh media without any drug or drug-loaded NPs and was used as negative controls. The media was removed after 72 h of incubation and cells were washed twice with sterile PBS. To each well 50 µl of MTT solution (0.5 mg/ml) was added and further incubated for 4 h at 37 °C and humidified atmosphere having 5% CO2. The medium was then removed and 100 µl of DMSO was added to each well to dissolve the blue formazan crystal converted from MTT. Cell viability was assessed by measuring the absorbance of the converted dye, at 570 nm on a micro plate reader (Varioskan Flash, Thermo Scientific, USA). The IC50 (concentration at which 50% of the growth of cells is inhibited) was calculated by GraphPad Prism 7.0 (GraphPad Software, La Jolla, CA) software.
Statistical analysis
Data were represented as mean ± standard deviation (SD) in determining the physicochemical characters (drug loading, encapsulation efficiency, particle size, zeta potential, in vitro drug release and cryoprotectant effect on particle size after lyophilization) and in the case of cytotoxicity study, the data were furnished as mean ± standard error means. The data of in vitro cytotoxicity were statistically analysed for significance by one-way analysis of variance (ANOVA) followed by post hoc Newman–Keuls multiple comparisons using GraphPad Prism 7.0 software (GraphPad Software, La Jolla, CA). p < .05 was considered statistically significant.
Results and discussion
PLGA-PEG-FA NPs have been used for effective delivery of chemotherapeutic agents with enhances efficacy because of both passive and active targeting of the NPs. We have synthesized PLGA-PEG-FA NPs loaded with GEN and examined its potency in the treatment of ovarian cancer.
Preparation and characterization of PLGA-PEG and PLGA-PEG-FA conjugates
The conjugates were synthesized as reported in earlier literatures with some modifications following the scheme below ().
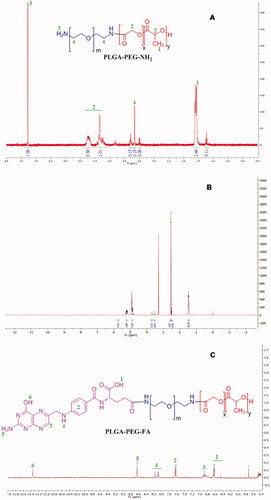
The synthesis of these conjugates involves the formation of amide bonds. For the formation of these bonds, first, a succinimide end group (PLGA-NHS) is produced from the carboxylic group of PLGA. Then, the succinimide end group is paired with the amine group of PEG-bis-amine. For conjugation of FA to the polymer, the succinimide derivative was formed by conversion of COOH group of FA to NHS group (which is more reactive) and finally, this reacted with the free primary NH2 group of PLGA-PEG copolymer. The terminal FA moiety has been found to reside on the surface when the NPs are formed and are able to bind with the folate receptors [Citation1,Citation6]. The terminal FA moiety is predicted to reside on the surface because it is bound to PEG and PEG is hydrophilic in nature. Conjugation of FA to PLGA-PEG was confirmed by 1H NMR analysis (). The characteristic peaks of PLGA are; δ(ppm) = 1.5 (3H, –CH3 of poly(glycolide)); δ(ppm) = 4.8–5.3 (H, poly(D,L-lactide) and poly(glycolide)). Peak at δ(ppm) = 3.6 (H, PEG-bis-amine); δ(ppm) = 7.3 (2H, NH2 of PEG-bis-amine) were assigned to PEG-bis-amine. Different peaks such as, δ(ppm) = 6.4–6.7 (COOH, folate); δ(ppm) = 6.9 (H, aromatic ring); δ(ppm) = 7.6 (m,4H, aromatic ring); δ(ppm) = 8.0–8.2 (NH, folate); δ(ppm) = 8.6 (NH2, folate) and δ(ppm) = 11.35 (OH, folate) of PLGA-PEG-FA conjugate as shown in Figure3(C) are characteristic of FA. shows the enlarged area of 5.4–12.0 ppm of . It shows the characteristic peaks of FA of the conjugate PLGA-PEG-FA. The peaks of FA are less intense as the number of protons and w/w percentage of FA (MW 441) in the conjugate molecule are much smaller than the number of protons and w/w percentage of PLGA-PEG polymer (MW 43,900). Furthermore, the percentage conjugation of FA to PLGA-PEG was determined by UV absorbance at 364 nm and was found to be 43.2% (molar ratio). We have not observed any release of FA from the conjugate during the preparation of the NPs formulation. Light will have effect on the stability of the NPs containing genistein, as it is a photosensitive isoflavone [Citation39]. Hence, the NPs were prepared under dark condition and lyophilized in amber-coloured glass vials.
Preparation and characterization of genistein loaded PLGA-based nanoparticles
Nanoparticles were fabricated by the nano-precipitation method with each of the polymers PLGA, PLGA-PEG and PLGA-PEG-FA. P407 at a concentration of 0.5% w/v was found to be the optimum stabilizer for preparing smaller size NPs and was used for preparing all the NPs [Citation37,Citation38]. NPs were characterized for particle size, polydispersity index (PDI) and zeta potential using dynamic light scattering (DLS) and encapsulation efficiency by UV-Visible spectroscopy. The particle size, PDI, zeta potential, drug loading and percent encapsulation efficiency of all the formulations are collated in . Particle size (before and after lyophilization) of PLGA-PEG and PLGA-PEG-FA NPs were smaller than the PLGA NPs, which may be due to the presence of hydrophilic PEG coating that enhances the stability of NPs and decreases the particle size. These findings agree with earlier reports [Citation1,Citation40]. The surface charge of the NPs is negative. The zeta potential of PLGA-PEG-FA NPs is lower compared to PLGA and PLGA-PEG NPs which may be attributed to the role of FA (which contains protonated amino acid group) in reducing the negative charge. Similar reports have been cited in previous studies [Citation1,Citation15]. The % DL (w/w, 7.8–8.8) and % EE (w/w, 78–88) were reasonably good. In contrast, % DL and % EE of both PLGA-PEG (8.8, 88) and PLGA-PEG-FA NPs (8.0, 80) were slightly higher compared to PLGA NPs (7.8, 78). The higher encapsulation of genistein in PLGA-PEG may be due to its non-covalent interaction with the free amine and other group of the PLGA-PEG molecule which is reduced in the PLGA-PEG-FA conjugate.
Table 1. Physicochemical characteristics of different polymeric nanoparticles containing genistein.
We have not performed transmission electron microscopy (TEM)/scanning electron microscopy (SEM) for surface morphology. Earlier studies have found that the NPs formed are usually spherical in shape and have smooth surface [Citation41].
In vitro release of genistein
In vitro release of genistein from the NPs was studied by the dialysis bag method in PBS (pH 7.4) containing 0.5% Tween 80 and at 37 °C as reported earlier [Citation6]. The 0.5% Tween 80 was used to maintain sink condition for the drug and may have increased the dissolution of the drug in the media. Genistein dissolved in DMSO was almost completely released from the dialysis bag within 8 h (). The release of GEN from the NPs (GEN-loaded PLGA NPs, GEN-loaded PLGA-PEG NPs and GEN-loaded PLGA-PEG-FA NPs) exhibited a similar pattern of around 20% of drug release after 1 h. After that the drug release was sustained. Around 75% GEN was released from the GEN-loaded PLGA NPs after six days. However, the release of GEN from the GEN-loaded PLGA-PEG and PLGA-PEG-FA NPs after six days was relatively higher than the PLGA NPs. The percentage release of GEN from GEN-loaded PLGA-PEG and PLGA-PEG-FA NPs was 88 and 86%, respectively after six days. The release of GEN from GEN-loaded PLGA-PEG and PLGA-PEG-FA NPs was significantly (p < .05) higher compared to PLGA NPs starting from 2 h until the end of the study. The initial burst release of GEN from the PLGA-based NPs may be due to the weakly bound drugs on the surface of the NPs, which is quickly released by diffusion. However, the remaining drug present inside the NP structure needed a break-down of the polymer and diffusion for sustained release of GEN. The faster drug release from PLGA-PEG and PLGA-PEG-FA NPs than PLGA NPs may be attributed to particle size and presence of greater hydrophilic surface due to PEG moiety. The hydrophilic surface can absorb more water by increasing interaction with the aqueous medium resulting in faster degradation of the polymer by hydrolysis and faster release of the entrapped drug [Citation1].
Figure 4. In vitro release profiles of GEN from PLGA-based NP in PBS (pH 7.4) at 37 °C. Data are presented as mean ± standard deviation (SD), (n = 3).
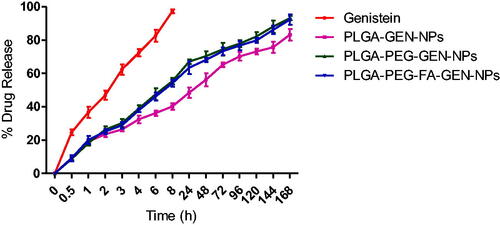
The correlation coefficient (r2) values for various release models such as zero order, first order and Higuchi model were calculated and is reported in . The r2 values suggests that the release of GEN from PLGA and PLGA-PEG-FA NPs from 24 to 120 h (when approximately 20 to 80% GEN is released from the NPs) followed Higuchi model. The overall 0-168 h release of GEN from the PLGA NP followed Higuchi model but the release of GEN from PLGA-PEG and PLGA-PEG-FA NPs seems to follow first order kinetics. The dialysis method eliminates the need for a separation step such as centrifugation, but the membrane itself may function as a diffusion barrier influencing the release kinetics of the poorly water-soluble GEN.
Table 2. In vitro release kinetic of different formulations containing genistein.
The encapsulated drug is released by simple diffusion from the NPs. The release of drug from PLGA NPs is slower due to hydrophobic nature of PLGA which obstruct the diffusion of medium in/out of NPs. However, NPs containing PEG are more hydrophilic and more permeable to water which facilitates diffusion of medium in/out of NPs resulting in faster drug release in case of PLGA-PEG and PLGA-PEG-FA NPs. Similar effect is seen in earlier studies, especially, with the use of higher molecular weight of PEG (PEG 5000 vs PEG 2000) in the conjugate resulted in faster release of the drug from the NPs [Citation42]. There is possibility of drug leakage if the NPs are not stable for a reasonable time and there is alteration of the NP morphology [Citation41].
The release of genistein from the NPs in the PBS (pH 7.4) containing 0.5% Tween 80 is sustained for a period of around eight days compared to only 8 h in case of pure genistein. The NPs has potential for use as sustained and targeted release of the drug in ovarian cancer. We are planning to conduct future studies on the effect of acidic pH on the in-vitro release of GEN from these NPs and IC50 values at 24, 48, 72 and 96 h. Also, we will study the efficacy and toxicity of these NPs in animal models of the ovarian cancer. In a study with athymic mice transplanted with MDA-MB-231 tumour, plasma concentration of indocyanine green (ICG) in FA-PLGA/mPEG2000-PLGA NPs (DM-NPs) treated group was significantly higher than the PLGA NPs (NM-NPs) treated group. After 4 h, the drug concentration in the tumour tissue in the DM-NP treated group was significantly higher than the NM-NP treated group (AUC0–12 h in plasma is increased by 245%). The tumour accumulation of the FA-conjugated DM-NP was significantly higher than the NM-NP (AUC0–12 h in tumour is increased by 194%). The increase was due to the long circulation and FA receptor-mediated uptake [Citation42]. The drug release could be sustained from the folate-targeted NPs in both physiological pH as well as in the acidic microenvironment of the tumour, which will maintain the therapeutic efficacy [Citation43,Citation44].
Effect of cryoprotectants on the particle size of nanoparticles after lyophilization
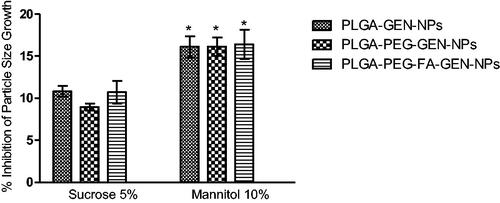
Lyophilization of the NPs caused an increase in the particle size as NPs tend to aggregate due to change in physical state and stress related to freeze-drying. In our earlier studies, we found that addition of sucrose (5%) and mannitol (10%) to the NPs suspension prior to lyophilization prevented the increase in the size of the NPs due to freeze-drying. Cryoprotectants, usually sugars, form hydrogen bonds with the NPs, which protects the NPs from aggregation and stresses due to freezing [Citation6]. In the present study, the decrease in average particle size growth by mannitol and sucrose was around 16% and 10%, respectively (). Like our earlier findings, mannitol (10%) was more effective in controlling the particle size of NPs compared to sucrose (5%) in the NP suspension [Citation6]. Since the cryoprotectant efficiency was not large enough, the lyophilized NPs without cryoprotectants were used for subsequent biological studies and physicochemical characterization.
Cellular uptake study
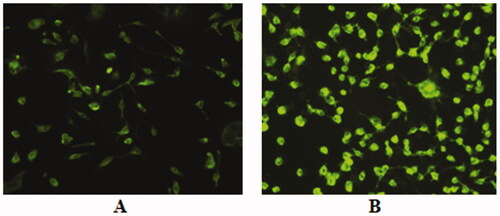
shows that the coumarin-6 loaded NPs are taken up by the SKOV-3 cells. The intensity of green fluorescence in the cells treated with NPs prepared from the folic acid-conjugated polymer (PLGA-PEG-FA) is much higher compared to the NPs prepared from non-conjugated PLGA-PEG (). Ovarian cancer cells over express FR and treatment with FR-targeted (folate-targeted) NPs would have a higher binding of the NPs with the receptor. The FR binding followed by receptor-mediated endocytosis may have caused enhanced cellular internalization of the folate-targeted NPs.
Figure 6. Fluorescence microscopic images of SKOV-3 human ovarian cancer cells after incubation of 4 h with coumarin-6 loaded PLGA-PEG nanoparticles (A) and PLGA-PEG-FA nanoparticles (B). The image B show higher uptake of the PLGA-PEG-FA nanoparticles in the cells.
Earlier studies have shown that the ovarian cancer cell line, SKOV-3, overexpresses folate receptors (FR). The finding of the overexpression of FR in SKOV-3 cells in this study was further verified by [3H] folic acid cell surface binding assay in the presence and absence of excess non-radioactive free folic acid, which is a functional measure for FR [Citation45].
FA decreases folate receptor-mediated endocytosis of FA-conjugated NPs due to competition. Also, the cellular intake of FA-conjugated NPs usually decrease in presence/pre-treatment with FR inhibitors [Citation46–48]. The affinity and specificity of FA-decorated NP was measured by assessing the radioactivity of SKOV-3 cells treated with 131I labelled pristine drug and folate-decorated NPs. It has been postulated that the folate-decorated nanocarriers have good affinity towards ovarian cancer cells [Citation49]. Furthermore, the folic acid-conjugated NPs have specificity towards SKOV-3 cells [Citation50].
In vitro cytotoxicity study
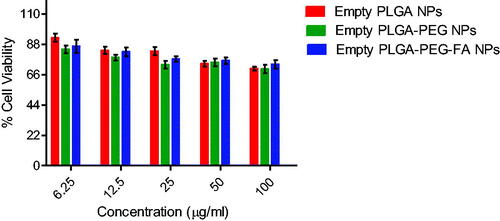
The findings of in vitro cytotoxicity of pure GEN and GEN-loaded PLGA, PLGA-PEG and PLGA-PEG-FA NPs at different concentrations of GEN in an ovarian cancer cell line (SKOV-3) are depicted in . The anticancer effect of different NPs containing GEN are in the order: PLGA-PEG-FA > PLGA-PEG > PLGA. GEN-loaded PLGA, PLGA-PEG and PLGA-PEG-FA NPs showed 1.9-, 2.2- and 4.3-fold higher cytotoxicity than the pristine drug. The anticancer effect of PLGA-PEG-FA NPs containing GEN is significantly higher (p < .05) compared to the effect of PLGA as well as PLGA-PEG NPs containing GEN. Furthermore, the empty (without GEN) PLGA-based nano-formulations exhibited negligible effect on the ovarian cancer cells () confirming the non-toxic nature of the polymer conjugates. From our study, the blank nanoparticles have very low cytotoxicity to SKOV-3 cells and only at high concentrations. The cytotoxicity observed is mostly due to genistein. The lower anticancer effect of GEN compared to GEN containing NPs may be attributed to the efflux of pure GEN from the cells by P-glycoprotein (P-gp) pumps. The enhanced cytotoxicity of GEN-loaded PLGA NPs may be due to their small size resulting in enhanced cell permeability and as nanoparticles are probably not subject to the p-glycoprotein efflux. GEN-loaded PLGA-PEG NPs showed slightly higher cytotoxicity compared to GEN-loaded PLGA NPs which may be due to their smaller size (because of PEGylation) and increased cellular uptake. Both the PLGA-PEG and PLGA-PEG-FA showed sustained release of genestin for around six days. The slightly higher release rate of GEN from both these NPs may have contributed towards the increased cytotoxic effect compared to the PLGA NPs. Finally, conjugation of folic acid with the GEN-loaded PLGA-PEG NPs further increased the cytotoxicity by two-fold. This effect may be due to enhanced cellular internalization (as observed in the previous section) via folate receptor-mediated endocytosis and circumventing the efflux of GEN by P-gp pumps. These effects augmented the anticancer effect of GEN-loaded PLGA-PEG-FA NPs in folate receptor overexpressed ovarian cancer cells, SKOV-3. All the NPs were effective as drug carrier for sustained release of GEN for around six days, but the PLGA-PEG-FA NPs was more cytotoxic due to its capability of targeting the FA receptors on the SKOV-3 ovarian cancer cells. In the present study, we have not compared the efficacy of the fabricated formulation with any standard drug (positive control). Earlier studies reported that the IC50 of paclitaxel in SKOV-3 cells after 48 h of incubation is 0.029 µM [Citation51]. In our current studies, the IC50 of GEN and GEN encapsulated NPs of PLGA, PLGA-PEG and PLGA-PEG-FA were 189, 98, 86 and 44 µM, respectively.
Figure 7. In vitro cytotoxicity of genistein (GEN) and GEN-loaded PLGA, PLGA-PEG and PLGA-PEG-FA NPs against ovarian cancer cell line (SKOV-3) after 72 h of incubation (mean ± SEM, n = 3). MTT assay for pure GEN was also carried out at 54.48 and 108.96 µg/ml concentrations (Data not shown). *p < .05, **p < .01, ***p <.001 versus GEN treatment at the same dose; ###p < .001 versus GEN containing PLGA NPs treatment at the same dose; ^^^p < .001 versus GEN containing PLGA-PEG NPs treatment at the same dose.
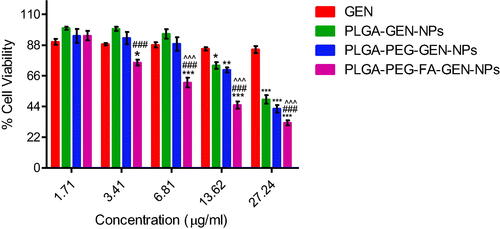
Figure 8. In vitro cytotoxicity of empty (without GEN) PLGA, PLGA-PEG and PLGA-PEG-FA NPs against ovarian cancer cell line (SKOV-3) after 72 h of incubation (mean ± SEM, n = 3).
Target-specific formulations produce enhanced cytotoxicity and minimize the adverse effects of drug molecules. Various target ligands such as transferrin, folate receptor, integrins, growth hormone receptor, glucose transporter, epidermal growth factor receptors, etc. have been utilized for active targeting of nanoparticles into various cancer cell lines. Out of the above folate receptor has been widely studied for targeted delivery of anticancer agents [Citation7,Citation52,Citation53]. Sustained drug release and enhanced anticancer property in FR overexpressed cancer cells can be achieved by surface modification of nanocarriers [Citation12]. Furthermore, small sized, stable and target-specific NPs show increased cellular internalization of the drug molecule [Citation54]. In the present study, the folate-conjugated PLGA-based NPs containing GEN, which is small, and having sustained release behaviour showed enhanced cellular internalization and cytotoxicity in ovarian cancer cells.
Conclusion
GEN containing PLGA-PEG and PLGA-PEG-FA NPs prepared were small (less than 150 nm) and exhibited sustained release of GEN. The folate-decorated PLGA-PEG NPs showed increased cellular uptake. The GEN containing PLGA-PEG-FA showed superior anticancer activity than the non-targeted PLGA-based NPs in folate receptor-overexpressed ovarian cancer cell line, SKOV-3. Folate-targeted PLGA nanoparticles could be utilized for target-specific delivery of GEN in ovarian cancer. However, the in vivo studies are warranted in future to validate the efficacy of the fabricated formulation. GEN could be delivered in the future with a combination of another targeting ligand/drug with FA or both ligand and drug. Furthermore, this formulation may also be employed for a co-delivery of imaging agents for theragnostic purposes.
Author contributions
Conception of the idea: MDH, AP; design of the work: MDH, AP, SS; acquisition, analysis and interpretation of data: AP, SS, MDH, MK, PKN; drafting the manuscript: AP, SS; revising the manuscript: MDH, AP, SS, MK, PKN; final approval of the manuscript: all; agreement to be accountable for all aspects of the work: all.
Acknowledgements
The authors are thankful to California State University, Fresno, CA for NMR analyses. The authors would also like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP-2021/301), King Saud University, Riyadh, Saudi Arabia.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All the data are included in the manuscript and the supporting data of the findings are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- El-Hammadi MM, Delgado AV, Melguizo C, et al. Folic acid-decorated and PEGylated PLGA nanoparticles for improving the antitumour activity of 5-fluorouracil. Int J Pharm. 2017;516(1-2):61–70.
- Athanasiou KA, Niederauer GG, Agrawal C. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials. 1996;17(2):93–102.
- Patra A, Satpathy S, Hussain MD. Nanodelivery and anticancer effect of a limonoid, nimbolide, in breast and pancreatic cancer cells. Int J Nanomed. 2019;14:8095–8104.
- Acharya S, Sahoo SK. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv Drug Deliv Rev. 2011;63(3):170–183.
- Song X, Zhao Y, Wu W, et al. PLGA nanoparticles simultaneously loaded with vincristine sulfate and verapamil hydrochloride: systematic study of particle size and drug entrapment efficiency. Int J Pharm. 2008;350(1-2):320–329.
- Saxena V, Naguib Y, Hussain MD. Folate receptor targeted 17-allylamino-17-demethoxygeldanamycin (17-AAG) loaded polymeric nanoparticles for breast cancer. Colloids Surf B Biointerfaces. 2012;94:274–280.
- Zwicke GL, Mansoori GA, Jeffery CJ. Utilizing the folate receptor for active targeting of cancer nanotherapeutics. Nano Rev. 2012;3(1):18496.
- Lutz RJ. Targeting the folate receptor for the treatment of ovarian cancer. Transl Cancer Res. 2015;4:118–126.
- Hijaz M, Das S, Mert I, et al. Folic acid tagged nanoceria as a novel therapeutic agent in ovarian cancer. BMC Cancer. 2016;16:220.
- Li S, Li X, Ding J, et al. Anti-tumor efficacy of folate modified PLGA-based nanoparticles for the co-delivery of drugs in ovarian cancer. Drug Des Devel Ther. 2019;13:1271–1280.
- Chen J, Li S, Shen Q. Folic acid and cell-penetrating peptide conjugated PLGA–PEG bifunctional nanoparticles for vincristine sulfate delivery. Eur J Pharm Sci. 2012;47(2):430–443.
- Luiz MT, Abriata JP, Raspantini GL, et al. In vitro evaluation of folate-modified PLGA nanoparticles containing paclitaxel for ovarian cancer therapy. Mater Sci Eng C. 2019;105:110038.
- Martin LP, Konner JA, Moore KN, et al. Characterization of folate receptor alpha (FR α) expression in archival tumor and biopsy samples from relapsed epithelial ovarian cancer patients: a phase I expansion study of the FR α-targeting antibody-drug conjugate mirvetuximasoravtansine. Gynecol Oncol. 2017;147:6–11.
- Wei K, Peng X, Zou F. Folate-decorated PEG-PLGA nanoparticles with silica shells for capecitabine controlled and targeted delivery. Int J Pharm. 2014;464(1-2):225–233.
- Liang C, Yang Y, Ling Y, et al. Improved therapeutic effect of folate-decorated PLGA-PEG nanoparticles for endometrial carcinoma. Bioorg Med Chem. 2011;19(13):4057–4066.
- Singh R, Kesharwani P, Mehra NK, et al. Development and characterization of folate anchored saquinavir entrapped PLGA nanoparticles for anti-tumor activity. Drug Dev Ind Pharm. 2015;41(11):1888–9011901.
- Chiu HI, Samad NA, Fang L, et al. Cytotoxicity of targeted PLGA nanoparticles: a systematic review. RSC Adv. 2021;11(16):9433–9449.
- Fatima A, Singh R. The chemistry and pharmacology of genistein. Nat Prod J. 2016;6:3–12.
- Taylor CK, Levy RM, Elliott JC, et al. The effect of genistein aglycone on cancer and cancer risk: a review of in vitro, preclinical, and clinical studies. Nutr Rev. 2009;67(7):398–415.
- Pavese JM, Farmer RL, Bergan RC. Inhibition of cancer cell invasion and metastasis by genistein. Cancer Metastasis Rev. 2010;29(3):465–482.
- Li QS, Li CY, Li ZL, et al. Genistein and its synthetic analogs as anticancer agents. Anticancer Agents Med Chem. 2012;12(3):271–281.
- Hafidh RR. A comprehensive anticancer molecular study for genistein the promising anticancer drug. J Contemp Med Sci. 2017;3:264–269.
- Lee JY, Kim HS, Song YS. Genistein as a potential anticancer agent against ovarian cancer. J Tradit Complement Med. 2012;2(2):96–104.
- Li HQ, Luo Y, Qiao CH. The mechanisms of anticancer agents by genistein and synthetic derivatives of isoflavone. Mini Rev Med Chem. 2012;12(4):350–362.
- Choi EJ, Kim T, Lee MS. Pro-apoptotic effect and cytotoxicity of genistein and genistin in human ovarian cancer SKOV-3 cells. Life Sci. 2007;80(15):1403–1408.
- Ahmed AA, Goldsmith J, Fokt I, et al. A genistein derivative, ITB-301, induces microtubule depolymerization and mitotic arrest in multidrug-resistant ovarian cancer. Cancer Chemother Pharmacol. 2011;68(4):1033–1044.
- Gercel-Taylor C, Feitelson AK, Taylor DD. Inhibitory effect of genistein and daidzein on ovarian cancer cell growth. Anticancer Res. 2004;24(2B):795–800.
- Rucinska A, Kirko S, Gabryelak T. Effect of the phytoestrogen, genistein-8-C-glucoside, on Chinese hamster ovary cells in vitro. Cell Biol Int. 2007;31(11):1371–1378.
- Chen X, Anderson JJ. Isoflavones inhibit proliferation of ovarian cancer cells in vitro via an estrogen receptor-dependent pathway. Nutr Cancer. 2001;41(1-2):165–171.
- Gossner G, Choi M, Tan L, et al. Genistein-induced apoptosis and autophagocytosis in ovarian cancer cells. Gynecol Oncol. 2007;105(1):23–30.
- Davis JN, Kucuk O, Djuric Z, et al. Soy isoflavone supplementation in healthy men prevents NF-kappa B activation by TNF-alpha in blood lymphocytes. Free Radic Biol Med. 2001;30(11):1293–1302.
- Cai L, Yu R, Hao X, et al. Folate receptor-targeted bioflavonoid genistein-loaded chitosan nanoparticles for enhanced anticancer effect in cervical cancers. Nanoscale Res Lett. 2017;12(1):509.
- Cheng J, Teply BA, Sherifi I, et al. Formulation of functionalized PLGA-PEG nanoparticles for in vivo targeted drug delivery. Biomaterials. 2007;28(5):869–876.
- Esmaeili F, Ghahremani MH, Ostad SN, et al. Folate-receptor-targeted delivery of docetaxel nanoparticles prepared by PLGA-PEG-folate conjugate. J Drug Target. 2008;16(5):415–423.
- Yoo HS, Park TG. Folate receptor targeted biodegradable polymeric doxorubicin micelles. J Control Release. 2004;96(2):273–283.
- Stephenson SM, Low PS, Lee RJ. Folate receptor-mediated targeting of liposomal drugs to cancer cells. Methods Enzymol. 2004;387:33–50.
- dos Santos AM, Meneguin AB, Fonseca-Santos B, et al. The role of stabilizers and mechanical processes on physico-chemical and anti-inflammatory properties of methotrexate nanosuspensions. J Drug Deliv Sci Technol. 2020;57:101638.
- Matteucci ME, Hotze MA, Johnston KP, et al. Drug nanoparticles by antisolvent precipitation: mixing energy versus surfactant stabilization. Langmuir. 2006;22(21):8951–8959.
- Yang S, Lee S, Chung H, et al. Stability of isoflavone daidzein and genistein in riboflavin, chlorophyll B, or methylene blue photosensitization. J Food Sci. 2008;73(2):C100–C105.
- Tao Y, Han J, Dou H. Surface modification of paclitaxel-loaded polymeric nanoparticles: evaluation of in vitro cellular behavior and in vivo pharmacokinetic. Polymer. 2012;53(22):5078–5086.
- Weiss B, Schaefer UF, Zapp J, et al. Nanoparticles made of fluorescence-labelled poly(l-lactide-co-glycolide): preparation, stability, and biocompatibility. J Nanosci Nanotechnol. 2006;6(9-10):3048–3056.
- Ma Y, Sadoqi M, Shao J. Biodistribution of indocyanine green-loaded nanoparticles with surface modifications of PEG and folic acid. Int J Pharm. 2012;436(1-2):25–31.
- Ramasamy S, David RJRS, Enoch IVMV. Folate-molecular encapsulator-tethered biocompatible polymer grafted with magnetic nanoparticles for augmented drug delivery. Artif Cells Nanomed Biotechnol. 2018;46(sup2):675–682.
- Kaliyamoorthi K, Pillai AS, Alexander A, et al. Designed poly(ethylene glycol) conjugate-erbiumdoped magnetic nanoparticle hybrid carrier: enhanced activity of anticancer drug. J Mater Sci. 2021;56(5):3925–3934.
- Hou Z, Gattoc L, O'Connor C, et al. Dual targeting of epithelial ovarian cancer via folate receptor α and the proton-coupled folate transporter with 6-substituted pyrrolo[2,3-d]pyrimidine antifolates. Mol Cancer Ther. 2017;16(5):819–830.
- Ince I, Yeliz Y, Güler G, et al. Synthesis and characterization of folic acid-chitosan nanoparticles loaded with thymoquinone to target ovarian cancer cells. J Radioanal Nucl Chem. 2020;324(1):71–85.
- Vergote IB, Marth C, Coleman RL. Role of the folate receptor in ovarian cancer treatment: evidence, mechanism, and clinical implications. Cancer Metastasis Rev. 2015;34(1):41–52.
- Cuong N-V, Li Y-L, Hsieh M-F. Targeted delivery of doxorubicin to human breast cancers by folate-decorated star-shaped PEG–PCL micelle. J Mater Chem. 2012;22(3):1006–1020.
- Tong L, Chen W, Wu J, et al. Folic acid-coupled nano-paclitaxel liposome reverses drug resistance in SKOV3/TAX ovarian cancer cells. Anti-Cancer Drugs. 2014;25(3):244–254.
- Lu T, Sun J, Chen X, et al. Folate-conjugated micelles and their folate receptor-mediated endocytosis. Macromol Biosci. 2009;9(11):1059–1068.
- Pashaei-Asl F, Pashaei-Asl R, Khodadadi K, et al. Enhancement of anticancer activity by silibinin and paclitaxel combination on the ovarian cancer. Artif Cells Nanomed Biotechnol. 2018;46(7):1483–1487.
- Kelemen LE. The role of folate receptor alpha in cancer development, progression and treatment: cause, consequence or innocent bystander. Int J Cancer. 2006;119(2):243–250.
- Kaliyamoorthy K, Pillai AS, Alexander A, et al. β-cyclodextrin-folate functionalized poly(lactic-co-glycolide)-superparamagnetic ytterbium ferrite hybrid nanocarrier for targeted delivery of camptothecin. Mater Sci Eng C Mater Biol Appl. 2021;122:111796.
- Tagde P, Kulkarni GT, Mishra DK, et al. Recent advances in folic acid engineered nanocarriers for treatment of breast cancer. J Drug Deliv Sci Technol. 2020;56:101613.