?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Securing a stable supply of dysprosium (Dy) is one of the major problems facing the automotive industry due to increased Dy consumption and low-volume production. Investigations of new recycling methods are therefore extremely important. In this study, we focused on mesoporous silica particles (MPS) with tunable nanoscale mesopores (2–50 nm), large surface areas, high pore volumes, and abundant surface chemistry. To perform adsorption experiments with several metal ions in the solution, the silica materials were treated via amino functionalization using silane coupling reagents, 3-aminopropyltriethoxysilane (APTES), N-(2-aminoethyl)-3-aminopropyltriethoxysilane (N2APTES), and N-(6-aminohexyl)-3-aminopropyltrimethoxysilane (N6APTES), as well as via carboxyl functionalization using diglycolic acid. Interestingly, MPS sheet-2HNHCOOH, a product with a sheet-like morphology, exhibited enhanced Dy ion adsorption (13.3 µg/mg). The sheets retained approximately100% in the initial capacity after 10 cycles. The results suggest that MPS sheet-2HNHCOOH is an appropriate a candidate material for selective Dy ion adsorption.
Graphical abstract
1. Introduction
Dysprosium (Dy), a rare earth element that is used as an additive in neodymium magnets, has been widely used in the automobile industry in recent years [Citation1]. In 2013, 90% of the global production of rare earth elements came from China. Their widespread use is therefore constantly being evaluated [Citation2]. Due to the increased consumption and high cost of Dy, investigations into new recycling methods are extremely important [Citation2]. Many studies have been reported on collected ion of metals, and rare earth ions have been reported [Citation3–Citation6]. In previous reports by Ogata et al., selective adsorption of Dy ions (0.146 mmol/g) from heavy metal ion mixtures was achieved using silica gel with carboxyl functionalization on the surface [Citation2]. This selective adsorption was thought to be based on the formation of complexes by long carboxyl functionalized groups and the outermost shells of Dy ions [Citation7,Citation8]. However, these materials have insufficient adsorption capacity.
Mesoporous silica (MPS) has unique properties, including a controlled, tunable pore size (2–50 nm), large surface area, and large pore volume, and its surface can be easily functionalized [Citation9–Citation15]. MPS particles were synthesized for the first time in 1992, which open the new application fields as enzyme carriers, columns, absorbents, etc., [Citation16–Citation20]. Generally speaking, increases in the adsorption amount depend on the improvement of the adsorption sites on the adsorbents. This makes MPS particles attractive for Dy adsorption. In our previous reports, we focused on the morphologies of three MPS particles, including spherical, sheet, and Stöber particles with DNA adsorbed on them [Citation21]. The results showed that the DNA adsorption was changed by varying the particle morphologies because the amount of DNA adsorption was greatly influenced by giving the MPS particles long chains and a large number of functionalized groups on the surface. We postulated that the functionalized groups on MPS surfaces could be optimized by changing the morphologies. Based on these results, it is expected that MPS with various morphologies could contribute to increasing the adsorptive capacity of Dy ions.
In this study, we prepared MPS particles with sheet and spherical morphologies as well as non-porous Stöber silica. Their surfaces were functionalized by replacing the silane coupling reagents, including 3-aminopropyltriethoxysilane (APTES), N-(2-aminoethyl)-3-aminopropyltriethoxysilane (N2APTES), and N-(6-aminohexyl)-3-aminopropyltrimethoxysilane (N6APTES), with amino and carboxyl groups as well as with carboxyl group-functionalization reagents, such as diglycolic anhydride. Subsequently, selective Dy ion adsorption experiments were carried out using the functionalized MPS particles. The aim of this study was to investigate selective Dy ions adsorption on amino- and, carboxyl-functionalized MPS particles with different morphologies.
2. Materials and methods
2.1. Materials and characterization
Palmitoyl chloride, l-alanine, cetyltrimethylammonium chloride (CTAC), nitric acid, metal ion (Cu, Dy, Fe, Nd, and Zn) standard solutions were obtained from Wako Pure Chemical Industries, Japan. Triblock copolymer Pluronic P123 was purchased from Sigma-Aldrich, St. Louis, MO. Tetraethoxysilane (TEOS, Shin-Etsu Chemical Co., Japan) was used as the silica source. 3-Aminopropyltriethoxysilane (APTES, Shin-Etsu Chemical Co., Country), N-(2-aminoethyl)-3-aminopropyltriethoxysilane (N2APTES), and N-(6-aminohexyl)-3-aminopropyltrimethoxysilane (N6APTES) (AZmax Co., Japan) were selected for functionalization of amino groups on silica. Diglycolic anhydride was purchased from Tokyo Chemical Industry Co., Japan.
Field emission scanning electron microscopy (FE-SEM, S4300, Hitachi Co., Japan) and transmission electron microscopy (JEM 2010, JEOL, Japan) were used at an acceleration voltage of 10 kV and an operating voltage of 200 kV, respectively, to characterize the morphologies of the synthesized silica particles. The silica pore characteristics were analyzed by the Brunauer-Emmett-Teller (BET) and Barret-Joyner-Halenda (BJH) methods from the data for nitrogen (N2) adsorption-desorption measurements [Citation22]. Amino- and carboxyl-functionalization of the particle surfaces were analyzed by Fourier transform-infrared (FT-IR) spectra using an FT/IR-4700 (JASCO Co., Japan). The samples were prepared by pelletizing silica with KBr. The amount of functionalization was estimated by thermogravimetry (TG) using a Thermo Plus TG 8120 (Rigaku Co., Japan). To investigate the surface charge, the zeta-potentials of the samples were investigated using ELS-Z (Otsuka Electronics Co., Japan). Metal ion concentrations in the solution were measured by ICP-OES using an IRIS Advantage (Thermo Fisher Scientific Inc., Waltham, MA).
2.2. Preparation of MPS
We synthesized sheet-like and spherical MPS particles (denoted MPS sheet and spherical MPS herein), and spherical colloidal silica (Stöber). These silica particles were functionalized by amino silanes containing aminopropyl (-NH2), ethylene-diamine (-2ENH2), and hexylene-diamine (-2HNH2) groups. Next, carboxyl-functionalization was performed on these amino-functionalized silica particles using diglycolic anhydride (-NHCOOH, -2ENHCOOH, and -2HNHCOOH), as shown in Scheme S1. MPS sheet with hexylene-diamine groups was denoted MPS sheet-2HNH2. MPS sheet-2HNH2 functionalized with diglycolic anhydride was denoted MPS sheet-2HNHCOOH. Spherical MPS and Stöber silica were denoted similarly.
2.2.1. MPS sheet
MPS sheet particles were synthesized using a dual templating route with N-palmitoyl-l-alanine (C16-l-Ala) consisting of surfactants prepared with palmitoyl chloride, l-alanine and Pluronic P123 [Citation23]. C16-l-Ala (0.31 g) was dissolved in a solution of deionized water (25.5 mL) and sodium hydroxide (0.1 M, 10 mL), and the mixture was stirred for 2 h. After dissolving another surfactant, P123 (0.3 g), and stirring the mixture for 1 h, hydrochloric acid (0.1 M, 4.5 mL) was added. TEOS (1.46 g) and APTES (0.28 g) were then added dropwise to the mixture at 4°C. The mixture was stirred for 3 h, and then left to stand for 24 h at room temperature. After centrifugation, the precipitate was heated for 24 h at 100°C. The solid product was washed with deionized water, ethanol, and acetone. To remove the surfactant from the particle pores, calcination was carried out at 500°C for 4 h.
2.2.2. Spherical MPS
CTAC (3.52 g) was added to sodium chloride (1 M, 2.28 mL), deionized water (440 g), and methanol (360 g). The mixture was stirred until the surfactant dissolved. TEOS (1.32 g) was dropped into the mixture under vigorous stirring. After the mixture had been stirred for 6 h and then left to stand overnight, the solids were collected by centrifugation and washed using ethanol and acetone. Finally, the surfactant was removed as described above [Citation24].
2.2.3. Stӧber silica
TEOS (1.46 g) and APTES (0.28 g) were added to a mixture of deionized water (17.7 g), methanol (175 mL), and ammonia solution (28%, 14.7 M, 7.2 g). After stirring for 3 s, the mixture was stored overnight. The precipitate was collected by centrifugation and washed using ethanol and acetone. The product was calcined as described above [Citation25].
2.3. Amino- and carboxyl-functionalization on silica
xCarboxyl-functionalized silica was prepared using diglycolic anhydride on amino-functionalized silica [Citation26,Citation27]. Synthesized silica (50 mg) was dispersed in toluene (10 mL) containing an amino-silane reagent (1.0 mL), such as (3-aminopropyl)triethoxysilane (APTES), N-(2-aminoethyl)-3-aminopropyltriethoxysilane (N2APTES) or N-(6-aminohexyl)-3-aminopropyltrimethoxysilane (N6APTES). The suspension was then refluxed for 6 h. The particles were centrifuged, washed using ethanol and acetone, and dried.
The amino-functionalized silica was dispersed again in a mixture of ethanol (5 mL) and 5 M HCl (5 mL). The suspension was heated at 80°C in an oil bath for 6 h. Toluene (15 mL) was added after the materials were collected, and the mixture was stirred in a water bath at 50°C for 10 min. Diglycolic anhydride (173 mg) was added, and the mixture stirred overnight at 20°C. The particles were separated by centrifugation, washed using ethanol and acetone, and dried.
2.4. Metal ion adsorption
Silica particles (2.0 mg) were added to deionized water (2 mL), and a metal ion solution containing Cu, Dy, Fe, Nd, and Zn (12.5 ppm, pH = 1.25, 1.5, and 1.75, 8 mL) was added. The mixture contained 2.0 mg of silica particles and 10 mL of metal ion solution (10 ppm). After stirring of the mixture at 20°C for 2 h, centrifugation was performed. The amount of metal ion adsorption was calculated from the ion concentrations of the supernatant measured by ICP-OES. The amount of Dy ions adsorbed on the prepared MPS was calculated using Equation (1):
where QI and QE are the initial and equilibrium Dy ions concentrations (µg/mL) in sample solutions, respectively. I0 is the initial Dy ion content in the solution.
2.5. Adsorption isotherms
To evaluate the adsorption mechanism, isotherm equilibrium adsorption experiments were performed at 20°C. The Langmuir isotherm model is defined as monolayer coverage of adsorbents over a homogenous surface, while the Freundlich isotherm expression is multilayer adsorption on the surface [Citation28]. The two kinds of isotherm models, Langmuir and Freundlich, are expressed as follows:
where Qe (µg/mg) is the adsorption capacity of metal ions on MPS expressed by the equations, and Cs (mg/L) represents the equilibrium concentration. In the above equation, Qm is the maximum adsorption capacity of the monolayer surface coverage on MPS. KL (L/g) and KF (mg/g) represent the affinity constants of the Langmuir and Freundlich plots, respectively. 1/n indicates the Freundlich coefficient.
2.6. Cycling test
The prepared carboxyl-functionalized MPS particles were cycled, as follows: Carboxyl-silica particles, MPS-2HNHCOOH, were dispersed in deionized water (2 mL). Dy ion solution (8 mL, pH 1.75, 12.5 ppm) was added to the suspension with stirring at 20°C for 2 h. After solid-liquid separation by centrifugation, the supernatant was collected to investigate the amount of Dy adsorption. To elute the Dy ions, 1 M HNO3 was added to the precipitate. The mixture was then stirred 20°C for 4 h. The particles and supernatants were recollected by centrifugation. The collected precipitate was washed twice with water. The Dy adsorption and elution processes were repeated 10 times. The amounts of Dy ion adsorption and elution were calculated from the amounts of Dy remaining in the supernatant after adsorption and elution. To evaluate the cycling properties of MPS-2HNHCOOH, the value of the cycling capacity (QC) was calculated by Equation (4), based on the amounts of Dy ion adsorption in the solution before (Qb) and after desorption (Qa):
3. Results and discussion
3.1. Characterization of synthesized MPSs
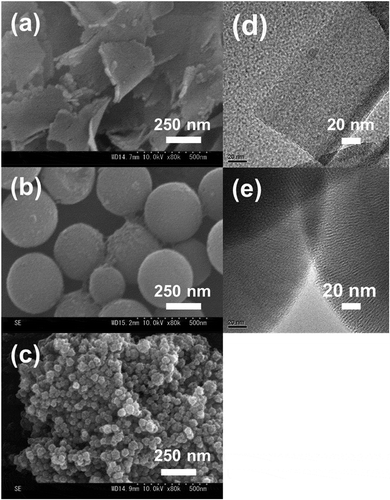
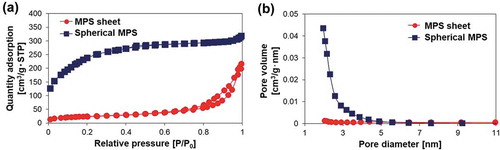
The synthesized silica particles were characterized using FE-SEM, TEM, and N2 adsorption-desorption isotherms. shows FE-SEM and TEM images of the synthesized silica particles. The MPS sheets with sheet-like morphologies had a thickness of ~100 nm and a width of ~1 μm (,d)). Spherical MPS particles with a particle size of approximately 400 nm can be observed in ,e). The Stöber silica contained large numbers of particle aggregations with a particle size of approximately 50 nm ()). Nitrogen adsorption-desorption isotherms were measured to investigate the presence of pores, pore size, surface area, and pore volume (). The MPS sheet and spherical MPS contained type IV isotherms, indicating the presence of mesopores on silica particles ()). Additionally, the hysteresis loops of MPS sheet and spherical MPS were displayed as types H1 and H4, respectively. The pore size obtained by pore size distribution ((b)) was <2.0 nm for both MPS sheet and spherical MPS. The values for the specific surface areas of MPS sheet, spherical MPS, and Stöber silica were 122, 888, and 120 m2/g, respectively. The pore volumes of MPS sheet and spherical MPS were 0.27 and 0.47 cm3/g. These results are listed in . Spherical MPS has a seven times larger surface area (888 m2/g) than MPS sheet (122 m2/g). The pore volume of spherical MPS was 0.47 cm3/g, which was larger than that of than MPS sheet (0.27 cm3/g).
Scheme 1. Schematics of amino-functionalized mesoporous silica (MPS) materials employing (a) APTES (-NH2), (b) N-(2-aminoethyl)-3-aminopropyltriethoxysilane (-2ENH2) and (c) N-(6-aminohexyl)-3-aminopropyltrimethoxysilane (-2HNH2), followed by carboxyl-functionalized MPS materials employing diglycolic anhydride: (c) -NHCOOH, (d) -2ENHCOOH, and (e) -2HNHCOOH, respectively.
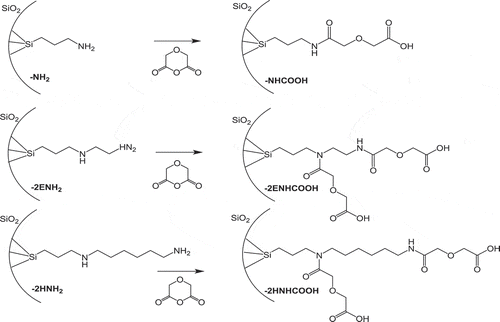
Table 1. Structure characteristics of MPS and silica particles.
3.2. Amino- and carboxyl functionalization on MPSs
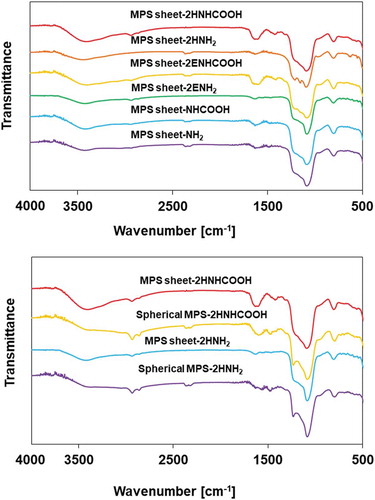
Amino- and carboxyl-functionalization on silica particles was confirmed by FT-IR and TG. Functional groups on silica particles were confirmed from the FT-IR spectra. shows the FT-IR spectra of silica-NH2 and -NHCOOH. Amino-functionalized silica particles have FT-IR peaks at approximately 2930 and 2850 cm−1, corresponding to the C-H stretching, and at approximately 1620 cm−1, corresponding to the N-H vibration. The FT-IR spectra display Si-O-Si vibrations at 1084 cm−1. After carboxyl functionalization, the transmittance at 2930 and 2850 cm−1 was lower than that of amino-silica particles. The FT-IR peak was slightly shifted to 1610 cm−1 and broadened. In addition, a peak appeared at 1430 cm−1. These results indicated that silica-NHCOOH contained carboxyl functional groups.
Figure 3. Fourier-transform IR (FT-IR) absorbance spectra of MPS sheet-2HNHCOOH, MPS sheet-2HNH2, MPS sheet-2ENHCOOH, MPS sheet-2ENH2, MPS sheet-NHCOOH, MPS sheet-NH2, MPS sheet-2HNHCOOH, spherical MPS-2HNHCOOH, MPS sheet-2HNH2, and spherical MPS-2HNH2, respectively.
Thermogravimetry (TG) was performed to estimate the amount of the functional group on the surface of silica particles. The functionalized amounts of amino and carboxyl groups are listed in . The spherical MPS contained amino groups of 1.59–3.36 μmol/mg. By contrast, the amino amounts on the MPS sheet and Stöber silica were decreased to 0.80–1.71 μmol/mg and 0.55–1.98 μmol/mg, respectively. It can be surmised that the spherical MPS had a larger surface area than the MPS sheet and Stöber silica particles. In all the amino-silica particles, the increased chain length resulted in a decrease in the amount of the amino-functionalization. On the other hand, the MPS sheet had the largest amount of carboxyl groups, i.e., 1.26, 1.29, and 1.80 μmol/mg for MPS sheet-NHCOOH, -2ENHCOOH, and -2HNHCOOH, respectively. The COOH/NH2 ratios were 1.05, 1.12, and 1.58. Even though spherical MPS had a large amount of amino functionalization, however, the amount of the carboxyl group and the COOH/NH2 ratio were greatly decreased to 0.31–0.44 μmol/mg and 0.13–0.2 μmol/mg, respectively. It is believed that pores 2 nm in size were filled with amino groups. It was difficult to introduce diglycolic anhydride into the pores of amino-functionalized spherical MPS. Stöber silica also had small carboxyl-functionalized amounts. In comparison with the structures of amino groups, the carboxyl-functionalized amounts could be increased by increasing the number of amino groups [from one (-NH2) to two (-2ENH2 and -2HNH2)]. Long amino chains (from -2ENH2 to -2HNH2) thus increased the amount of carboxyl on silica particles.
Table 2. The amount of amino and carboxyl functionalization on silica particles on various processes.
3.3. Metal ion adsorption on carboxyl-MPSs
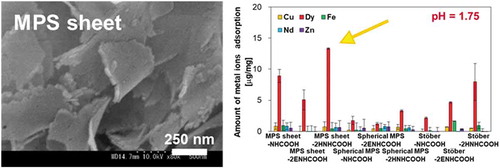
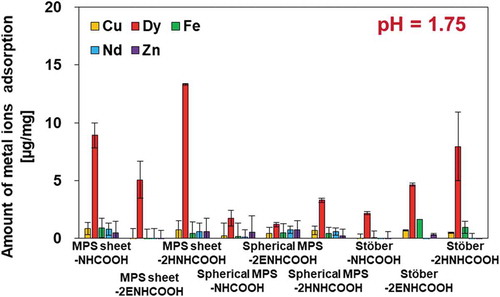
Metal ions were adsorbed on prepared silica-NHCOOH, and the adsorption amounts were investigated. From a practical point of view, the adsorption experiment was carried out on a heavy metals mixture (Cu, Dy, Fe, Nd, and Zn) at 20°C. Metal ions are thought to be adsorbed on silica particles on carboxyl groups, and the electric charge of the carboxyl groups is important for the adsorption of metal ions. We, therefore, investigated the amounts of metal ions adsorbed on prepared carboxyl-silica particles at pH = 1.25, 1.5, and 1.75. shows the amount adsorbed on carboxyl silica particles at pH = 1.75, and the results of adsorption at pH = 1.25 and 1.5 are shown in Figure S2. Carboxyl-silica particles can selectively adsorb Dy ions from metal ion mixtures at these pH values. The amount of metal ions other than Dy adsorbed was less than 1.7 μg/mg. At pH = 1.75, MPS sheet-2HNHCOOH showed the highest adsorption capacity (13.3 μg/mg). The amount of Dy adsorption on MPS sheet-NHCOOH was 8.9 μg/mg. However, MPS sheet-2ENHCOOH had a lesser amount of Dy adsorption (5.1 μg/mg). The amount of Dy adsorbed on carboxyl-Stöber silica particles was increased (to 2.2–8.0 μg/mg) by increasing the number of amino- and carboxyl-groups and the chain length (from-NHCOOH to -2HNHCOOH). Carboxyl-spherical MPS samples, on the other hand, showed considerably lower Dy adsorption amounts (less than 3.3 μg/mg). The functionalized amount of carboxyl groups decreased due to their long, narrow pores, while Dy was hard to load into the pores on spherical MPS. Based on these results, it was clear that the sheet morphology showed a better Dy adsorption ability than the sphere morphology. Finally, changing the morphologies of spherical and sheet type MPS and Stöber influenced the Dy ion capacity on the surface. It was expected that MPS-sheet has better adsorption performance than spherical adsorption because the Dy ion binding sites were greatly exposed [Citation29,Citation30]. Thus, we concluded that the surface structure of MPS-sheet was effective for Dy ion adsorption.
Figure 4. Adsorption of metal ions on functionalized MPS particles from the mixture. Five metal ions (Cu, Dy, Fe, Nd, and Zn) were used in this experiment at pH = 1.75.
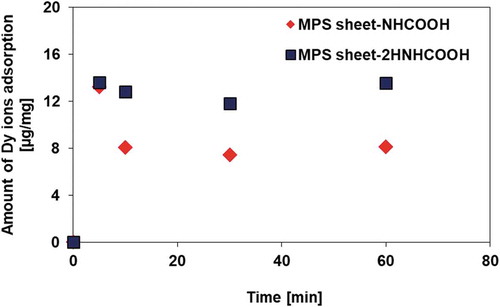
At a pH 1.25 of metal ion solution, the Dy amounts on carboxyl-MPS sheet were decreased (Figure S2). Carboxyl-Stöber silica particles, Stöber-2HNHCOOH, showed a good Dy adsorption ability (9.8 μg/mg) at pH = 1.5. The adsorption amount on Stöber-2HNHCOOH was, however, lower than that on MPS sheet-2HNHCOOH. Additionally, amino-silica particles without carboxyl-functionalization using diglycolic anhydride adsorbed extremely small amounts of metal ions, suggesting that carboxyl groups are essential for the adsorption of Dy ions (Figure S3). To confirm the adsorption equilibrium, adsorption experiments were carried out for 60 min using carboxyl-silica particles ().
3.4. Adsorption behavior
Understanding the mechanism of Dy ion adsorption onto functionalized MPS is of great importance to further optimization for future applications. The equilibrium data described the applicable adsorption isotherms. To evaluate the adsorption mechanism, various concentrations of Dy adsorption isotherms on MPS sheet-NHCOOH and MPS sheet-2HNHCOOH were plotted, as shown in Fig. S4. These data were fitted to Langmuir and Freundlich adsorption models. The correlation coefficients for the Langmuir and Freundlich models and the maximum amounts of Dy adsorption (Qmax) on MPS were calculated and listed in . The results showed that the R2 values for Langmuir plots for MPS sheet-NHCOOH and MPS sheet-2HNHCOOH were both 0.99, while the R2 values for the Freundlich plots were 0.69 and 0.85, respectively. It was therefore considered that the mechanism of Dy ion adsorption on MPS sheet-NHCOOH and MPS sheet-2HNHCOOH might be related to monolayer adsorption. The Qmax values for MPS sheet-NHCOOH and MPS sheet-2HNHCOOH were 8.9 and 13.8 µg/mg.
Table 3. Langmuir and Freundlich constants for Dy ions adsorption.
3.5. Cycling test
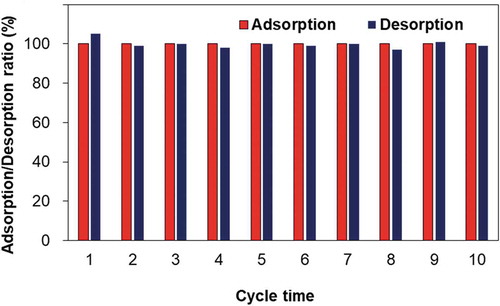
Cycling efficiency is one of the most important characteristics of adsorbents from the ecological point of view. A cycling test was carried out using the sheet-like MPS with the highest Dy adsorption capacity. The adsorption capacity was recorded after each cycle, and the corresponding results are shown in . It can be observed that there was a minimal loss of adsorption, even after 10 cycles. This result demonstrated that the functionalized groups on MPS surfaces were retained during each desorption process.
Figure 6. Cycling tests of MPS sheet particles were performed 10 times for the adsorption and desorption processes. The values were calculated from the supernatant concentration of Dy ions using Equation (4).
4. Conclusions
The aim of this present study was to develop MPS materials with highly selective Dy ion adsorption characteristics. In the study, MPS particles with sheet and spherical morphologies as well as Stöber silica were synthesized. Particles were treated by silane coupling reagents of APTES, N2APTES, and N6APTES, and carboxyl functionalization was conducted using diglycolic acid. In the ion adsorption experiments, the amount of Dy ion adsorbed was greater on each silica particle with large numbers of amino and carboxyl groups (-2HNHCOOH). In addition, MPS particles with sheet structures exhibited the greatest Dy adsorption capacity compared with the changed morphologies of silica particles such as spherical and Stöber silica after treatment. MPS sheet-2HNHCOOH exhibited the maximum adsorption capacity for Dy ions (13.3 µg/mg). In the isotherm equilibrium data, MPS sheet-2HNHCOOH with a monolayer applied obeyed the Langmuir model. Finally, the cycle performance of MPS sheet-2HNHCOOH was evaluated. The results suggested that its high carboxyl-functionalization makes the MPS sheet-2HNHCOOH an excellent candidate for use as a selective recovery agent for Dy ions. Thus, this study revealed a route to significantly improving Dy ion recovery materials that will undoubtedly be useful for the recycling of rare earth elements.
Supplemental Material
Download MS Word (263.4 KB)Acknowledgments
This work was supported in part by Grant-in-Aid for Scientific Research (C) No. 15K06474 from the Japan Society for the Promotion of Science (JSPS).
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Aghayan H, Mahjoub AR, Khanchi AR. Samarium and dysprosium removal using 11-molybdo-vanadophosphoric acid supported on Zr modified mesoporous silica SBA-15. Chem Eng J. 2013;225:509–519.
- Ogata T, Narita H, Tanaka M. Adsorption behavior of rare earth elements on silica gel modified with diglycol amic acid. Hydrometallurgy. 2015;152:179–182.
- Aguado J, Arsuaga JM, Arencibia A, et al. Aqueous heavy metals removal by adsorption on amine-functionalized mesoporous silica. J Hazard Mater. 2009;163:213–221.
- Liu AM, Hidajat K, Kawi S, et al. A new class of hybrid mesoporous materials with functionalized organic monolayers for selective adsorption of heavy metal ions. Chem Commun. 2000;1145–1146.
- Binnemans K, Jones PT, Blanpain B, et al. Recycling of rare earth: a critical review. J Cleaner Prod. 2013;51:1–22.
- Moldoveanu GA, Papangelakis VG. Recovery of rare earth elements adsorbed on clay minerals: leaching with ammonium sulfate. Hydrometallurgy. 2013;131–132:158–166.
- Zheng X, Liu E, Zhang F, et al. Efficient adsorption and separation of dysprosium from NdFeB magnets in an acidic system by ion imprinted mesoporous silica sealed in a dialysis bag. Green Chem. 2016;18:5031–5040.
- Zhang N, Hu B, Huang C. A new ion-imprinted silica gel sorbent for on-line selective solid-phase extraction of dysprosium (Ⅲ) with detection by inductively coupled plasma-atomic emission spectrometry. Anal Chim Acta. 2017;597:12–18.
- Yu Z, Liu B, Zhou H, et al. Mesoporous ZrO2 fibers with enhanced surface area and the application as recyclable absorbent. Appl Surf Sci. 2017;399:288–297.
- Orita T, Tomita M, Harada M, et al. Binding activity of avidin to the biotin within mesoporous silica materials for bioanalytical application. Anal Biochem. 2012;425:1–9.
- Zhao S, Zhang Y, Zhou Y, et al. One-step synthesis of core-shell structured mesoporous silica spheres templated by protic ionic liquid and CTAB. Mater Lett. 2016;178:35–38.
- Sohrabnezhad S, Jafarzadeh A, Pourahmad A, et al. Synthesis and characterization of MCM–41 ropes. Mater Lett. 2018;212:16–19.
- Dong X, Wang Y, Dan H, et al. A facile route to synthesize mesoporous SBA–15 silica spheres from powder quartz. Mater Lett. 2017;204:97–100.
- Fois E, Gamba A, Tabacchi G, et al. Ab initio study of defect sites at the inter surface of mesoporous silicas. J Phys Chem B. 2003;107:10767–10772.
- Zhao S, He M, Zhou Y, et al. Synthesis of micro/mesoporous silica material by dual-template method as a heterogeneous catalyst support for alkylation. RSC Adv. 2015;5:28124–28132.
- Santos SMI, Cecilia JA, Garcia EV, et al. Adsorption of biomolecules in porous silicas modified with zirconium. Effect of the textural properties and acidity. Micropor Mesopor Mater. 2018;260:146–154.
- Kato K, Suzuki M, Tanemura M, et al. Preparation and catalytic evaluation of cytochrome c immobilized on mesoporous silica materials. J Ceram Soc Jpn. 2010;118:410–416.
- Wu L, Zhang H, Wu M, et al. Dual-templating synthesis of multi-shelled mesoporous silica nanoparticles as catalyst and drug carrier. Micropor Mesopor Mater. 2016;228:318–328.
- Park S, Jeon Y, Jun K, et al. Synthesis and characterization of the surface modified SBA–15 with dicobaltcarbonyl complex. Bull Korean Chem Soc. 2014;35:2077–2080.
- Hikosaka R, Nagata F, Tomita M, et al. Optimization of pore structure and particle morphology of mesoporous silica for antibody adsorption for use in affinity chromatography. Appl Surf Sci. 2016;384:27–35.
- Hikosaka R, Nagata F, Tomita M, et al. Adsorption and desorption characteristics of DNA onto the surface of amino functional mesoporous silica with various particle morphologies. Colloids Surf B Biointerfaces. 2016;140:262–268.
- Brunauer S, Emmett PH, Teller E. Adsorption of gases in multimolecular layers. J Am Chem Soc. 1938;60:309–319.
- Nakanishi K, Tomita M, Kato K. Improvement in the catalytic activity of cytochrome c by immobilization on a novel mesoporous silica sheet. RSC Adv. 2014;4:4732–4735.
- Yano K, Fukushima Y. Synthesis of mono-dispersed mesoporous silica spheres with highly ordered hexagonal regularity using conventional alkyltrimethylammonium halide as a surfactant. J Mater Chem. 2004;14:1579–1584.
- Kambara K, Shimura N, Ogawa M. Larger scale syntheses of surfactant templated nanoporous silica spherical particles by the Stӧber method. J Ceram Soc Jpn. 2007;115:315–318.
- Chong ASM, Zhao XS. Functionalization of SBA–15 with APTES and characterization of functionalized materials. J Phys Chem B. 2003;107:12650–12657.
- An Y, Chen M, Xue Q, et al. Preparation and self-assembly of carboxylic acid-functionalized silica. J Colloid Interface Sci. 2007;311:507–513.
- Levan MD, Vermeulen T. Binary Langmuir and Freundlich isotherm for ideal adsorbed solutions. J Phys Chem. 1981;85:3247–3250.
- Nakanishi K, Tomita M, Kato K. Synthesis of amino-functionalized mesoporous silica sheets and their application for metal ion capture. J Asian Ceram Soc. 2015;3:70–76.
- Doadrio A, Sánchez-Montero J, Doadrio J, et al. A molecular model to explain the controlled release from SBA-15 functionalized with APTES. Micropor Mesopor Mater. 2014;195:43–49.