Abstract
High translucent zirconia (HTZ) has excellent mechanical properties, biocompatibility, and good semi-translucency making it an ideal material for aesthetic anterior dental implant abutments without antibacterial properties. In the oral environment, the surface of the abutment material is susceptible to microbial adhesion and biofilm formation, which can lead to infection or peri-implantitis and even implant failure. This study aims to promote the formation of a biological seal at the implant-soft tissue interface by modifying the HTZ surface, using the load-bearing capacity of the aluminosilicate porous structure and the broad-spectrum antibacterial effect of silver nanoparticles to prevent peri-implant bacterial infection and inflammation and to improve the success rate and prolong the use of the implant. FE-SEM (field emission scanning electron microscopes), EDS (energy dispersive spectroscopy), and XPS (X-ray photoelectron spectroscopy) results showed that aluminosilicate non-vacuum sintering can form open micro- and nanoporous structures on HTZ surfaces, and that porous aluminosilicate coatings obtain a larger number, smaller size, and more uniformly shaped silver nanoparticles than smooth aluminosilicate coatings, and could be deposited deeper in the coating. The ICP-AES (inductively coupled plasma-atomic emission spectroscopy) results showed that the early silver ion release of both the smooth silver coating and the porous silver coating was obvious, the silver ion concentration released by the former was higher than that of the latter. However, the silver ion concentration released by the porous silver coating was higher than that of the smooth coating when the release slowed down. Both smooth and porous silver coatings both inhibited E. coli (Escherichia coli), S. aureus (Staphylococcus aureus), and L. acidophilus (L. acidophilus), and porous silver coatings had stronger antibacterial properties. The silver coating was successfully constructed on the surface of HTZ, through aluminium silicate sintering and silver nitrate solution impregnation. It was found that the high concentration environment of silver nitrate solution was more advantageous for nano-Ag deposition, and the non-vacuum sintered porous surface was able to obtain a larger number of nano-Ag particles with smaller sizes. The porous Ag coating exhibited superior antibacterial properties. It was suggested that the HTZ with silver coating had clinical application, and good antibacterial properties that can improve the survival rate and service life of implants.
Introduction
Biomaterials are materials with natural organ tissue’s function or partial function and are the latest branch of biomedical science with a wide range of applications [Citation1,Citation2]. Owing to their outstanding biocompatibility, durability, aesthetics, and other benefits, bioceramics are preferred and are frequently utilized in clinical settings for dental prostheses [Citation3–5].
As a new type of fine ceramic, zirconia has good mechanical properties (fracture toughness, strength, and hardness), biocompatibility and stability, aesthetics, thermal conductivity, and formability, which effectively solve the problem of insufficient strength and toughness of conventional all-ceramic crown materials [Citation6–9]. Zirconia restorations have the following advantages over other conventional restorative materials [Citation10,Citation11]: zirconia has mechanical properties comparable to those of metal and can fully withstand the chewing forces of posterior teeth; zirconia improves aesthetics, with material and colour close to the surrounding tissue, and the absence of metal supports in all-porcelain crowns enhances patient satisfaction with their appearance; zirconia has good histocompatibility and will not be corroded by saliva or gingival sulcus after placement, and has no toxic effects on soft tissues in the oral cavity; it has made great strides in the development of all-ceramic crown restorative materials (single crowns and fixed prostheses), implant materials, and core materials, and has become a research hotspot in the field of dentistry [Citation11–14].
The oral cavity is a complex microbial environment; under normal conditions, oral microorganisms survive and multiply as biofilms on the teeth or mucosa, maintaining a dynamic balance through symbiosis, competition, and antagonism, thus maintaining oral health [Citation15,Citation16]. When the body’s immune system is low, the environment of the oral cavity changes, allowing certain bacteria to become dominant and the balance between microorganisms to be disrupted, resulting in a range of oral diseases [Citation17,Citation18]. The prevention and treatment of oral diseases by regulating the micro-ecological environment have become a hot topic of research in the field of dentistry. Antibiotics are now commonly used clinically as antibacterial agents, but the short duration of action and low bioavailability of antibiotics also contribute to their relatively low antibacterial efficiency [Citation19]. Therefore, it is imminent to find new antibacterial and antibacterial solutions. In recent years, with the in-depth study of nanomaterials, their superior antibacterial properties have attracted more and more attention [Citation20,Citation21]. In addition, the silver ions released by the silver coating interact with bacterial cell membranes and thus produce bioactive oxygen species, inhibiting the reproduction and metabolism of bacteria and ultimately having a bactericidal effect [Citation22].
In this study, silver-coated high translucent zirconia (HTZ) implant abutment materials were prepared by modifying the surface of HTZ, and the surface morphology of the modified samples was characterized by field emission scanning electron microscopes (FE-SEM), energy dispersive spectroscopy (EDS) and X-ray photoelectron spectroscopy (XPS). Comparison of the silver ion release capacity of surface coatings on smooth and porous silver-carrying materials using inductively coupled plasma-atomic emission spectroscopy (ICP-AES). In vitro, inhibition of Escherichia coli (E. coli), Staphylococcus aureus (S. aureus), and Lactobacillus acidophilus (L. acidophilus) by different modified coatings was observed by inhibition zone method, flat colony counting method and SEM observation of bacterial morphology.
Materials and methods
Sintering of basic HTZ ceramic tiles (V)
The VITA YZ HTColor (ECDY24981436, Vita, Germany) HTZ porcelain blocks were cut to a thickness of 1 mm and a diameter of 20 mm using CAD/CAM technology [Citation23] and sandblasted [Citation24,Citation25]. They were then ultrasonically cleaned in acetone, absolute ethanol, and deionized water, twice each to remove organic matter and impurities on the surface of the material, and then placed it in an oven for drying. The material was sintered in a VITA Zyrcomat oven (Vita, Germany) at 1000 °C for 15 min according to the basic sintering process under vacuum sintering conditions for the sintering stream.
Sintering of aluminosilicate coatings (V/sAS and V/pAS)
The previously produced HTZ (V) was further cut into rectangles of 10 mm in length and 7 mm in width, cleaned with deionized water under ultrasonic vibration for 5 min, and then dried. The aluminosilicate VITA VM9 ceramic powder (Zahnfabrik, Germany) was evenly coated on the surface of V/s and V/p to a layer thickness of less than 0.5 mm. Non-vacuum sintered aluminosilicate coated porcelain tiles (V/sAS) were obtained via a non-vacuum procedure using special ceramic roasting support in a porcelain oven at 980 °C. Vacuum-sintered aluminosilicate-coated porcelain tiles (V/pAS) were obtained by roasting in a ceramic roasting support at 980 °C in a porcelain-baking oven under vacuum conditions.
Impregnated with a silver nitrate solution
Pure silver nitrate crystals were dissolved in deionized water and prepared to a concentration of 1 mol/l silver nitrate solution (Sigma-Aldrich, USA) and dissolved with the aid of ultrasound. V/sAS and V/pAS were submerged below the surface of silver nitrate. V/sAS and V/pAS were impregnated in an ultrasonic water bath for 30 min in a light-proof environment with an ultrasonic output of 60 W.
Secondary sintering of HTZ without silver (V/sAS/Ag and V/pAS/Ag)
The V/pAS impregnated with 1 mol/l silver nitrate solution was sintered twice in a porcelain oven, protected from light, at a rate of 50 °C/min and a sintering temperature of 450 °C (above the decomposition temperature of silver nitrate at 440 °C) for 5 min to produce a non-vacuum sintered HTZ (V/pAS/Ag) loaded with silver. Secondary vacuums intering of V/sAS impregnated with 1 mol/l silver nitrate to produce silver-laden vacuum-sintered HTZ (V/sAS/Ag).
Surface morphology analysis
The completed samples were scanned by a field emission scanning electron microscope (SEM, Hitachi, Japan) at an accelerating voltage of 10 kV to observe the surface morphology of each group of samples, including the distribution, morphology, and size of the silver particles.
Surface chemical elemental analysis
The elemental components of the V/sAS/Ag and V/pAS/Ag coatings were identified using EDS (Genesis XM2, EDAX, USA). The chemical composition and content of the V/sAS/Ag and V/pAS/Ag coating surfaces were analyzed by XPS (ESCALAB250X, Thermmo, USA) using a 75 W AlKα-ray source. The sample was first exhausted and then scanned at a pass energy of 160 eV for the full spectrum, with 0–1200 eV as the scan range. To obtain detailed information on each element, a high-resolution scan of each element was performed using 150 W Al rays at a passing energy of 20 eV and energy corrected using the C1s binding energy (284.8 eV) as a standard.
Performance analysis and silver ion release detection
The transmittance (T) of V/sAS/Ag and V/pAS/Ag were measured and compared using a by spectrophotometer. The transmittance was calculated using the formula: T = It/I0. I0 is the incident light intensity and It is the transmitted light intensity. The transmittance range is 0-–00%, and the greater the transmittance, the better the light transmission. V/sAS/Ag and V/pAS/Ag were prepared in a tube containing 10 ml of PBS solution, and the tube was shaken continuously for 96 h at 37 °C in a constant temperature shaker. At 2, 4, 8, 12, 24, 36, 48, 72, and 96 h, respectively, 1 ml of PBS solution was used to detect the release of silver ions. Silver ions in PBS solutions taken at different sampling times were measured by ICP-AES (Varian, USA) and each sample was repeated 3 times.
Activation of strains and preparation of bacterial suspensions
Strain activation: S. aureus (TX21651-ATCC25923, yingxinbio, China), E. coli (ATCC8099-1EA, yingxinbio, China), and L. acidophilus (ATCC435, babio, China) were inoculated into LB solid medium (babio, China) and passaged 3 times in succession to ensure bacterial viability. After being determined to be free of contamination by morphological characterization and biochemical tests, it was used for experimental antibacterial studies on HTZ with silver.
Preparation of bacterial suspensions: The activated bacteria were inoculated into LB liquid medium (babio, China) and shaken overnight at 37 °C. The optical density value of the bacterial suspension was adjusted to 0.5 using the medium, at which point the bacterial concentration was approximately 1 × 109 CFU/L.
Inhibition zone method
LB solid media plates and V/sAS/Ag and V/pAS/Ag with silver-free, HTZ materials (V/sAS and V/pAS) were sterilized by UV light for 20 min in an ultra-clean bench (KENTON, China). 2 ml of each prepared suspension was evenly applied to the surface of the solid medium and V/sAS/Ag and V/pAS/Ag were placed in the medium plates, with the HTZ without silver (V/sAS and V/pAS) as controls, and incubated in a constant temperature incubator at 37 °C for 24 h, and the formation of antibacterial zones around the materials in each group was observed and photographed (olympus, Japan).
Flat colony counting method
V/sAS/Ag and V/pAS/Ag with HTZ without silver (V/sAS and V/pAS) were sterilized under a UV lamp for 20 min. The material was then placed in 2 ml of the prepared bacterial suspension and incubation for 24 h. The material was removed from the suspension and rinsed off with sterile PBS to remove surface floating bacteria. Each material was then added to a centrifuge tube containing 5 ml of sterile PBS and vortexed, and shaken for 5 min to dislodge the bacteria on the surface of the material into the PBS solution. 0.1 ml of each bacterial suspension was evenly spread on a solid medium plate (LB) and left overnight at 37 °C. The number of colonies was recorded and the surface bacterial inhibition rate was calculated for each material. Inhibition rate (%) = (number of colonies in the non-silvered group − number of colonies in the silvered group)/number of colonies in the non-silvered group × 100%.
SEM observation of bacterial morphology
SEM was used to observe the changes in bacterial morphology after the co-cultureing with each material. V/sAS/Ag, V/pAS/Ag, and HTZ without Ag (V/sAS and V/pAS/Ag) were added to 1 ml of the three prepared spare bacterial suspensions for 24 h. After completion of treatment, each material was washed 3 times with sterile PBS for 2 min. Next, the bacteria were fixed with 2.5% glutaraldehyde for 2 h. The fixed bacteria were then subjected to 50, 60, 70, 80, 90, 95, and 100% alcohol dehydration for 15 min each time and CO2 critical point drying treatment, and the structural characteristics of the bacteria on the surface of each material were observed by SEM.
Statistical methods
The results were analyzed using SPSS 22.0 and expressed as mean ± standard deviation (x ± s). Independent samples t-test and one-way ANOVA were used to compare the statistical significance of the differences in data between groups, with p < 0.05 indicating statistical significance.
Results
Microscopic surface morphology of coatings impregnated with silver nitrate
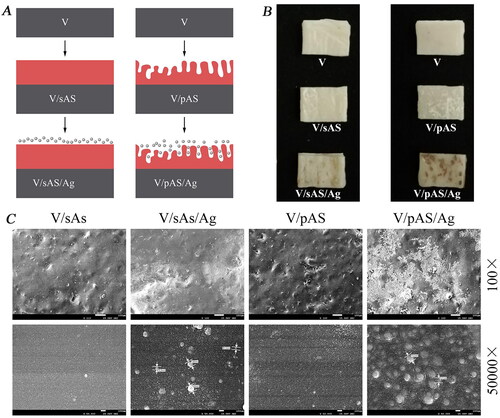
A schematic diagram of the vacuum- and non-vacuum sintered silver-laden HTZ samples construction is shown in . V/sAS/Ag is a vacuum-sintered coating impregnated with silver nitrate at a concentration of 1 mol/l and V/pAS/Ag is a non-vacuum-sintered coating impregnated with silver nitrate at a concentration of 1 mol/l (). SEM scan of the coating impregnated and sintered in silver solution was shown in , with the non-vacuum sintered coating forming a micro-nano composite porous structure visible at a magnification of 100×. Both the V/sAS/Ag and V/pAS/Ag surfaces showed silver particles on the surface, with the V/sAS/Ag and V/pAS/Ag showing an aggregated silver particle morphology. At a magnification of 50,000×, the average diameter of the silver particles on the V/sAS/Ag surface was approximately 90 nm, with the silver particles in the V/pAS/Ag group varying from 40 nm to 80 nm in diameter. The morphology of the silver particles deposited on the surface of V/sAS/Ag was spherical and scattered, while V/pAS/A showed some areas of aggregation of silver particles, consistent with the distribution of silver particles observed under low magnification.
Figure 1. V/sAS/Ag and V/pAS/Ag Fabrication processes and surface micromorphology of each material. A: Schematic of the construction of a silver HTZ sample downloaded under vacuum and non-vacuum conditions, with the base ceramic sheet in grey, the aluminosilicate coating in red, and the silver particles in particles. B: Representative images of sintered materials. C: Representative images of observation of the surface morphology of different materials.
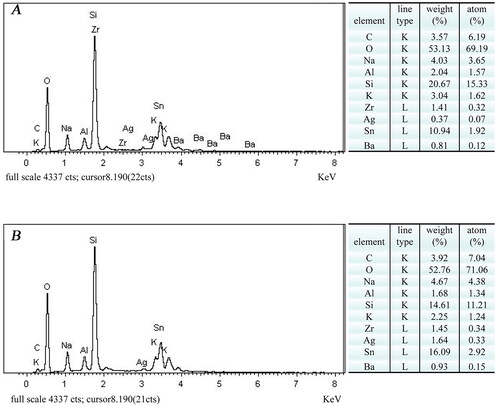
EDS elemental analysis of V/sAS/Ag and V/pAS/Ag surfaces
The EDS spectra of the surface elements of V/sAS/Ag and V/pAS/Ag are shown in . We detected the main elements such as oxygen (O), silicon (Si), and tin (Sn) on the surface of V/sAS/Ag and V/pAS/Ag. Carbon (C), sodium (Na), aluminium (Al), potassium (K), zirconium (Zr), and barium (Ba) were also present in the samples. These elements originated from the coating material aluminosilicate (VITA VM9), indicating that the aluminosilicate coating was successfully constructed on the HTZ substrate. V/pAS/Ag had more silver atoms than V/sAS/Ag, and the trend of increasing silver loading of V/sAS/Ag and V/pAS/Ag was consistent with the SEM results.
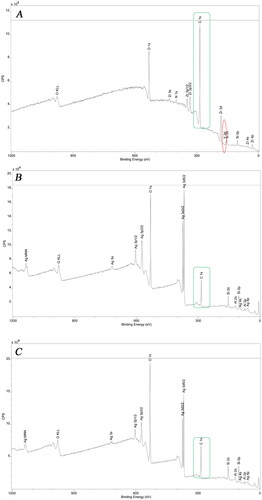
XPS elemental analysis of material surfaces
The surface chemical elemental compositions of the control V and silver-loaded samples from the V/sAS/Ag and V/pAS/Ag were detected by XPS. As shown in , C, the elements on the surface of the V samples were mainly Zr and O. Elemental Y (yttrium) peaks appeared on the surface of the V samples, while there were no Y peaks on the surface of the V/sAS/Ag and V/pAS/Ag coatings, but there are obvious peaks of elemental Si and Al, indicating that the aluminosilicate coating successfully covered the surface of the HTZ. The Ag signal (Ag3d peak) was present on the surface of the samples in the V/sAS/Ag and V/pAS/Ag groups. Therefore, it is assumed that the silver ions have been reduced and successfully loaded onto the aluminosilicate-coated surface.
Figure 3. XPS detection of Material surface elements. A: Representative images of full spectrum of vacuum sintered unloaded silver sample V; B: Representative images of full spectrum of vacuum sintered coated V/sAS/Ag sample. C: Representative images of full spectrum of non-vacuum sintered coated V/pAS/Ag sample.
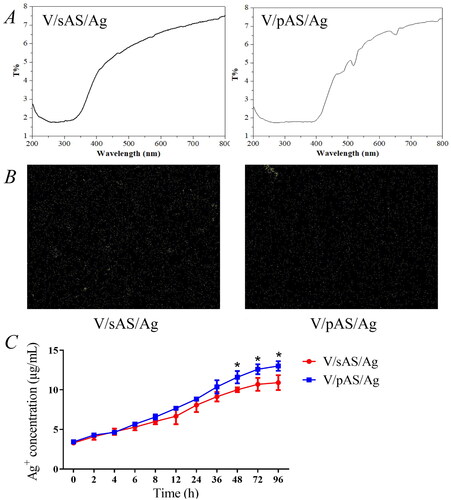
V/pAS/Ag and V/sAS/Ag performance analysis and surface silver element detection
The average transmittance of the two silver-loaded materials was 6.6 ± 0.5% for V/sAS/Ag and 6.7 ± 0.7% for V/pAS/Ag in the wavelength range of 200-800 nm, and the two materials had good transmittance (). As shown in for the electron microscopic surface scan of the silver solution-impregnated sample, the V/sAS/Ag-modified coating showed a superficial distribution of silver nanoparticles compared to V/pAS/Ag, and V/pAS/Ag silver nanoparticles were deposited deeper in the aluminosilicate coating. shows the release rates of silver ions from the two silver-loaded samples at 37 °C. The release of silver ions was more pronounced in both samples between 2 and 48 h, after which it gradually stabilized. The concentration of silver ions released from V/pAS/Ag after 6 h was higher than that released from V/sAS/Ag. The concentration of silver ions released from V/pAS/Ag and V/sAS/Ag reached (12.47 ± 1.11) μg/mL and (10.90 ± 1.51) μg/mL respectively at the 96th hour, suggesting a faster rate of silver ion release from the surface of the V/pAS/Ag.
Figure 4. Silver element detection. A: Representative images of transmittance of modified V/sAS/Ag and V/pAS/Ag; B: Representative images of V/sAS/Ag and V/pAS/Ag silver particle distribution; C: Silver ion release curves in V/sAS/Ag and V/pAS/Ag. T-test was used for expression differences between the two groups, * p < 0.05.
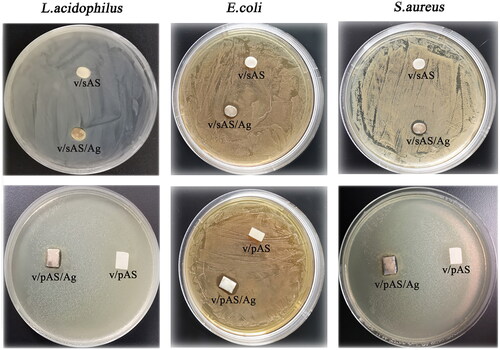
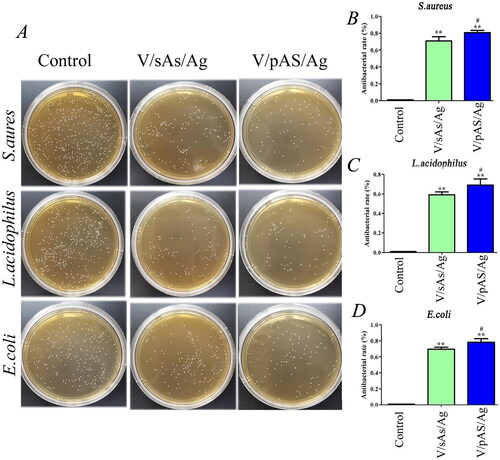
Antibacterial performance evaluation of the inhibition zone method
The antibacterial properties of V/sAS/Ag and V/pAS/Ag were examined using the inhibition zone method. As shown in , after 24 h of culture on a solid culture plate, there were obvious inhibition zones around V/sAS/Ag and V/pAS/Ag, and the inhibition zones of S.aureus and L.acidophilus were more obvious; in contrast, the HTZ without silver (V/sAS and V/pAS) did not show inhibitory circles on any of the three bacterial culture plates. The above experiments may indicate that silver ions were released from the surface of both V/sAS/Ag and V/pAS/Ag materials into the surrounding medium, thus inhibiting the growth of the three bacteria.
Antibacterial performance evaluation
The antibacterial properties of the surfaces of each group of materials were further investigated using the flat colony counting method. S.aureus, L.acidophilus, and E.coli co-cultured with HTZ without silver (control group) for 24 h grew vigorously on LB medium plates, whereas co-culture with V/sAS/Ag and V/pAS/Ag materials significantly inhibited the growth of S.aureus, L.acidophilus, and E.coli (). After bacterial enumeration, the inhibition rate of S.aureus (), L.acidophilus (), and E.coli () co-cultured with V/sAS/Ag and V/pAS/Ag was more than 90% compared with the control group, and the inhibition rate between the V/sAS/Ag and V/pAS/Ag groups had statistically significant differences (p < 0.05).
Figure 6. The sterilisation effect of silver-loaded materials. A: Bactericidal effect of V/sAS/Ag and V/pAS/Ag treatment on S.aureus, L.acidophilus, and E.coli. Statistical plots of the bactericidal effects of V/sAS/Ag and V/pAS/Ag on S.aureus (B), L. acidophilus (C), and E.coli (D). compared with the control group, **p < 0.01; compared with the V/sAS/Ag group, #p < 0.05.
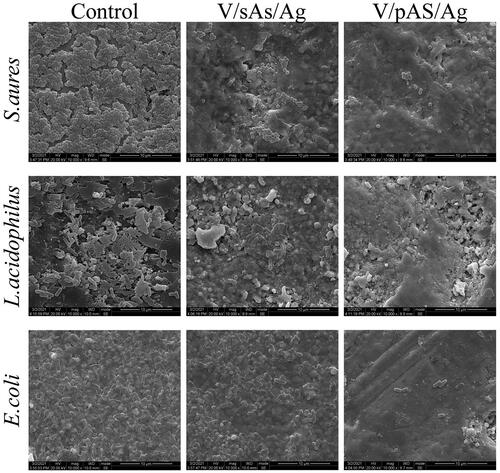
SEM observation of morphological changes in each group of bacteria
As shown in , S.aureus had a spherical structure, and the control group had a regular morphology with an intact cell wall and neat arrangement, suggesting that HTZ without silver (the control group) had no destructive effect on S.aureus. V/sAS/Ag treated S.aureus was significantly deformed, showing a lower degree of hollowness, disrupted bacterial structure, and a disorganized arrangement. The degree of morphological deformation and disruption was more pronounced in V/pAS/Ag-treated S.aureus and fewer bacteria with a normal morphology. L.acidophilus and E.coli had rod-like structures; the cell walls of L.acidophilus and E.coli in the control group were intact, and the bacteria were neatly arranged, suggesting that HTZ without silver had no cytotoxic effect on L.acidophilus and E.coli. V/sAS/Ag-treated L. acidophilus and E.coli showed a small amount of bacterial deformation, with some degree of hollowness and disruption of the connection between bacteria. The morphological disruption of L. acidophilus and E.coli treated with V/pAS/Ag was more severe, with massive deformation and disorganized arrangement. The above results confirmed that both V/sAS/Ag and V/pAS/Ag were effective in killing S. aureus, L. acidophilus, and E.coli, and the V/pAS/Ag material was more effective.
Disscussion
Although zirconia materials are widely used in the oral cavity, they exhibit no antibacterial activity [Citation26]. The implant is placed in the bone through the soft tissue of the skin and is directly exposed to the external environment, making it vulnerable to bacterial infection during the implant placement and healing process. Direct exposure of the percutaneous area to the external environment makes it susceptible to bacterial infections during the implant placement and healing processes [Citation27]. Therefore, it is of great clinical importance to improve the antibacterial capacity of zirconium oxide implant abutments by modifying the surface of the material in the gingival penetration area to maintain oral health.
Zirconia has a good elastic modulus, mechanical properties, and corrosion resistance, and its translucency is advantageous for improving the aesthetics of dental implants [Citation6–11]. Zirconia abutments have comparable implantation success rates and impact on oral plaque and bleeding as titanium abutments [Citation28]. HTZ is a new type of yttrium (Y)-stabilized zirconia that is ideal as an aesthetic implant abutment for anterior teeth and has further improved aesthetic properties compared to titanium abutments, making it a promising material for aesthetic implant restorations [Citation29,Citation30]. In this study, the VITA YZ HTColor with yttrium-stabilized HTZ all-ceramic material was used to create the implant abutment. The addition of Y2O3 to the zirconia material improved its translucency and high-temperature resistance of the material.
Previous studies have shown that the micro-gap between the implant abutments provides space for bacteria to grow, inducing infection and inflammation of the peri-implant tissue affecting the aesthetic and functional functioning of the restoration and even the success of the implant [Citation31,Citation32]. Although antibiotics can inhibit bacterial growth at the implant-abutment interface to a certain extent and reduce the incidence of peri-implant tissue-related infections, the long-term application of antibiotics tends to cause resistance problems [Citation19]. If the abutment material kills surface bacteria and reduces bacterial adhesion, it can further control infection, prevent inflammation and prevent bacterial resistance.
Adding a functional surface coating is the most common way to modify the surface of planting materials, either as a direct antimicrobial coating material or as an antimicrobial coating material that releases antimicrobial substances [Citation33]. The incorporation of antimicrobial peptides into the surface of implant materials is a common method for constructing direct antimicrobial coating materials, which are less likely to cause multi-drug resistance in bacteria and can effectively address the resistance challenges faced by traditional antibiotics [Citation34]. However, antimicrobial peptides are inherently cytotoxic and are therefore less commonly used in clinical practice [Citation35]. In addition, composite antimicrobial coating materials capable of releasing antimicrobial substances are an important option for addressing the bacterial adhesion and proliferation of implant materials. Composite coating materials that release antibacterial substances are mostly based on metal ions such as silver, copper, and zinc [Citation36]. In this study, VM9 porcelain powder was used as the coating porcelain powder for VITA YZ HTColor. Both vacuum and non-vacuum sintering procedures were used in the experiments, and the vacuum sintering resulted in a denser aluminosilicate coating. The surface of HTZ was sintered with aluminosilicate under non-vacuum conditions to construct a micro-nano porous structure, and then the prepared material was immersed in silver nitrate (AgNO3) solution, and through secondary sintering, the porous aluminosilicate was used as a carrier silver ion are reduced in situ to build a silver particle coating on the surface of HTZ. In addition, V/pAS/Ag can detect more silver ion release because its surface is more silver-loaded. The modification process of impregnation combined with sintering is simple and easy to implement, improving the material properties while avoiding the intrusion of harmful substances as far as possible and laying the foundation for the coating to adhere firmly and continue to perform its antimicrobial function steadily in the future.
Ag nanoparticles are favoured in biomedical material modification research due to their good antibacterial properties and biocompatibility, coupled with their large specific surface area, strong penetration, and low toxic side effects on human cells, and are widely used in oral and orthopaedic surgical endophytes for anti-infection [Citation37,Citation38]. Previous studies have found that the proliferation rates of various bacteria, such as Klebsiella Pneumoniae and S.aureus, were reduced when cultured on the surface of silver-loaded implants [Citation39]. Streptococci, staphylococci, lactobacilli, enterobacteria, and spirochaetes in the oral cavity can cause endodontic periapical disease, periodontal disease, oral mucosal disease, oral and maxillofacial interstitial infections, and postoperative infections [Citation40–42]. In this study, the release of Ag ions from V/sAS/Ag and V/pAS/Ag in PBS was examined. It was found that the release of silver ions from the surfaces of V/sAS/Ag and V/pAS/Ag continued at a rapid rate for 48 h and then tended to moderate, while the release of silver ions from the surface of V/pAS/Ag was higher than that of V/sAS/Ag. This may be related to the porous structure of the V/pAS/Ag material coating surface, which may also explain the different antimicrobial properties of the two materials.
Conclusions
In summary, the silver-loaded V/sAS/Ag and V/pAS/Ag in this study consistently released silver ions with high bactericidal efficacy, resulting in antibacterial effects against S. aureus, L. acidophilus, and E. coli. The results of the study provide a solution to inhibit plaque adhesion; V/sAS/Ag and V/pAS/Ag were expected to be alternative materials for new implant abutments.
Authors’ contributions
Min Peng and Jun-Lan Chuan performed the experiments, Gao-ping Zhao and Qiang Fu designed the experiments, and all authors wrote the manuscript.
Acknowledgments
Not applicable.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Sun W, Gregory DA, Tomeh MA, et al. Silk fibroin as a functional biomaterial for tissue engineering. Int J Mol Sci. 2021;22(3):1499. https://doi.org/10.3390/ijms22031499
- Bose S, Ke D, Sahasrabudhe H, et al. Additive manufacturing of biomaterials. Prog Mater Sci. 2018;93:45–111. doi:10.1016/j.pmatsci.2017.08.003.
- Beheshtizadeh N, Azami M, Abbasi H, et al. Applying extrusion-based 3D printing technique accelerates fabricating complex biphasic calcium phosphate-based scaffolds for bone tissue regeneration. J Adv Res. 2022;40:69–94. doi:10.1016/j.jare.2021.12.012.
- Hertz A, Bruce IJ. Inorganic materials for bone repair or replacement applications. Nanomedicine. 2007;2(6):899–918. doi:10.2217/17435889.2.6.899.
- Sun H, Chan Y, Li X, et al. Multi-omics analysis of oral bacterial biofilm on titanium oxide nanostructure modified implant surface: in vivo sequencing-based pilot study in beagle dogs. Mater Today Bio. 2022;15:100275. doi:10.1016/j.mtbio.2022.100275.
- Han JM, Lin H, Hong G. Zirconia dental implant: a review of literature on clinical application and animal studies. Zhonghua Kou Qiang Yi Xue Za Zhi. 2013;48(12):769–771.
- Sanon C, Chevalier J, Douillard T, et al. A new testing protocol for zirconia dental implants. Dent Mater. 2015;31(1):15–25. doi:10.1016/j.dental.2014.09.002.
- Bankoğlu GüNGöR M, AYDıN C, YıLMAZ H, et al. An overview of zirconia dental implants: basic properties and clinical application of three cases. J Oral Implantol. 2014;40(4):485–494. doi:10.1563/AAID-JOI-D-12-00109.
- Gahlert M, Roehling S, Sprecher CM, et al. In vivo performance of zirconia and titanium implants: a histomorphometric study in mini pig maxillae. Clin Oral Implants Res. 2012;23(3):281–286. doi:10.1111/j.1600-0501.2011.02157.x.
- Eraslan O, Aykent F, YüCEL MT, et al. The finite element analysis of the effect of ferrule height on stress distribution at post-and-core-restored all-ceramic anterior crowns. Clin Oral Investig. 2009;13(2):223–227. doi:10.1007/s00784-008-0217-5.
- Yamashita D, Machigashira M, Miyamoto M, et al. Effect of surface roughness on initial responses of osteoblast-like cells on two types of zirconia. Dent Mater J. 2009;28(4):461–470. doi:10.4012/dmj.28.461.
- Shijo Y, Shinya A, Gomi H, et al. Studies on mechanical strength, thermal expansion of layering porcelains to alumina and zirconia ceramic core materials. Dent Mater J. 2009;28(3):352–361. doi:10.4012/dmj.28.352.
- Asmussen E, Peutzfeldt A, Heitmann T. Stiffness, elastic limit, and strength of newer types of endodontic posts. J Dent. 1999;27(4):275–278. doi:10.1016/s0300-5712(98)00066-9.
- Sailer I, Gottnerb J, Kanelb S, et al. Randomized controlled clinical trial of zirconia-ceramic and metal-ceramic posterior fixed dental prostheses: a 3-year follow-up. Int J Prosthodont. 2009;22(6):553–560.
- Davis NM, Proctor DM, Holmes SP, et al. Simple statistical identification and removal of contaminant sequences in marker-gene and metagenomics data. Microbiome. 2018;6(1):226. doi:10.1186/s40168-018-0605-2.
- Kolenbrander PE, Palmer R J JR, Periasamy S, et al. Oral multispecies biofilm development and the key role of cell-cell distance. Nat Rev Microbiol. 2010;8(7):471–480. doi:10.1038/nrmicro2381.
- Signoretto C, Bianchi F, Burlacchini G, et al. Drinking habits are associated with changes in the dental plaque microbial community. J Clin Microbiol. 2010;48(2):347–356. doi:10.1128/JCM.00932-09.
- Vellappally S, Fiala Z, SMEJKALOVá J, et al. Influence of tobacco use in dental caries development. Cent Eur J Public Health. 2007;15(3):116–121. doi:10.21101/cejph.a3431.
- Norrby SR, Nord CE, Finch R. Lack of development of new antimicrobial drugs: a potential serious threat to public health. Lancet Infect Dis. 2005;5(2):115–119. doi:10.1016/S1473-3099(05)70086-4.
- Wang Y, Yang Y, Shi Y, et al. Antibiotic-free antibacterial strategies enabled by nanomaterials: progress and perspectives. Adv Mater. 2020;32(18):e1904106. doi:10.1002/adma.201904106.
- Wadu-Mesthrige K NA, Bulychev A, et al. High-resolution atomic force microscopy studies of the Escherichia coli outer membrane: structural basis for permeability. Langmuir. 2000; 6(16): 2789-2796. https://doi.org/10.1021/la991013x.
- Silver S, Phung LT, Silver G. Silver as biocides in burn and wound dressings and bacterial resistance to silver compounds. J Ind Microbiol Biotechnol. 2006;33(7):627–634. doi:10.1007/s10295-006-0139-7.
- Bichu YM, Alwafi A, Liu X, et al. Advances in orthodontic clear aligner materials. Bioact Mater. 2023;22:384–403. doi:10.1016/j.bioactmat.2022.10.006.
- Aleksandrowicz P, Żelechowska P, Agier J, et al. Evaluation of metalloproteinase-8 levels in crevicular fluid of patients with healthy implants or periodontitis. Mediators Inflamm. 2017;2017:4920847. doi:10.1155/2017/4920847.
- Parlar A, Bosshardt DD, Cetiner D, et al. Effects of decontamination and implant surface characteristics on re-osseointegration following treatment of peri-implantitis. Clin Oral Implants Res. 2009;20(4):391–399. doi:10.1111/j.1600-0501.2008.01655.x.
- Lee KR, Choe HC, Heo YR, et al. Effect of different grinding burs on the physical properties of zirconia. J Adv Prosthodont. 2016;8(2):137–143. doi:10.4047/jap.2016.8.2.137.
- Arciola CR, Campoccia D, Montanaro L. Implant infections: adhesion, biofilm formation and immune evasion. Nat Rev Microbiol. 2018;16(7):397–409. doi:10.1038/s41579-018-0019-y.
- Arciola CR, Campoccia D, Ehrlich GD, et al. Biofilm-based implant infections in orthopaedics. Adv Exp Med Biol. 2015;830:29–46. doi:10.1007/978-3-319-11038-7_2.
- Zarone F, Russo S, Sorrentino R. From porcelain-fused-to-metal to zirconia: clinical and experimental considerations. Dent Mater. 2011;27(1):83–96. doi:10.1016/j.dental.2010.10.024.
- Piconi C, Maccauro G. Zirconia as a ceramic biomaterial. Biomaterials. 1999;20(1):1–25. doi:10.1016/s0142-9612(98)00010-6.
- Carinci F, Lauritano D, Bignozzi CA, et al. A new strategy against Peri-Implantitis: antibacterial internal coating. Int J Mol Sci. 2019;20(16):3897.doi: 10.3390/ijms20163897.
- Siadat H, Najafi H, Alikhasi M, et al. Effect of lateral oblique cyclic loading on microleakage and screw loosening of implants with different connections. J Dent Res Dent Clin Dent Prospects. 2018;12(3):183–189. doi:10.15171/joddd.2018.028.
- Zhu N, Ji H, Yu P, et al. Surface modification of magnetic iron oxide nanoparticles. Nanomaterials. 2018;8(10):810. doi:10.3390/nano8100810.
- Manrique-Moreno M, Suwalsky M, PATIñO-GONZáLEZ E, et al. Interaction of the antimicrobial peptide ΔM3 with the Staphylococcus aureus membrane and molecular models. Biochim Biophys Acta Biomembr. 2021;1863(2):183498. doi:10.1016/j.bbamem.2020.183498.
- Su BC, Wu TH, Hsu CH, et al. Distribution of positively charged amino acid residues in antimicrobial peptide epinecidin-1 is crucial for in vitro glioblastoma cytotoxicity and its underlying mechanisms. Chem Biol Interact. 2020;315:108904. doi:10.1016/j.cbi.2019.108904.
- Lemire JA, Harrison JJ, Turner RJ. Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat Rev Microbiol. 2013;11(6):371–384. doi:10.1038/nrmicro3028.
- Prasath S, Palaniappan K. Is using nanosilver mattresses/pillows safe? A review of potential health implications of silver nanoparticles on human health. Environ Geochem Health. 2019;41(5):2295–2313. doi:10.1007/s10653-019-00240-7.
- Singh H, Du J, Yi TH. Biosynthesis of silver nanoparticles using aeromonas sp. THG-FG1.2 and its antibacterial activity against pathogenic microbes. Artif Cells Nanomed Biotechnol. 2017;45(3):584–590. doi:10.3109/21691401.2016.1163715.
- Umar Farooq M, Pervez Mughal M, Ahmed N, et al. On the investigation of surface integrity of Ti6Al4V ELI using Si-Mixed electric discharge machining. Materials. 2020;13(7):1549. doi:10.3390/ma13071549.
- Ishihata K, Seong CH, Kibe T, et al. Lipoteichoic acid and lipopolysaccharides are affected by p38 and inflammatory markers and modulate their promoting and inhibitory effects on osteogenic differentiation. Int J Mol Sci. 2022;23(20):12633. doi:10.3390/ijms232012633.
- Khemaleelakul S, Baumgartner JC, Pruksakorn S. Identification of bacteria in acute endodontic infections and their antimicrobial susceptibility Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94(6):746–755. doi:10.1067/moe.2002.129535.
- Peng X, Cheng L, You Y, et al. Oral microbiota in human systematic diseases. Int J Oral Sci. 2022;14(1):14. doi:10.1038/s41368-022-00163-7.