?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
We began this study by synthesizing apatite fibers (AFs) and silicon-containing apatite fibers (Si-AFs) using a homogeneous precipitation method. Next, we have successfully fabricated three-dimensional apatite fiber scaffolds (AFSs) and silicon-containing apatite fiber scaffolds (Si-AFSs) using the above the AFs and Si-AFs, respectively. Finally, we successfully enhanced the mechanical properties of the scaffolds using carbon beads (CBs) with diameters of 20 μm and 150 μm, and conducted uniaxial pressing of the green compacts. These were named “AFS100,” “AFS300,” “Si-AFS100,” and “Si-AFS300” based on the AF (or Si-AF)/CB ratio. In the study, these scaffolds with enhanced mechanical property were implanted into porcine tibia, muscle and fat, and their osteoconduction and osteoinduction were examined. The bone-formation rates of Si-AFS100 and Si-AFS300 were higher than those of AFS100 and AFS300, while the bone-formation rates of AFS100 and Si-AFS100, were higher than those of AFS300 and Si-AFS300. These results indicated that addition of Si and controlled porosity may promote osteoconduction. Furthermore, AFS100 and Si-AFS100 exhibited osteoinductivity in muscle in the absence of osteoblasts. In conclusion, Si-AFS100 with enhanced mechanical properties has high osteoconductivity and osteoinductivity and can be expected to be used as a scaffold material and next-generation artificial bone filler for promoting bone formation.
1. Introduction
Hydroxyapatite [Ca10(PO4)6(OH)2; HAp] is a major inorganic component of human bone and teeth. That can bond directly with the host tissue; it has therefore been used as an implantation and scaffold material for bone tissue engineering [Citation1–Citation3]. Recent studies have shown the bioactivity of HAp to increase with the substitution of trace elements such as magnesium and zinc into its crystal lattice [Citation4,Citation5]. In particular, it is known that silicon (Si) can promote bone formation [Citation6]. Furthermore, Si ions can stimulate human umbilical vein endothelial cells to express the vascular endothelial growth factor strongly and promote angiogenesis [Citation7]. In fact, Si-substituted HAp (Si-HAp) ceramics exhibit higher bioactivity than pure HAp ceramics [Citation8].
The aim of the present study was to develop a novel scaffold material that promotes bone formation for bone tissue engineering. We successfully synthesized apatite fibers (AFs) using a homogeneous precipitation method [Citation9]. We then used these AFs to fabricate an apatitefiber scaffold (AFS), which is suitable for three-dimensional cell culture [Citation2]. Finally, we successfully enhanced the mechanical properties of these AFSs while maintaining their fiber integrity through uniaxial pressing of green compacts [Citation10].
Recently, we successfully synthesized silicon-containing apatite fibers (Si-AFs) and fabricated a silicon-containing apatitefiber scaffold (Si-AFS) [Citation11]. Our previous research showed that Si-AFS had a three-dimensional cell culture environment and that osteoblastic cells in/on Si-AFS achieved greater proliferation better than those in/on AFS due to eluted Si ions [Citation12,Citation13]. This result is in good agreement with a report by Keeting et al. [Citation14].
In this study, Si-AFSs with enhanced mechanical properties were implanted into porcine tibia, muscle and fat. The purpose of the study was to clarify the osteoconductivity and osteoinductivity of Si-AFSs by implantation into tibia and fat/muscle, respectively.
2. Materials
2.1. Fabrication of AFSs and Si-AFSs
As previously reported, Si-AFs were synthesized by a homogeneous precipitation method [Citation8]. The composition of Si-AF is described as Ca10(PO4)6-x(SiO4)x(OH)2-x. The starting solution was prepared by mixing Ca(NO3)2 · 4H2O (reagent grade, Wako Co., Japan), (NH4)2HPO4 (reagent grade, Wako Co.), Si(OC2H5)4 (TEOS; reagent grade, Wako Co.), (NH2)2CO (reagent grade, Wako Co.) and HNO3 (reagent grade, Wako Co.). The concentrations of TEOS were 0 (AF) and 0.8 (Si-AF) mass%, which are reported to promote bone formation and to be free from toxicity [Citation15,Citation16]. AF was prepared by heating the starting solution at 80°C for 24 h, followed by heating at 90°C for 72 h, while Si-AF was prepared by heating the starting solution at 80°C for 24 h, followed by heating at 95°C for 144 h [Citation11,Citation12].
Spherical CBs (Nikabeads; Nihon Carbon Company, Japan) with diameters measuring approximately 20 μm and 150 μm were added to the Si-AF slurry, which comprised 1 mass% Si-AFs in a mixed solvent of ethanol/water = 1/1 (v/v) at a CB/Si-AF (w/w) ratio of 1/1 or 3/1. The compacts for scaffolds were fabricated by pouring and vacuum pumping the CB-containing Si-AF slurry into the vinyl-chloride molds. The compacts were than uniaxially pressed at 30 MPa in metal molds with an internal diameter of 3.8 mm for implantation or of 4.2 mm for characterization. The resulting green compacts were fired at 1300°C for 5 h in a water-vapor atmosphere to fabricate Si-AFSs with enhanced mechanical properties. The completed scaffolds were named “AFS100,” “AFS300,” “Si-AFS100” and “Si-AFS300” depending on the amounts of CBs added.
The scaffolds were characterized as follows; The phase composition was determined by X-ray diffractometry (XRD; MiniFlex, Rigaku Co., Japan). The X-ray generator with Cu Kα was operated at 30 kV and 15 mA. Data were collected in the range of 2θ 4.0–50.0° with a step size of 0.04° and a count speed of 4°·min−1. Phase identification was achieved by comparing the diffraction patterns with the International Centre for Diffraction Data standard. Fourier transform infrared (FT-IR; IR Prestige21, Shimadzu Co., Japan) spectra were measured by the KBr tablet method. Spectra were obtained at a 4 cm−1 resolution over the range of 400–4000 cm−1 with a scan speed of 2.5 mm·s−1. The microstructures were observed by scanning electron microscopy (SEM; JSM6390LA, JEOL Ltd., Japan). The porosity and compressive strength of AFS and Si-AFS specimens (4.2 mm diameter) were determined, together with the specific surface area and water absorption. The specific surface area of specimens was determined by the Brunauer-Emmett-Teller method using a surface area analyzer (Flowsorb III 2310, Shimadzu Co.) [Citation17]. Water absorption was measured according to the Japanese Industrial Standard R 2205. Specifically, the water absorption rate was calculated from the amount of water absorbed into the scaffolds by vacuum aspiration divided by the dry mass of the scaffolds (following formula);
In the calculation, the water density was set at 1 g·cm−3. Moreover, the amounts of dissolved Ca2+, PO43- and SiO44- ions in α-minimum essential medium (α-MEM) with 10% fetal bovine serum (FBS) were measured by inductively coupled plasma atomic emission spectrometry (ICP-AES; SPS7800, SII Nano Technology, Japan). The α-MEM was exchanged every 2 days, and the amounts of dissolved ions were cumulated.
2.2. Implantation method
This study was approved by the Institutional Animal Care and Use Committee, Meiji University, and carried out in accordance with the Meiji University School of Agriculture Animal Experimentation Regulations. Three female pigs [one wild pig (sus scrofa) and two transgenic cloned pigs with the humanized Kusabira-Orange (huKO) gene [age: 8 months, body weight: approximately 140 kg] were used in this study [Citation18]. All the pigs were in good health during the experimental period. Prior to surgery, all scaffolds were dry-sterilized at 160°C for 90 min. In the wild pig, three samples each of AFS300 and Si-AFS300 were implanted into the tibia, muscle and fat. In the huKO pigs, two samples each of AFS100, AFS300, Si-AFS100 and Si-AFS300 were implanted into the tibia, muscle and fat.
The pigs were put to sleep with general anesthesia. After shaving, a tibia was exposed by incising the skin and periosteum. Cylindrical defects measuring 3.8 mm in diameter were thin drilled into the tibia. The scaffolds were inserted into the drill holes before closing the skin and periosteum. The scaffolds were then inserted into the pocket after incising the muscle or fat, and the skin and muscle or fat were closed. All the pigs were sacrificed 12 or 13 weeks after surgery.
2.3. Histological evaluation
The specimens removed from the tibia were observed using X-ray micro-computed tomography (micro-CT; inspeXio SMX-90CT, Shimadzu Co.). The specimens were then sectioned without decalcification and stained with Villanueva bone (VB) staining to measure the bone formation rates using image analysis software (WinROOF, Mitani Co., Japan). The bone formation rates were calculated after extracting the stained portions of in the macropores as bone formation areas by means of the following formula:
Specimens taken from the muscle and fat were sectioned after decalcification and stained with hematoxylin and eosin (HE) and Masson’s trichrome (MT) for microscopic observation.
3. Results
3.1. Characterization of AFSs and Si-AFSs
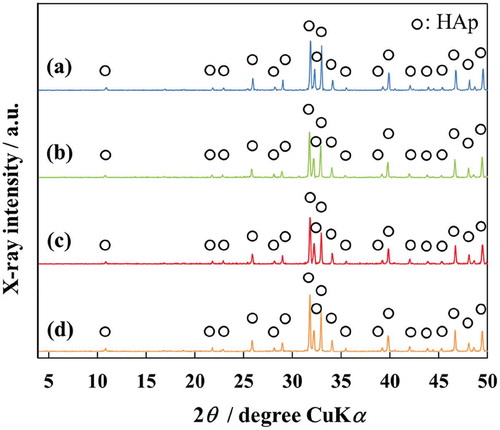
XRD patterns of AFSs and Si-AFSs are shown in . The XRD patterns indicate that all the scaffolds were single-phase HAp. They also show that, the substitution of AFS with Si did not affect phase compositions. The FT-IR spectra of AFSs and Si-AFSs show absorptions of PO43- groups at 1300–900, 600 and 570 cm−1 and absorptions of OH− groups at 3570 cm−1. SEM images of AFSs and Si-AFSs are shown in . All the scaffolds contain many macropores due to burning out of CBs and many interconnected micropores resulting from entangled AFs.
Figure 2. SEM micrographs of scaffolds: (a) AFS100, (b) AFS300, (c) Si-AFS100, and (d) Si-AFS300. Macropores and micropores were present (dashed red circles and green asterisks).

The other scaffold characteristics are shown in . As the amounts of CBs were increased, porosity, specific surface area and water absorption increased, while compressive strength decreased. The substitution of AFS with Si did not affect these characteristics.
Table 1. Some properties of AFSs and Si-AFSs.
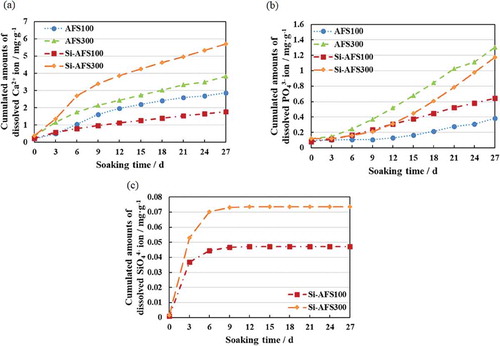
The amounts of dissolved Ca2+, PO43- and SiO44- ions in α-MEM are shown in . As the amounts of CBs were increased, the amounts of all dissolved ions increased. Si-AFS300 had the largest amount of dissolved Ca2+ ions and Si-AFS100 the smallest amount. When the amounts of CBs were equal, on the other hand, Si-AFS eluted more PO43- ions than AFS.
3.2. Histological evaluation
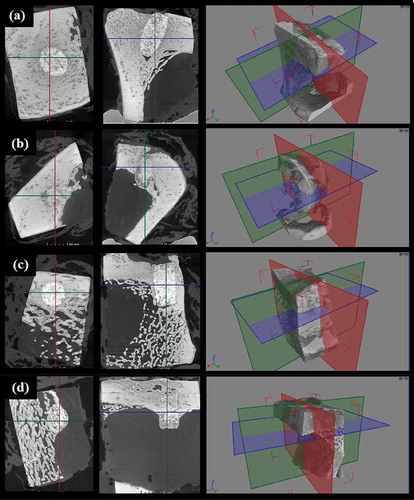
Micro-CT images of specimens taken from the tibia are shown in . Biointegration occurred between the bone and scaffold. In addition, scaffolds were present across the cortical bone and medullary canal without collapsing.
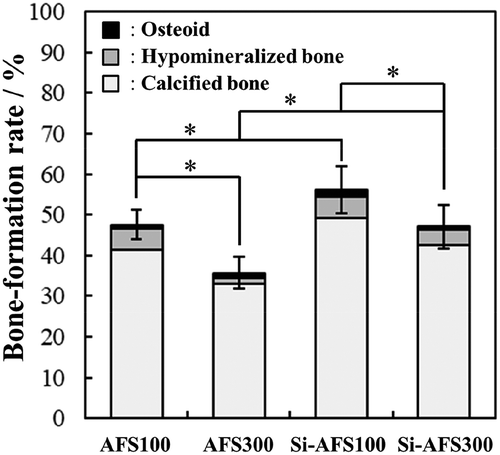
The VB staining results of specimens taken from the tibia are shown in and . Histological observation of the visible light showed that the cells with a light purple appearance penetrated into the center of the scaffolds. In addition, the edges of the scaffolds were dissolved, and the dissolution area increased as the amount of CBs increased. Histological observation under fluorescent light showed the presence of calcified bone and the partial presence of hypomineralized bone and osteoid. The bone formation rates calculated by image analysis are shown in . The bone formation rates for AFS100, Si-AFS100, AFS300 and Si-AFS300 were 47.6%, 35.7%, 56.2% and 47.0%, respectively.
Figure 5. VB staining of AFS100 and AFS300. M: materials, O: osteoid, H: hypomineralized bone, C: calcified bone. High magnification: highly magnified images of dashed square areas. Visible light: osteoid: purplish red; hypocalcified bone: orange; calcified bone: light brown. Fluorescence: osteoid: red; hypocalcified bone: orange; calcified bone: green.
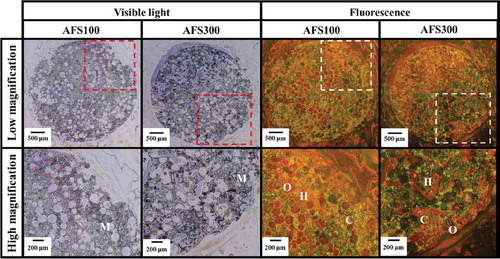
Figure 6. VB staining of Si-AFS100 and Si-AFS300. M: materials, O: osteoid, H: hypomineralized bone, C: calcified bone. High magnification: highly magnified images of dashed square areas. Visible light: osteoid: purplish red; hypocalcified bone: orange; calcified bone: light brown. Fluorescence: osteoid: red; hypocalcified bone: orange; calcified bone: green.
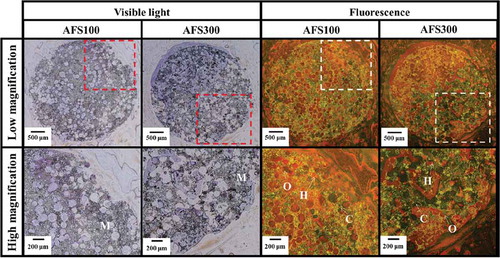
Figure 7. Bone-formation rates of AFSs and Si-AFSs. Error bars represent the standard deviation. (n = 4 (AFS100, Si-AFS100) or 7 (AFS300, Si-AFS300), *p < 0.05).
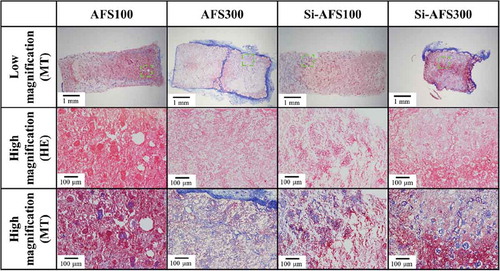
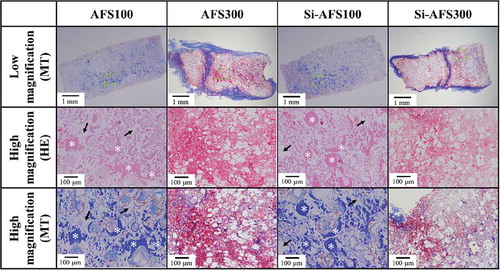
The HE and MT staining results for specimens taken from the muscle are shown in . Histological observation showed that, among specimens implanted in the muscles, bone formed inside the pores of AFS100 and Si-AFS100. In addition, the pores of specimens implanted in the fat were filled with fibrous tissue, as shown in . As evidenced in , osteogenesis occurred in the case of muscle implantation, but scarcely occurred in the case of fat implantation.
Table 2. Scores of bone-formed specimens after implantation.
Figure 8. HE and MT staining of specimens removed from the muscle (decalcified section). High magnification: highly magnified images of dashed square areas. HE staining: nucleus: blue; cell membrane: red. MT staining: muscle fiber: red; collagen fiber: violet. White asterisk: bone formation area. Black arrow: residual material.
4. Discussion
In this study, scaffolds of pure HAp and 0.8 mass% Si-HAp were fabricated. All scaffolds used in the study were single-phase HAp, and no secondary phases were detected for Si-AFS100 and Si-AFS300. These results indicated that SiO44- ions were incorporated into the PO43- site of the HAp lattice [Citation8,Citation19].
All four kinds of scaffolds tested, AFS100, AFS300, Si-AFS100 and Si-AFS300, had large numbers of macropores and micropores. Although the compressive strength of all the scaffolds was lower than that of to cortical bone, that of AFS100 and Si-AFS100 was higher than that of cancellous bone [Citation20,Citation21]. Macropores are suitable for three-dimensional cell culture and enable bone ingrowth [Citation22]. They also permit nutrient circulation. Hence, their higher porosity is considered to be advantageous for bone formation. The scaffolds’ porosity and other mechanical properties were dependent on the amounts of CBs. Increases in the amounts of ions dissolved from the scaffolds may be due to the increase of the specific surface area. Measurement of ion amounts was carried out in α-MEM with FBS to mimic the in vivo environment. The amounts of dissolved Ca2+ and PO43- ions were lower than these present in the original α-MEM. Based on these results, it was considered that calcium phosphate was precipitated on the scaffolds. The release of SiO44- ions plateaued within a week. The amounts of SiO44- ions in α-MEM were close to levels that promote cell proliferation and bone formation [Citation23]. Elution of SiO44- ions is therefore thought to enhance the bone formation ability of Si-AFS100 and Si-AFS300.
The in vivo results showed that bone formation occurred in all specimens implanted in the tibia and in AFS100 and Si-AFS100 implanted in muscle. While AFSs had excellent osteoconductivity, Si-AFSs showed significantly higher osteogenesis than AFS. It is considered that dissolved/remaining SiO44- ions promoted bone formation. The bone-formation rates of AFS100 and Si-AFS100 were higher than those of AFS300 and Si-AFS300. This is due to their high porosity; thus, there is a possibility that the bone formation rates may increase when scaffolds are implanted for a longer term.
Yuan et al. have reported that osteoinduction occurs in the order of vascular invasion, monocyte differentiation, osteoclast resorption and new bone formation [Citation24]. In order for osteoinduction to occur, moreover micropores for nutrient circulation, macropores for bone formation and mild dissolution of materials to provide a Ca/P ratio suitable for osteogenesis are important [Citation24]. The four kinds of scaffolds tested in the present study have interconnected micropores for angiogenesis and three-dimensional macropores for osteogenesis. Eluted Si ions are particularly effective on promoting angiogenesis in Si-AFSs [Citation7]. In order for osteoclasts to adhere and material remodeling to occur, however, the macropores must be protected from fast body fluid circulation. Therefore, bone formation did not occur in AFS300 and Si-AFS300. No bone formation occurred in any specimens implanted in fat, moreover, because the spaces suitable for osteogenesis were filled with fibrous tissue. These results show that scaffolds with an appropriate porosity (approximately 70%) such as AFS100 and Si-AFS100 invite osteoinduction.
5. Conclusion
AFSs and Si-AFSs with similar mechanical properties were fabricated. The in vivo results of this study show that AFSs and Si-AFSs have excellent osteoconductivity. Si-AFS100, in particular showed a significantly higher bone formation rate than the other scaffolds tested. In experiments investigating osteoinduction, osteogenesis was observed in the pores of AFS100 and Si-AFS100 in muscle tissue. These results indicate that Si-AFS100 has exceptional potential for use as a novel bioceramic with both excellent osteoconductivity and osteoinductivity for bone tissue engineering and next-generation artificial bone grafting.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Hench LL. Bioceramics. J Am Ceram Soc. 1998;81:1705–1728.
- Aizawa M, Shinoda H, Uchida H, et al. In vitro biological evaluations of three-dimensional scaffold developed from single-crystal apatite fibres for tissue engineering of bone. Phosphorus Res Bull. 2004;17:262–268.
- Honda M, Fujimi T, Izumi S, et al. Topographical analyses of proliferation and differentiation of osteoblasts in micro- and macro pores of apatite-fiber scaffold. J Biomed Mater Res A. 2010;94A:937–944.
- Landi E, Logroscino G, Proietti L, et al. Biomimetic Mg-substituted hydroxyapatite: from synthesis to in vivo behaviour. J Mater SciMater Med. 2008;19:239–247.
- Santos MH, Valerio P, Goes AM, et al. Biocompatibility evaluation of hydroxyapatite/collagen nanocomposites doped with Zn+2. Biomed Mater. 2007;2:135–141.
- Carlisle EM. Silicon: a possible factor in bone calcification. Science. 1970;167:279–280.
- Li H, Chang J. Bioactive silicate materials stimulate angiogenesis in fibroblast and endothelial cell co-culture system through paracrine effect. Acta Biomater. 2013;9:6981–6991.
- Gibson IR, Best SM, Bonfield W. Chemical characterization of silicon-substituted hydroxyapatite. J Biomed Mater Res. 1999;44:422–428.
- Aizawa M, Poter AE, Best SM, et al. Syntheses of silicon-containing apatite fibres by a homogeneous precipitation method and their characterization. Key Eng Mater. 2006;309–311:1129–1132.
- Motojima S, Morisue H, Matsumoto M, et al. Development of Apatite-fiber scaffold with enhanced mechanical property via Uniaxial Pressing and Their Biocompatibility. Bioceramics. 2009;22:177–180.
- Aizawa M, Poter AE, Best SM, et al. Ultrastructural observation of single-crystal apatite fibres. Biomaterials. 2005;26:3427–3433.
- Kinoshita Y, Best SM, Aizawa M. Fabrication And Evaluation Of Silicon-Containing Apatite Fiber Scaffolds For Bone Tissue Engineering. Phosphorus Res Bull. 2012;26:101–104.
- Kinoshita Y, Best SM, Aizawa M. In vitro evaluation of silicon-containing apatite fiber scaffolds for bone tissue engineering. Key Eng Mater. 2012;529–530:391–396.
- Keeting PE, Oursler MJ, Wiegand KE, et al. Zeolite a increases proliferation, differentiation, and transforming growth factor beta production in normal adult human osteoblast-like cells in vitro. J Bone Miner Res. 1992;7:1281–1289.
- Hing KA, Revell PA, Smith N, et al. Effect of silicon level on rate, quality and progression of bone healing within silicate-substituted porous hydroxyapatite scaffolds. Biomaterials. 2006;27:5014–5026.
- Zou S, Ireland D, Brooks RA, et al. The effects of silicate ions on human osteoblast adhesion, proliferation, and differentiation. J Biomed Mater Res Part B Appl Biomater. 2009;90:123–130.
- Brunauer S, Emmett PH, Teller E. Adsorption of gases in multimolecular layers. J Am ChemSoc. 1938;60:309–319.
- Matsunari H, Onodera M, Tada N, et al. Transgenic-cloned pigs systemically expressing red fluorescent protein, Kusabira-Orange. Cloning Stem Cells. 2008;10:313–323.
- Patel N, Best SM, Bonfield W, et al. A comparative study on the in vivo behavior of hydroxyapatite and silicon substituted hydroxyapatite granules. J Mater SciMater Med. 2002;13:1199–1206.
- Bayraktar HH, Morgan EF, Niebur GL, et al. Comparison of the elastic and yield properties of human femoral trabecular and cortical bone tissue. J Biomech. 2004;37:27–35.
- Evans FG. Mechanical properties of bone. USA: Charles C. Tomas Publisher; 1973. p. 83.
- Morisue H, Matsumoto M, Chiba K, et al. In vivo bone formation using three-dimensional scaffoldsdeveloped from a single crystal apatite fiber. J Biomed Mater Res A. 2008;90:811–818.
- Reffitt DM, Ogston N, Jugdaohsingh R, et al. Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro. Bone. 2003;32:127–135.
- Yuan H, Yang Z, Li Y, et al. Osteoinduction by calcium phosphate biomaterials. J Mater SciMater Med. 1998;9:723–726.