Abstract
The overview describes the synergy between biological sciences and cellular structures processed by additive manufacturing to elucidate the significance of cellular structured implants in eliminating stress shielding and in meeting the bio-mechanical property requirements of elastic modulus, impact resistance, and fatigue strength in conjunction with the biological functionality. The convergence of additive manufacturing, computer-aided design, and structure-property relationships is envisaged to provide the solution to the current day challenges in the biomedical arena. The traditional methods of fabrication of biomedical devices including casting and mechanical forming have limitations because of the mismatch in micro/microstructure, mechanical, and physical properties with the host site. Additive manufacturing of cellular structured alloys via electron beam melting and laser powder bed fusion has benefits of fabricating patient-specific design that is obtained from the computed tomography scan of the defect site. The discussion in the overview consists of two aspects – the first one describes the underlying reason that motivated 3D printing of implants from the perspective of minimising stress shielding together with the mechanical property requirements, where the mechanical properties of cellular structured implants depend on the cellular architecture and percentage cellular porosity. The second aspect focuses on the biological response of cellular structured devices.
Additive manufacturing of biomedical devices
Additive manufacturing is currently an overstated adjective and is the fashion of the day. It is based on the concept of rapid prototyping (RP) and involves layer-by-layer fabrication of 3D physical models generated from the computer-aided design (CAD) [Citation1–3]. A few steps are followed in the 3D printing of biomedical medical devices with complex architecture. In brief, a computed tomography (CT) scan of the defect site is first obtained to visualise a 3D image of the region of interest. Next, the region of interest from the CT scan data is extracted and converted into a 3D design using a software, for instance MIMICS. The STL (STereoLithography) file is subsequently used as an input file by the 3D printing equipment to print the biomedical device [Citation1]. In 3D printing of the device by additive manufacturing for instance by electron beam melting or laser powder fusion, the architecture of the unit cell impacts the properties of the biomedical device. An important benefit of additive manufacturing is the flexibility to create a patient-specific implant [Citation1, Citation4–6].
Bio-mechanical functionalities in 3D printed cellular structured titanium alloy biomedical devices
A unique class of cellular structured metallic alloys processed by additive manufacturing has been proposed in the context of biomedical devices to primarily address the challenge of stress shielding. The genesis of cellular structured arises from the fact that the modulus of elasticity of widely used titanium alloy (Ti-6Al-4V) is greater than the bone. Obtaining desirable mechanical properties in 3D printed cellular structured biomedical devices by additive manufacturing that are compatible with the host tissue is an absolute necessity that merits serious consideration. For instance, the compressive strength of natural human bone is approximately 2–12 MPa for cancellous human bone and approximately 100–230 MPa for cortical bone. The elastic modulus of cancellous bone is approximately 0.1–4.5 GPa and cortical bone is approximately 15–25 GPa, while that of titanium alloy is approximately 110 GPa [Citation7,Citation8]. The underlying cause of stress shielding and bone restoration is this mismatch or incompatibility in elastic modulus between natural bone and titanium alloy, where the elastic modulus is greater than the host tissue. Consequently, it is crucial that the elastic modulus and strength be comparable to those of human bone [Citation7–9].
A viable approach to reduce the elastic modulus is either to design a new alloy or fabricate cellular structures (porous/mesh structure) with modulus comparable to bone. In this regard, additive manufacturing is appropriate to fabricate cellular structured implants that enable us to accurately control the porous architecture, shape, and functionalities, that render them ideal for patient-specific implants [Citation1, Citation7,Citation8]. This is crucial for cellular structures because of several reasons. In this regard, additive manufacturing provides us the splendid opportunity to fabricate customised cellular structures that are impossible to obtain via traditional manufacturing methods. The ability to tailor the internal architecture of biomedical device enables us to design structures with specific mechanical properties and functionalities, making them ideal for patient-specific implants [Citation1, Citation4–9]. Moreover, the selection of specific lattice design of interconnected cellular structures with clinically relevant geometry enables tuning of mechanical properties, such as strength, stiffness, and toughness, together with enhanced osseointegration, bone ingrowth, and improved biological performance. The porous nature of architecture provides nutrients into cells or pores, facilitates nutrient exchange, oxygen diffusion, efficient cell seeding and production of an extracellular matrix (ECM), all of which are important for tissue regeneration and healing [Citation1, Citation7, Citation10–15]. By modulating the pore size, selection of unit cell, uniform/graded/gradient structure, the modulus of cellular structure can be tuned in the range of 0.5 – 15 GPa, which is comparable to trabecular (0.1–4.5 GPa) and cortical bone (15–25 GPa) [Citation1, Citation7, Citation16]. Thus, through appropriate design of constituent unit cells, graded/gradient cellular structures can be additively fabricated to meet not only the mechanical property requirements, elastic modulus, high fatigue strength and impact toughness, but also biological functions.
It is optimal to build cellular-structured biomedical devices as a structure with numerous layers whose mechanical characteristics differ across the 3D-printed structure, taking into account the nature of the human bone. This can be achieved by designing a graded (porous structure with variable porosity along a particular direction) or gradient (gradual change in porosity) structures, which enables a change in elastic modulus [Citation16].
In fact, the concept of functionally graded structure is consistent with natural bone, which has an outer highly dense region (cortical bone) and the inner significantly less dense porous region (cancellous bone). The bone marrow cavity is present in the central region [Citation1, Citation7,Citation8, Citation17]. Thus, there is a gradual increase in porosity (hence modulus) variation from cortical to cancellous transition, especially at the end of long bones. Therefore, to reproduce the architecture of natural bone, biomedical device with graded porosity is preferred [Citation1, Citation18]. Graded cellular structure of Ti-alloys used as hip stems meet the requirements of tissue engineering with advantage of stress shielding over homogenous porous structure [Citation1, Citation19]. Here the highly porous surface is in contact with the host bone and the porosity gradually decreases until it approaches a solid core material for high load bearing application or a porous core for low load bearing application to facilitate faster tissue ingrowth [Citation1].
In general, the unit cell, pore architecture, and percentage porosity control the mechanical properties of 3D cellular formations. They can be adjusted in additive manufacturing to achieve the appropriate strength and energy absorption capacity, overcoming the conflict between the desired strength-toughness combination and the requirement for a certain proportion of porosity in the cellular structure.
Selection of an appropriate unit cell (cubic, G7 (materialize magics unit cell, rhombic dodecahedron) [Citation20] provides an approach to obtain the elastic modulus, strength, impact toughness, and fatigue resistance in conjunction with biological functions. From the perspective of fatigue resistance, rhombic dodecahedron unit cells offered good impact energy absorption capability and lower cyclic ratcheting rate, with greatly reduced susceptibility to stress and strain [Citation20]. Furthermore, in graded cellular structures, stress (and strain) can be distributed across different unit cells, which is beneficial for fatigue resistance. presents a summary of density, modulus, and strength for the three different unit cells [Citation20].
Table 1. Physical and mechanical properties of Ti-6Al-4V titanium alloy fabricated by electron beam melting (EBM) with different unit cell structures [Citation20].
shows schematic of unit cells together with SEM micrographs of unit cells and compressive fatigue deformation behaviour.
Figure 1. (a) CAD files and (b) scanning electron micrographs of different unit cell elements with (c) stress-strain plots showing brittle (cubic and rhombic dodecahedron) and ductile (G7) characteristics of unit cells. (d) Compression deformation behaviour showing different deformation modes and angle of fracture/crush bands for cubic (bucking and 90), G7 (bending and 45), and rhombic dodecahedron (bending and 45) unit cell elements [adapted from reference Citation20].
![Figure 1. (a) CAD files and (b) scanning electron micrographs of different unit cell elements with (c) stress-strain plots showing brittle (cubic and rhombic dodecahedron) and ductile (G7) characteristics of unit cells. (d) Compression deformation behaviour showing different deformation modes and angle of fracture/crush bands for cubic (bucking and 90), G7 (bending and 45), and rhombic dodecahedron (bending and 45) unit cell elements [adapted from reference Citation20].](/cms/asset/87dc38fc-5a14-4363-b440-ef8caab20bfc/ianb_a_2278156_f0001_c.jpg)
It was noted that both low- and high-density regions of the graded cellular structure initially witnessed uniform deformation, followed by fracture in the low density region, when the crush band was formed [Citation7]. However, finite element modelling indicated a discontinuous stress transfer in graded materials at the nodes, but not in the gradient material () [Citation21]. This presumably implied the superiority of gradient design over graded and uniform structural design in terms of high strength-high ductility-high energy absorption. Gradients and graded materials are therefore expected to have the best fusion of elastic modulus, strength, fatigue resistance, and energy absorption capacity.
Figure 2. Finite volume method (FVM) stress analysis of graded and gradient mesh structures performed using ANSYS software, indicating the stress discontinuity along the interface between the two different layers in graded mesh (G1-G2 and G2-G3) during deformation. Such discontinuities were absent in gradient mesh structures [adapted from reference Citation21].
![Figure 2. Finite volume method (FVM) stress analysis of graded and gradient mesh structures performed using ANSYS software, indicating the stress discontinuity along the interface between the two different layers in graded mesh (G1-G2 and G2-G3) during deformation. Such discontinuities were absent in gradient mesh structures [adapted from reference Citation21].](/cms/asset/c541dfce-3f37-4f9a-83d9-24d1c3e32a2a/ianb_a_2278156_f0002_c.jpg)
It is known that mechanical strength and energy absorption ability of uniform cellular structured materials degrade with decrease in density and with increase in the porosity of cellular structured materials [Citation22]. In this regard, functionally graded cellular structures were considered appropriate (). According to research on the compression-compression fatigue behaviour of functionally graded Ti-6Al-4V structures under almost identical bulk stress conditions, the local stress distribution in the struts was not uniform due to variations in the constituent cells’ mechanical characteristics. The constituent with the lowest strength was where fatigue cracks initially appeared, and they later spread until failure happened. But during the procedure, no structural damage was seen in other components [Citation22].
Figure 3. Graded and uniform cellular structures (physical models and cellular structures) [adapted from reference Citation22].
![Figure 3. Graded and uniform cellular structures (physical models and cellular structures) [adapted from reference Citation22].](/cms/asset/10703ed8-ff1f-44df-9f96-eaa62bee32d0/ianb_a_2278156_f0003_c.jpg)
The strain accumulation curves () [Citation22] of four meshes include three stages. In the first stage, the strain was slightly increased and then in the second stage it increased at a constant rate. After the knee point, the strain in G1, G2, G3 uniform meshes increased linearly, leading to rapid collapse of porous structure in the third stage. shows that the strain curve of graded meshes increased linearly for a number of cycles, and then became non-linear. In brief, the fatigue life increased according to the sequence of G1, G2, G3, and in the graded structure was similar to G1 at low and high stresses [Citation22].
Figure 4. Strain accumulation curves under the same stress level. (a) 2 MPa and (b) 3.8 MPa [adapted from reference Citation22].
![Figure 4. Strain accumulation curves under the same stress level. (a) 2 MPa and (b) 3.8 MPa [adapted from reference Citation22].](/cms/asset/a5c0fcf5-c1b0-44c2-ba98-d3eb37e1200c/ianb_a_2278156_f0004_c.jpg)
Based on the aforementioned explanation, we may achieve the ideal and necessary balance of low specific modulus and higher specific strength by adjusting the relative density and cellular architecture of Ti-6Al-4V cellular structures [Citation23–30]. The elastic modulus, compressive strength, and deformation behaviour of cellular structures are properly influenced by the pore size and cell shape, which are governed by the architecture of cellular structures (unit cell, gradient/graded structure) [Citation20, Citation31–35]. Effective ways to guarantee the desired fatigue performance life of additively made Ti-6Al-4V cellular structures include the unit cell and pore size. It is suggested that the unit cell, surface roughness, strut flaws, and the loading state of the struts all influence fatigue strength [Citation35]. Based on the suggested mechanisms, the appropriate unit cell design was chosen, and the struts’ buckling and bending deformation was caused. The buckling deformation behaviour of the struts can be controlled by optimising the design of the unit cell, which is intended to provide high compressive fatigue strength at a relatively low density because local stress in the struts is eliminated, and there is a decrease in the cyclic retching of struts during cyclic loading ()[Citation23].
Figure 5. Cubic, G7 and rhombic dodecahedron unit cells (a–c) and corresponding Ti-6Al-4V prototype structures processed by electron beam melting (d–f), and (g–i) are the schematic illustration of buckling and bending vectors of applied stress on cell struts. (j) stress versus number of cycles to failure of mesh structures with cubic, G7 and rhombic dodecahedron structures [adapted from reference Citation23].
![Figure 5. Cubic, G7 and rhombic dodecahedron unit cells (a–c) and corresponding Ti-6Al-4V prototype structures processed by electron beam melting (d–f), and (g–i) are the schematic illustration of buckling and bending vectors of applied stress on cell struts. (j) stress versus number of cycles to failure of mesh structures with cubic, G7 and rhombic dodecahedron structures [adapted from reference Citation23].](/cms/asset/c250837e-7096-4778-b10b-18380155e797/ianb_a_2278156_f0005_c.jpg)
In summary, by tuning the porosity and appropriately selecting the unit cell of graded cellular structured biomedical devices, including spine interbody fusion cages, the desired mechanical properties and biological functions can be obtained.
Cellular structures: biofunctions
We now succinctly describe the interplay between 3D printed cellular structure and biological functionality (osteoblast functions) and compare with solid Ti-6Al-4V alloy [Citation36,Citation37]. The seeding efficiency was low for cellular structures, but there was significant cell spreading and cell bridging of osteoblasts (), where the alkaline phosphate assay results suggested increase of enzyme activity with time for cellular structures. The interconnected porous structure in cellular structured titanium alloys enabled repair and reconstruction of tissue, even though there is change in the microenvironment [Citation8]. For instance, functionally graded cellular structured titanium alloy processed to minimize stress shielding and exhibit high energy absorption suggested that the structure facilitated flow of nutrients and cell proliferation across the entire structure [Citation38,Citation39].
Figure 6. Scanning electron micrographs of Ti-6Al-4V cellular structures with different porosity fabricated by electron beam melting (EBM) and corresponding osteoblast function [adapted from references Citation36,Citation37].
![Figure 6. Scanning electron micrographs of Ti-6Al-4V cellular structures with different porosity fabricated by electron beam melting (EBM) and corresponding osteoblast function [adapted from references Citation36,Citation37].](/cms/asset/a1783f5d-649a-4267-a5fc-ffde94193a13/ianb_a_2278156_f0006_b.jpg)
Rendering the surface of cellular structures bioactive is an aspect of significant interest to the biomedical community [Citation39,Citation40]. In this regard the cellular structures were modified via micro-arc oxidation and anodisation ( and ) [Citation41,Citation42]. Here the unique aspect of different surface oxidised characteristics of porous structure at different levels (macro-, micro, and nano), combined with bioactive titania, interconnected cellular architecture, and multimodal surface roughness was observed to increase the bioactivity [Citation41,Citation42] and provided an environment that was favourable to biological functions. Furthermore, the ability to transfer and move physiological fluid, oxygen, nutrients, and cells, simulating the fluid flow conditions of real bone, is another benefit of interconnected cellular architecture. The confluent sheet connects and integrates the cellular structure’s pores, which makes mechanical interlocking easier [Citation41,Citation42].
Figure 7. Computer-aided design profile of micro-arc oxidised Ti-6Al-4V alloy cellular structure (also referred as mesh structure) and SEM of micropores grown on the strut surface, bioactive apatite formation, and osteoblast-micropore interaction. Also presented are the fluorescent micrograph showing cell adhesion and expression of extracellular fibronectin, and optical micrograph of mineralised scaffold [adapted from reference Citation41].
![Figure 7. Computer-aided design profile of micro-arc oxidised Ti-6Al-4V alloy cellular structure (also referred as mesh structure) and SEM of micropores grown on the strut surface, bioactive apatite formation, and osteoblast-micropore interaction. Also presented are the fluorescent micrograph showing cell adhesion and expression of extracellular fibronectin, and optical micrograph of mineralised scaffold [adapted from reference Citation41].](/cms/asset/b195f95b-fbad-4551-aaf6-d93602c98e47/ianb_a_2278156_f0007_c.jpg)
Figure 8. Computer-aided design profile of anodised Ti-6Al-4V alloy cellular structure (also referred as mesh structure) and SEM of nanotubes grown on the strut surface, bioactive nanoscale apatite formation, and osteoblast-nanotube interaction. Also presented are the fluorescent micrograph representing cell proliferation and optical micrograph of mineralised scaffold [adapted from reference Citation42].
![Figure 8. Computer-aided design profile of anodised Ti-6Al-4V alloy cellular structure (also referred as mesh structure) and SEM of nanotubes grown on the strut surface, bioactive nanoscale apatite formation, and osteoblast-nanotube interaction. Also presented are the fluorescent micrograph representing cell proliferation and optical micrograph of mineralised scaffold [adapted from reference Citation42].](/cms/asset/674d6860-930e-4d72-8033-fc15a4ba9521/ianb_a_2278156_f0008_c.jpg)
Another approach that was adopted to biologically modify the surface was the use of bone morphogenetic protein-2 (BMP-2) and decellularized extracellular matrix. The bioactivity of the surface was not only enhanced but was also envisaged to increase the long-term stability and fixation of biomedical device with the surrounding bone [Citation7, Citation43,Citation44]. Surface modification with the osteoinductive growth factor, BMP, was viewed as an approach to enhance osteoinductivity and facilitate regeneration of bone tissue. The cellular structure together with pre-adsorbed protein (BMP-2) provided conditions that were favourable for bone tissue generation involving cell attachment and proliferation, synthesis of proteins, and mineralisation. The osteoinductive potential of cellular structures was increased, positively impacting cell signalling cascade to differentiate osteoblast cells into a more matured osteogenic phenotype. Other possibilities that were considered were incorporation of therapeutic drugs such as dexamethasone in the 3D printed cellular architecture [Citation43–46]. There is thus an opportunity to load therapeutic drugs during post-fabrication treatment [Citation7, Citation47–50].
Given that the vascularisation directly influences in vivo bone formation, in this regard, the interconnected porous cellular structure is favourable for the ingrowth of blood capillaries [Citation8, Citation51,Citation52] and the long-term success of 3D cellular structures under in vivo conditions is governed by the ability of the cellular structure to support angiogenesis (i.e. formation of new blood capillaries from the pre-existing blood vessels at the host site) [Citation8, Citation53–55].
In summary, additive manufacturing of 3D cellular structures with predefined and optimised architecture to obtain the desired mechanical properties enables cell alignment, spatial organisation of cells and vascularisation [Citation8, Citation56–58]. Moreover, in order to expedite the development of blood capillaries, especially when dealing with intricate 3D cellular constructs, it is typically advisable to undergo pre-treatment. This can be achieved by introducing growth factors like vascular endothelial growth factor (VEGF), which actively contribute to angiogenesis and hasten the healing process. This pre-treatment establishes a crucial connection between the host tissue’s blood vessels and endothelial cells, thereby facilitating the process of vascularisation [Citation8, Citation59,Citation60].
Illustrations of synthesis of protein (actin, vinculin, and fibronectin) and mineralisation in cellular structured titanium alloys is presented in [Citation36]. It is clear from these figures that capability of cells to migrate through the interconnecting cellular architecture led to colonisation of the complete structure. The cells penetrated and were embedded in the extracellular mineralised matrix and at later stages, the cells were embedded in a confluent layer of the extracellular matrix bridging the pores [Citation36].
Figure 9. Low and high magnification fluorescence micrographs illustrating the organisation and assessment of actin cytoskeleton (red) and vinculin focal contacts (green) of pre-osteoblasts cultured for 14 days on flat-Ti (control) and cellular Ti-mesh 3D-structures of different porosity (mesh 2 mm: porosity∼55%, mesh 3 mm: porosity∼77%, mesh 4 mm: porosity∼85%). (adapted from reference Citation36).
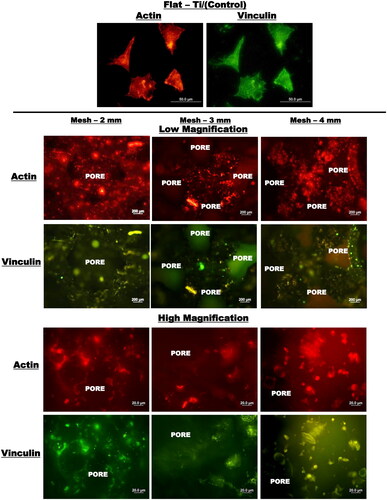
Figure 10. Low and high magnification fluorescence micrographs illustrating the organisation and assessment of actin cytoskeleton (red) and vinculin focal contacts (green) of pre-osteoblasts cultured for 14 days on flat-Ti (control) and cellular Ti-mesh 3D-structures of different porosity (mesh 2 mm: porosity∼55%, mesh 3 mm: porosity∼77%, mesh 4 mm: porosity∼85%) [adapted from reference Citation36].
![Figure 10. Low and high magnification fluorescence micrographs illustrating the organisation and assessment of actin cytoskeleton (red) and vinculin focal contacts (green) of pre-osteoblasts cultured for 14 days on flat-Ti (control) and cellular Ti-mesh 3D-structures of different porosity (mesh 2 mm: porosity∼55%, mesh 3 mm: porosity∼77%, mesh 4 mm: porosity∼85%) [adapted from reference Citation36].](/cms/asset/78081012-f5a9-4edf-98a7-70e8ae62ad06/ianb_a_2278156_f0010_c.jpg)
Figure 11. Low (left) and high (right) magnification optical micrographs of ARS-stained osteoblasts representing mineralisation of ECM on 3D-porous Ti-alloy mesh structure with different porosity (mesh 4 mm: porosity∼85%) after 14 days of incubation. High magnification areas are indicated by dotted squares in the low magnification images. Calcium-rich zones (red colour portion) on the structures represent the mineralised extracellular matrix. Dense mineralised ECM filling the pores on mesh structure of 2 mm and 3 mm indicate higher calcium content, in comparison to other structures [adapted from reference Citation36].
![Figure 11. Low (left) and high (right) magnification optical micrographs of ARS-stained osteoblasts representing mineralisation of ECM on 3D-porous Ti-alloy mesh structure with different porosity (mesh 4 mm: porosity∼85%) after 14 days of incubation. High magnification areas are indicated by dotted squares in the low magnification images. Calcium-rich zones (red colour portion) on the structures represent the mineralised extracellular matrix. Dense mineralised ECM filling the pores on mesh structure of 2 mm and 3 mm indicate higher calcium content, in comparison to other structures [adapted from reference Citation36].](/cms/asset/3cce37da-e4b5-42d7-9856-14a538e0a17a/ianb_a_2278156_f0011_c.jpg)
is an example of cellular structured implant of Ti-6Al-4V alloy replacements (acetabular cups and rod for osteonecrosis of femoral head) fabricated by electron beam melting. The cellular structured titanium alloy implant was characterised by elastic modulus similar to human bone, high mechanical stability, and good bone-material integration [Citation23].
Figure 12. An example of cellular structure implant of Ti-6Al-4V alloy fabricated by electron beam melting [adapted from reference Citation23].
![Figure 12. An example of cellular structure implant of Ti-6Al-4V alloy fabricated by electron beam melting [adapted from reference Citation23].](/cms/asset/86f17477-1edc-40a6-86fb-30ca8746b1cf/ianb_a_2278156_f0012_c.jpg)
Concluding remarks
Biomedical devices with a cellular structure, created using additive manufacturing, offer a promising and viable method for emulating the intricate, interconnected porous framework essential for achieving bone-like physical characteristics (such as low density) and mechanical attributes (including high strength, resistance to fatigue, and energy absorption). These desirable properties, coupled with enhanced biocompatibility, can be achieved through careful unit cell design, optimising relative density, and controlling pore volume fractions within the cellular structures. Moreover, from a biological perspective, this interconnected porous architecture promotes essential cellular functions such as attachment, proliferation, differentiation, and mineralisation.
Acknowledgements
R.D.K. Misra gratefully acknowledges numerous stimulating discussions with colleagues, notably, Professor L.E. Murr, Dr. K.C. Nune, Dr. A. Kumar, Dr. S.J. Li and many others with whom he had the privilege of working in recent years. This overview would not have been possible without the unstinted support of co-workers. The authors have attempted to consider selected diverse range of articles that have been published in the last one decade or so. Any omission is unintentional, particularly when only selected articles were considered during the preparation of overview. Furthermore, it is pertinent to mention that the present article is a brief or succinct overview with focus on highlighting the salient aspects of the current day trends in the fabrication of patient specific biomedical devices and not a comprehensive review article.
Data availability
Given that it is an overview article, data availability is not relevant. The authors have done their best in citing the relevant articles. However, inadvertently there may be situations where an article has not been appropriately cited. All figures adapted from references and reference number is indicated in the figure captions.
Disclosure statement
There are no conflicts of interest associated with this overview article and there are no financial or non-financial support that could have influenced its outcome.
Additional information
Funding
References
- Kumar A, Nune KC, Murr LE, et al. Biocompatibility and mechanical behavior of three-dimensional scaffolds for biomedical devices. Int Mater Rev. 2016;62(2):20–45.
- Vasireddi R, Basu B. Conceptual design of Three-Dimensional scaffolds for bone tissue engineering. Rapid Prototyp J. 2015;21(6):716–724. doi: 10.1108/RPJ-12-2013-0123.
- Schubert C, van Langeveld MC, Donoso LA. Innovations in 3D printing: a 3D overview from optics to organs. Br J Ophthalmol. 2014;98:159–161. doi: 10.1136/bjophthalmol-2013-304446.
- Klammert U, Gbureck U, Vorndran E, et al. 3D powder printed calcium phosphate implants for reconstruction of cranial and maxillofacial defects. J Cranio-Maxillofac Surg. 2010;38(8):565–570. doi: 10.1016/j.jcms.2010.01.009.
- Wang J, Yang M, Zhu Y, et al. Phage nanofibers induce vascularized osteogenesis in 3D printed bone scaffolds. Adv Mater. 2014;26(29):4961–4966.
- Zhao S, Zhu M, Zhang J, et al. Three dimensionally printed mesoporous bioactive glass and poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) composite scaffolds for bone regeneration. J Mater Chem B. 2014;2(36):6106–6118. doi: 10.1039/C4TB00838C.
- Nune KC, Li SJ, Misra RDK. Advancements in 3D titanium alloy mesh scaffolds. Sci China Mater. 2018;61(4):455–474. doi: 10.1007/s40843-017-9134-x.
- Murr LE, Gaytan SM, Medina F, et al. Next-generation biomedical implants using additive manufacturing of complex, cellular and functional mesh arrays. Philos Trans A Math Phys Eng Sci. 2010;368(1917):1999–2032. doi: 10.1098/rsta.2010.0010.
- Liulan L, Huicun AT, Qingxi Z, et al. F. The mechanical properties of bone tissue engineering scaffold fabricating via selective laser sintering. Life Syst Model Simulat. 2007;4689:146–152.
- Kumar A, Biswas K, Basu B. On the toughness enhancement in hydroxyapatite-based composites. Acta Mater. 2013;61(14):5198–5215. doi: 10.1016/j.actamat.2013.05.013.
- Kumar A, Biswas K, Basu B. Hydroxyapatite-Titanium bulk composites for bone tissue engineering applications: a review. J Biomed Mater Res-Part A. 2015;103(2):791–806. doi: 10.1002/jbm.a.35198.
- Wolff J. The law of bone remodelling., Translated by P. Maquet and R. Furlong’, 1986, Heidelberg, Springer-Verlag.
- Prendergast PJ, Huiskes R. The biomechanics of wolff’s law: recent advances. Ir J Med Sci. 1995;164(2):152–154.
- Dujovne AR, Bobyn JD, Krygier JJ, et al. Mechanical compatibility of noncemented hip prostheses with the human femur. J Arthroplast. 1993;8(1):7–22. doi: 10.1016/S0883-5403(06)80102-6.
- Engh CA, Bobyn JD. The infuence of stem size and extent of porous coating on femoral bone resorption after primary cementless hip arthroplasty. Clin Orthop Relat Res. 1988;231(&NA):7???28. doi: 10.1097/00003086-198806000-00002.
- Bignon A, Chouteau J, Chevalier J, et al. Effect of micro and macroporosity of bone substitutes on their mechanical properties and cellular response. J Mater Sci Mater Med. 2003;14(12):1089–1097. doi: 10.1023/b:jmsm.0000004006.90399.b4.
- Pompe W, Worch H, Epple M, et al. Functionally graded materials for biomedical applications. Mater Sci Eng A. 2003;362(1-2):40–60. doi: 10.1016/S0921-5093(03)00580-X.
- Xigeng M, Dan S. Graded/gradient porous biomaterials. Materials. 2010;3:26–47.
- Kelly A, Hideo N. Metallic scaffolds for bone regeneration. Materials. 2009;2:790–832.
- Zhao S, Li SJ, Hou WT, et al. The influence of cell morphology on the compressive behavior of Ti-6Al-4V meshes fabricated by electron beam melting. J Mech Behav Biomed Mater. 2016;59:251–264. doi: 10.1016/j.jmbbm.2016.01.034.
- Li SJ, Zhao S, Hou WT, et al. Functionally graded Ti-6Al-4V meshes with high strength and energy absorption. Adv Eng Mater. 2016;18(1):34–38. doi: 10.1002/adem.201500086.
- Wang QS, Li SJ, Hou WT, et al. Mechanistic understanding of Compression-Compression fatigue behavior of functionally graded Ti-6Al-4V mesh structure fabricated by electron beam melting. J Mech Behav Biomed Mater. 2020;103:103590. doi: 10.1016/j.jmbbm.2019.103590.
- Ren D, Li SJ, Wang H, et al. Fatigue behavior of Ti-6Al-4V cellular structures fabricated by additive manufacturing. J Mater Sci Technol. 2019;35(2):285–294. doi: 10.1016/j.jmst.2018.09.066.
- Cheng XY, Li SJ, Murr LE, et al. Compression deformation behavior of Ti-6Al-4V alloy with cellular structures fabricated by electron beam melting. J Mech Behav Biomed Mater. 2012;16:153–162. doi: 10.1016/j.jmbbm.2012.10.005.
- Li SJ, Xu QS, Wang Z, et al. Influence of cell shape on mechanical properties of Ti-6Al-4V meshes fabricated by electron beam melting method. Acta Biomater. 2014;10(10):4537–4547. doi: 10.1016/j.actbio.2014.06.010.
- Sun JF, Yang YQ, Wang D. Mechanical properties of a Ti6Al4V porous structure produced by selective laser melting. Mater. Des. 2013;49:545–552. doi: 10.1016/j.matdes.2013.01.038.
- Parthasarathy J, Starly B, Raman S, et al. Mechanical evaluation of porous titanium (Ti6Al4V) structures with electron beam melting (EBM). J Mech Behav Biomed Mater. 2010;3(3):249–259. doi: 10.1016/j.jmbbm.2009.10.006.
- Niendorf T, Brenne F, Schaper M. Lattice structures manufactured by SLM: on the effect of geometrical dimensions on microstructure evolution during processing. Metall Mater Trans B. 2014;45(4):1181–1185. doi: 10.1007/s11663-014-0086-z.
- Kadkhodapour J, Montazerian H, Darabi AC, et al. Failure mechanisms of additively manufactured porous biomaterials: effects of porosity and type of unit cell. J Mech Behav Biomed Mater. 2015;50:180–191. doi: 10.1016/j.jmbbm.2015.06.012.
- Ahmadi SM, Campoli G, Yavari SA, et al. Mechanical behavior of regular open-cell porous biomaterials made of diamond lattice unit cells. J Mech Behav Biomed Mater. 2014;34:106–115. doi: 10.1016/j.jmbbm.2014.02.003.
- Li K, Gao XL, Subhash G. Int. J. Solides. Struct. 42 (2005) 1777–1795. [61] K. Li, X.L. Gao, G. Subhash. J Mech Phys Solids. 2006;54(4):783–806. doi: 10.1016/j.jmps.2005.10.007.
- Côté F, Deshpande VS, Fleck NA, et al. The compressive and shear responses of corrugated and diamond lattice materials. Int J Solids Struct. 2006;43(20):6220–6242. doi: 10.1016/j.ijsolstr.2005.07.045.
- Melancon D, Bagheri ZS, Johnston RB, et al. Comp. Mater. Sci. 55 (2012) 1–9. [64] D. Melancon, Z.S. Bagheri, R.B. Johnston, L. Liu, M. Tanzer, D. Pasini. Acta Biomater. 2017;63:350–368. doi: 10.1016/j.actbio.2017.09.013.
- Zhang LC, Liu YJ, Li SJ, et al. Additive manufacturing of titanium alloys by electron beam melting: a review. Adv Eng Mater. 2018;20(5):1700842. doi: 10.1002/adem.201700842.
- Yavari SA, Ahmadi SM, Wauthle R, et al. Relationship between unit cell type and porosity and the fatigue behavior of selective laser melted meta-biomaterials. J Mech Behav Biomed Mater. 2015;43:91–100. doi: 10.1016/j.jmbbm.2014.12.015.
- Nune KC, Misra RDK, Gaytan SM, et al. Biological response of Next-Generation of 3D Ti-6Al-4V biomedical devices using additive manufacturing of cellular and functional mesh structures. J Biomater Tissue Eng. 2014;4(10):755–771. doi: 10.1166/jbt.2014.1232.
- Nune KC, Misra RDK, Gaytan SM, et al. Interplay between cellular activity and three‐dimensional scaffold‐cell constructs with different foam structure processed by electron beam melting. J Biomed Mater Res A. 2015;103(5):1677–1692. doi: 10.1002/jbm.a.35307.
- Nune KC, Kumar A, Misra RDK, et al. Functional response of osteoblasts in functionally gradient Ti-6Al-4V alloy mesh arrays processed by 3D additive manufacturing. Colloids Surf B Biointerfaces. 2017;150:78–88. doi: 10.1016/j.colsurfb.2016.09.050.
- Ponader S, Vairaktaris E, Heinl P, et al. Effects of topographical surface modifications of electron beam melted Ti-6Al-4V titanium on human fetal osteoblasts. J Biomed Mater Res A. 2008;84(4):1111–1119. doi: 10.1002/jbm.a.31540.
- Rapuano BE, Lee JJ, MacDonald DE. Titanium alloy surface oxide modulates the conformation of adsorbed fibronectin to enhance its binding to alpha(5) beta(1) integrins in osteoblasts. Eur J Oral Sci. 2012;120(3):185–194. doi: 10.1111/j.1600-0722.2012.954.x.
- Nune KC, Misra RDK, Li SJ, et al. The functional response of bioactive titania modified three-dimensional Ti-6Al-4V mesh structure toward providing a favorable pathway for intercellular communication and osteoincorporation. J Biomed Mater Res A. 2016;104(10):2488–2501. doi: 10.1002/jbm.a.35789.
- Nune KC, Misra RDK, Gai X, et al. The role of surface nanotopography on enhanced bioactivity and osteoconductive potential of anodized 3D printed Ti-6Al-4V alloy mesh structure. J Biomater Appl. 2018;32(8):1032–1048. doi: 10.1177/0885328217748860.
- Nune KC, Kumar A, Murr LE, et al. Interplay between self‐assembled structure of bone morphogenetic protein‐2 (BMP‐2) and osteoblast functions in three‐dimensional titanium alloy scaffolds: stimulation of osteogenic activity. J Biomed Mater Res A. 2016;104(2):517–532. doi: 10.1002/jbm.a.35592.
- Kumar A, Nune KC, Misra RDK. Biological functionality and mechanistic contribution of extracellular matrix-ornamented three dimensional Ti-6Al-4V mesh scaffolds. J Biomed Mater Res A. 2016;104(11):2751–2763. doi: 10.1002/jbm.a.35809.
- Liu H, Li W, Liu C, et al. Incorporating simvastatin/poloxamer 407 hydrogel into 3D-printed porous Ti-6Al-4V scaffolds for the promotion of angiogenesis, osseointegration and bone ingrowth. Biofabrication. 2016;8(4):045012. doi: 10.1088/1758-5090/8/4/045012.
- Kumar A, Nune KC, Murr LE, et al. Biocompatibility and mechanical behavior of three-dimensional scaffolds for biomedical devices: process-structure-property paradigm. Int Mater Rev. 2016;61(1):20–45. doi: 10.1080/09506608.2015.1128310.
- Wu BM, Borland SW, Giordano RA, et al. Solid free-form fabrication of drug delivery device. J Controlled Release. 1996;40(1–2):77–87. doi: 10.1016/0168-3659(95)00173-5.
- Katstra WE, Palazzolo RD, Rowe CW, et al. Oral dosage forms fabricated by three dimensional printing. J Control Release. 2000;66(1):1–9. doi: 10.1016/s0168-3659(99)00225-4.
- Wu W, Zheng Q, Guo X, et al. The controlled-releasing drug implant based on the three dimensional printing technology: fabrication and properties of drug releasing in vivo. J Wuhan Univ Technol-Mat Sci Edit. 2009;24(6):977–981. doi: 10.1007/s11595-009-6977-1.
- Yu DG, Zhu LM, Branford-White CJ, et al. Three-Dimensional printing in pharmaceutics: promises and problems. J Pharm Sci. 2008;97(9):3666–3690. doi: 10.1002/jps.21284.
- Kuboki Y, Jin Q, Takita H. Geometry of carriers controlling phenotypic expression in BMP-induced osteogenesis and chondrogenesis. J Bone Joint Surg. 2001;83(1 suppl 2):S1–105-S1-115. doi: 10.2106/00004623-200100002-00005.
- Tsuruga E, Takita H, Itoh H, et al. Pore size of porous hydroxyapatite as the cell-substratum controls BMP-induced osteogenesis. J Biochem. 1997;121(2):317–324. doi: 10.1093/oxfordjournals.jbchem.a021589.
- Yarlagadda P, Chandrasekharan M, Shyan J. Recent advances and current developments in tissue scaffolding. Biomed Mater Eng. 2005;15(3):159–177.
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6(4):389–395. doi: 10.1038/74651.
- Laschke MW, Harder Y, Amon M, et al. Angiogenesis in tissue engineering: breathing life into constructed tissue substitutes. Tissue Eng. 2006;12(8):2093–2104. doi: 10.1089/ten.2006.12.2093.
- Hutmacher DW, Sittinger M, Risbud M. Scaffold-based tissue engineering: rationale for computer-aided design and solid free-form fabrication systems. Trends Biotechnol. 2004;22:454–462.
- Novosel EC, Kleinhans C, Kluger PJ. Vascularization is the key challenge in tissue engineering. Adv Drug Deliv Rev. 2011;63(4-5):300–311. doi: 10.1016/j.addr.2011.03.004.
- Liu WF, Chen CS. Engineering biomaterials to control cell function. Mater Today. 2005;8(12):28–35. doi: 10.1016/S1369-7021(05)71222-0.
- Anderson CR, Ponce AM, Price RJ. Immunohistochemical identification of an extracellular matrix scaffold that microguides capillary sprouting in vivo. J Histochem Cytochem. 2004;52(8):1063–1072. doi: 10.1369/jhc.4A6250.2004.
- Ochman S, Frey S, Raschke MJ, et al. Local application of VEGF compensates callus deficiency after acute soft tissue trauma-results using a limb‐shortening distraction procedure in rabbit tibia. J Orthop Res. 2011;29(7):1093–1098. doi: 10.1002/jor.21340.
